Controlling the geometry of Cu(II) tectons to build one-dimensional hydrogen bonded chains†‡
Sergio
Martínez-Vargas
,
Simón
Hernández-Ortega
,
Rubén A.
Toscano
,
Domingo
Salazar-Mendoza
and
Jesús
Valdés-Martínez
*
Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, 04510, Coyoacán, Cd. México, D.F. México. E-mail: jvaldes@servidor.unam.mx
First published on 10th September 2007
Abstract
Through an analysis of the Cambridge Structural Database, CSD, we found that Cu(II) complexes with planar tridentate amines and two monodentate ligands tend to present a square pyramidal geometry. We decided to test the 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine, trpy, as a ternary ligand to build [Cu(trpy)(CA)(H2O)] tectons, CA representing an organic carboxylate, in the supramolecular synthesis of an H-bonded coordination compound. Six new crystal structures are reported and analyzed with other similar compounds reported in the CSD. In all of them a square pyramidal geometry and 1-D supramolecular structures are obtained through an O–H⋯O hydrogen bond between the coordinated water molecule and one O atom from the carboxylate ligand. Thus, molecular and supramolecular control over the Cu(II) complexes is achieved.
6′2″-terpyridine, trpy, as a ternary ligand to build [Cu(trpy)(CA)(H2O)] tectons, CA representing an organic carboxylate, in the supramolecular synthesis of an H-bonded coordination compound. Six new crystal structures are reported and analyzed with other similar compounds reported in the CSD. In all of them a square pyramidal geometry and 1-D supramolecular structures are obtained through an O–H⋯O hydrogen bond between the coordinated water molecule and one O atom from the carboxylate ligand. Thus, molecular and supramolecular control over the Cu(II) complexes is achieved.
1 Introduction
The synthesis of designed solid state functional materials is an important field of research in modern chemistry. One of the more successful strategies used in this field, usually called crystal engineering, is the so called Desiraju–Wuest approach. This approach requires on the one hand reliable molecular building blocks with simple geometries that will be maintained during the synthesis process, and on the other hand robust intermolecular interactions that will organize the molecular building blocks through self-assembly processes into networks with predictable connectivity. The molecular building blocks containing the self-recognition functional groups were called tectons by Wuest.1,2 The geometry arrangement obtained through intermolecular interactions were defined by Desiraju as supramolecular synthons.3The most widely used intermolecular interactions are hydrogen bonds, this is due to the fact that they are relatively strong and directional. In metal containing systems, the metal–ligand bond is also used as a supramolecular interaction. The synthesis of coordination frameworks (or coordination polymers) is a very active field of research. Such metal centers are introduced into networks mainly for two reasons, firstly, their presence incorporates the properties of the metal into the network, the second reason is that metal centers provide geometric features not found in organic chemistry. Copper(II) is a very interesting metal ion due to its magnetic properties. Unfortunately, from the point of view of structural consistency, Cu(II) displays a very varied and unpredictable coordination chemistry, which makes it all the more difficult to incorporate such ions into extended assemblies with a predetermined topology and connectivity. As an example of this situation, in a recent study we were able to control the supramolecular structure of Cu(II) complexes with carboxylic acids using isonicotinamide as a second ligand with two functions. On the one hand, the pyridine N atom coordinates to the Cu(II) ion, and on the other hand the amide group forms self-complementary H-bonds generating 1D chains. Unfortunately, we had no control on the geometry around the metal ion, so a diversity of geometries were obtained.4 Looking for a solution to this problem we found some reports which indicate that tridentate amines induce pentacoordination around Cu(II) ions;5 however, to the best of our knowledge, there are no systematic studies that confirm this possible control on the geometry of the metal ion. An analysis of the Cambridge Structural Database, CSD, reported here, indicates that in fact a square pyramid is the favored geometry for Cu(II) complexes with tridentate amines. Thus, we decided to start a study of different tridentate amines with the hypothesis that these ligands will act as blocking ligands and control the geometry of the metal complex, and in this way we will obtain reliable tectons for supramolecular synthesis. As a starting point we decided to use a rather rigid ligand, the 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine (trpy), to guarantee the planarity of the ligand.
6′2″-terpyridine (trpy), to guarantee the planarity of the ligand.
As a second ligand we decided to use carboxylic acids, based on the fact that they are accessible ligands with a very interesting coordination chemistry. If through the use of tridentate amines we are able to control the geometry of the metal ion, we will be able to study the rich chemistry of copper complexes with carboxylic acids in systems where there will be control of at least one variable, namely the geometry around the metal center. This understanding will allow the design of molecular materials with specific properties, for example, molecular magnets. In this study we used monocarboxylic (HCA) and dicarboxylic acids (H2DCA). The H2DCA have two interesting features: on the one hand, if they act as dianions (DCA) with copper(II) there will be no need of other counter-anions which may interfere unpredictably with the H-bonding. On the other hand, it is possible, by changing the pH, to obtain the monoanion (HDCA) of the dicarboxylic acid, increasing in this way the number of H-bond donors available to organize the molecules in the crystal. The expected tectons as well as the different carboxylic acids used are shown in Scheme 1.
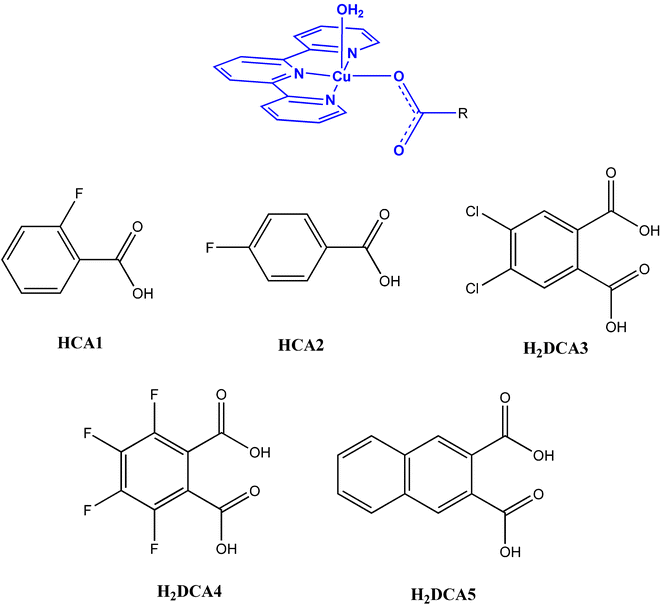 | ||
| Scheme 1 Tecton and the different carboxylic acids used in this study. | ||
Aiming at molecular and supramolecular control in Cu(II) complexes, in this paper we present and analyze the crystal structures of the following compounds: (aqua)(2-fluorobenzoato-k O)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine k3N,N′,N″)copper(II), perchlorate, 1, (aqua)(4-fluorobenzoato-kO)-(2,2′
6′2″-terpyridine k3N,N′,N″)copper(II), perchlorate, 1, (aqua)(4-fluorobenzoato-kO)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine k3N,N′,N″)copper(II), perchlorate, 2, (aqua)(4,5-dichlorophthalato-k2O,O′)(2,2′
6′2″-terpyridine k3N,N′,N″)copper(II), perchlorate, 2, (aqua)(4,5-dichlorophthalato-k2O,O′)(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine-k3 N,N′,N″)copper(II), trihydrate, 3, (aqua)(4,5-dichlorophthalato-k2O,O′)-(2,2′
6′,2″-terpyridine-k3 N,N′,N″)copper(II), trihydrate, 3, (aqua)(4,5-dichlorophthalato-k2O,O′)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine-k3N,N′,N″)-copper(II), 4,5-dichlorophthalato, hydrate, 4, (aqua)(tetrafluorophthalato-k2O,O′)-(2,2′
6′2″-terpyridine-k3N,N′,N″)-copper(II), 4,5-dichlorophthalato, hydrate, 4, (aqua)(tetrafluorophthalato-k2O,O′)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine-k3N,N′,N″)copper(II), perchlorate, 5, (aqua)(2,3-naphthalato-k2O,O′) -(2,2′
6′2″-terpyridine-k3N,N′,N″)copper(II), perchlorate, 5, (aqua)(2,3-naphthalato-k2O,O′) -(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine-k3N,N′,N″)copper(II), trihydrate, 6.
6′2″-terpyridine-k3N,N′,N″)copper(II), trihydrate, 6.
2 Experimental
All chemicals were purchased from Aldrich and used without further purification.The compounds were prepared by adding the stoichiometric amounts of aqueous solution of copper(II) perchlorate hexahydrate to 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine dissolved in ethanol with continuous stirring, followed by the addition of the corresponding carboxylic acid dissolved in the minimum volume of hot water. The reaction mixtures were stirred while gently heating for 30 min. The solutions obtained were let to evaporate slowly at room temperature, to obtain single crystals suitable for X-ray structure determination.
6′,2″-terpyridine dissolved in ethanol with continuous stirring, followed by the addition of the corresponding carboxylic acid dissolved in the minimum volume of hot water. The reaction mixtures were stirred while gently heating for 30 min. The solutions obtained were let to evaporate slowly at room temperature, to obtain single crystals suitable for X-ray structure determination.
Aqua-(2-fluorobenzoato-k O)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine k3N,N′,N″)-copper(II), perchlorate, 1. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.0 mg, 0.1 mmol) was added to an ethanolic solution (10 ml) of 2,2′
6′2″-terpyridine k3N,N′,N″)-copper(II), perchlorate, 1. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.0 mg, 0.1 mmol) was added to an ethanolic solution (10 ml) of 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine (23.3 mg, 0.10 mmol) whilst stirring, followed by the addition of 2-fluorobenzoic acid (HCA1,14.0 mg, 0.10 mmol) dissolved in hot water (5 ml) to form a light-blue solution. Light-blue single crystals, suitable for X-ray structure determination, were obtained by slow evaporation at room temperature after a week. Yield: 40.9 mg, 74%.
6′,2″-terpyridine (23.3 mg, 0.10 mmol) whilst stirring, followed by the addition of 2-fluorobenzoic acid (HCA1,14.0 mg, 0.10 mmol) dissolved in hot water (5 ml) to form a light-blue solution. Light-blue single crystals, suitable for X-ray structure determination, were obtained by slow evaporation at room temperature after a week. Yield: 40.9 mg, 74%.
Aqua-(4-fluorobenzoato-k O)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine-k3N,N′,N″)-copper(II), perchlorate, 2. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol) was mixed with an ethanolic solution (10 ml) of 2,2′
6′2″-terpyridine-k3N,N′,N″)-copper(II), perchlorate, 2. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol) was mixed with an ethanolic solution (10 ml) of 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine (23.3 mg, 0.10 mmol). 4-Fluorobenzoic acid (HCA2, 14.0 mg, 0.10 mmol) dissolved in hot water (5 ml) was then added whilst stirring. The solution turned light-blue. After a week at room temperature light-blue single crystals suitable for X-ray structure determination were produced by slow evaporation. Yield: 39.8 mg, 72%.
6′,2″-terpyridine (23.3 mg, 0.10 mmol). 4-Fluorobenzoic acid (HCA2, 14.0 mg, 0.10 mmol) dissolved in hot water (5 ml) was then added whilst stirring. The solution turned light-blue. After a week at room temperature light-blue single crystals suitable for X-ray structure determination were produced by slow evaporation. Yield: 39.8 mg, 72%.
Aqua-(4,5-dichlorophthalato-k2O,O′)(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″- terpyridine-k3N,N′N″)copper(II), trihydrate, 3. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.10 mmol) was mixed with 2,2′
6′,2″- terpyridine-k3N,N′N″)copper(II), trihydrate, 3. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.10 mmol) was mixed with 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml), followed by 4,5-dichloro-phthalato (H2DCA3, 23.5 mg, 0.10 mmol) dissolved in hot water (5 ml) whilst stirring. NaOH 1.0 N was added until a pH value of ca. 6. The solution turned light-green. Sky-blue single crystals, suitable for X-ray structure determination, were obtained after a week by slow evaporation at room temperature. Yield: 46 mg, 70%.
6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml), followed by 4,5-dichloro-phthalato (H2DCA3, 23.5 mg, 0.10 mmol) dissolved in hot water (5 ml) whilst stirring. NaOH 1.0 N was added until a pH value of ca. 6. The solution turned light-green. Sky-blue single crystals, suitable for X-ray structure determination, were obtained after a week by slow evaporation at room temperature. Yield: 46 mg, 70%.
Aqua-(4,5-dichlorophthalato-k2O,O′)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine k3N,N′,N″)-copper(II), 4,5-dichlorophthalato, hydrate, 4. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.10 mmol) was mixed with 2,2′
6′2″-terpyridine k3N,N′,N″)-copper(II), 4,5-dichlorophthalato, hydrate, 4. An aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.10 mmol) was mixed with 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml), 4,5-dichloro-phthalato, (H2DCA3, 23.5 mg, 0.10 mmol) dissolved in hot water (5 ml) was then added whilst stirring. The solution turned light-green. Sky-blue single crystals suitable for X-ray structure determination were yielded after a week of slow evaporation at room temperature. Yield: 51.3 mg, 64%.
6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml), 4,5-dichloro-phthalato, (H2DCA3, 23.5 mg, 0.10 mmol) dissolved in hot water (5 ml) was then added whilst stirring. The solution turned light-green. Sky-blue single crystals suitable for X-ray structure determination were yielded after a week of slow evaporation at room temperature. Yield: 51.3 mg, 64%.
Aqua-(tetrafluorophthalato-k2O,O′)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine-k3N,N′,N″)-copper(II), perchlorate, 5. 2,2′
6′,2″-terpyridine-k3N,N′,N″)-copper(II), perchlorate, 5. 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml) and an aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol), was mixed with tetrafluoro-phthalic acid (H2DCA4, 23.8 mg, 0.10 mmol) dissolved in hot water (5 ml) to obtain a light-green solution. The solution was set aside at room temperature until blue single crystals suitable for X-ray structure determination were obtained. Yield: 173 mg, 68%.
6′,2″-terpyridine (30.9 mg, 0.10 mmol) dissolved in ethanol (10 ml) and an aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol), was mixed with tetrafluoro-phthalic acid (H2DCA4, 23.8 mg, 0.10 mmol) dissolved in hot water (5 ml) to obtain a light-green solution. The solution was set aside at room temperature until blue single crystals suitable for X-ray structure determination were obtained. Yield: 173 mg, 68%.
Aqua-(2,3-naphthalene-dicarboxylato-k2O,O′)-(2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′2″-terpyridine-k3N,N′,N″)-copper(II), trihydrate, 6. 2,2′
6′2″-terpyridine-k3N,N′,N″)-copper(II), trihydrate, 6. 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-Terpyridine (30.9 mg, 0.10 mmol) dissolved in methanol (10 ml) and an aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol), were mixed with 2,3-naphthalene-dicarboxylic acid (H2DCA5, 21.6 mg, 0.10 mmol) dissolved in hot water (5 ml) to obtain a light-blue solution. The solution was set aside at room temperature until blue single crystals were obtained. Yield: 46 mg, 72%.
6′,2″-Terpyridine (30.9 mg, 0.10 mmol) dissolved in methanol (10 ml) and an aqueous solution (5 ml) of Cu(ClO4)2·6H2O (37.2 mg, 0.1 mmol), were mixed with 2,3-naphthalene-dicarboxylic acid (H2DCA5, 21.6 mg, 0.10 mmol) dissolved in hot water (5 ml) to obtain a light-blue solution. The solution was set aside at room temperature until blue single crystals were obtained. Yield: 46 mg, 72%.
2.1 Crystallography
Single crystals of 1–6 were mounted in random orientation on a glass fiber. The crystal data were collected on a Brucker SMART APEX CCD-based three-circle diffractometer (1, 2, 4, 5) or a Siemens P4/PC diffractometer (3, 6) using graphite monochromated Mo Kα (λ = 0.71073 Å) radiation at 293(2) K with SMART software.6a or XSCANS.6b The data were corrected by absorption effects with analytical (1, 2, 4, 5) or ψ-scans (3, 6). The structures were solved by direct methods (SHELXS-97)7 and refined with all data by full matrix least squares using SHELXL.8 All non-hydrogen atoms were localized from the difference electron density map and refined anisotropically. The hydrogen atoms were placed in geometrically idealized positions [0.97 Å (CH2) and 0.96 Å (CH3)] tied to the parent atom with Uiso(H) = 1.2 UeqC(sp2) and 1.5UeqC(sp2) for C(sp3) and refined using the riding model. Geometric calculations were done using Platon.9Table 1 provides crystallographic details for 1–6. Selected bond distances and angles for 1–6 are presented in Table 2. Tables of hydrogen bonds and π–π interactions are submitted as supplementary material.‡ CCDC 617692–617698 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viahttp://www.ccdc.cam.ac.uk/data_request/cif.| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Formula | C22H16ClFN3OCu | C22H17ClFN3O7Cu | C23H21Cl2N3O8Cu | C31H21Cl4N3O10Cu | C46H33ClF8N6O18Cu2 | C27.6H26.2N3O8Cu |
| M r/g mol–1 | 552.38 | 553.39 | 601.87 | 800.85 | 1272.31 | 591.46 |
| Crystal system | Triclinic | Monoclinic | Triclinic | Monoclinic | Monoclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P2/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c | P21 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 7.4640(5) | 8.4714(7)) | 7.359(1) | 7.3808(6) | 10.216(1) | 7.032(1) |
| b/Å | 11.7638(8) | 20.276(2) | 12.348(1) | 32.937(3) | 18.514(1) | 12.785(2) |
| c/Å | 13.5851(9) | 13.315(1) | 13.870(2) | 13.184(1) | 27.606(2) | 14.381(2) |
| α/° | 81.132(1) | 90 | 87.08(1) | 90 | 90 | 86.59(2) |
| β/° | 79.124(1) | 100.051(2) | 81.66(1) | 99.534(2) | 96.488(1) | 85.08(2) |
| γ/° | 73.027(1) | 90 | 78.55(1) | 90 | 90 | 84.95(1) |
| V/Å3 | 1114.1(1) | 2251.9(3) | 1221.9(3) | 3160.7(5) | 2420.2(2) | 1281.4(3) |
| Z | 2 | 4 | 2 | 4 | 2 | 2 |
| Reflections collected | 9162 | 18210 | 4327 | 25681 | 19951 | 4907 |
| Indep. reflections | 3914 | 3963 | 3998 | 5553 | 8524 | 4504 |
| R int | 0.033 | 0.0869 | 0.0644 | 0.1027 | 0.0388 | 0.0315 |
| Obs. refl. [I > 2σ(I)] | 3148 | 2046 | 3243 | 4844 | 6760 | 3786 |
| R/wR [I > 2σ(I)]2 | 0.0472/0.1336 | 0.0597/0.1146 | 0.0369/0.0847 | 0.0546/0.0821 | 0.0395/0.0700 | 0.0465/0.1198 |
| R/wR (all reflect.) | 0.0554/0.1291 | 0.1111/0.1245 | 0.0520/0.0922 | 0.1091/0.0914 | 0.0502/0.0725 | 0.0609/0.1354 |
| 1 | |||||||
| Cu1–O1 | 1.916(2) | Cu1–N3 | 2.011(3) | O1–Cu1–O3 | 86.9(1) | O3–Cu1–N3 | 98.3(1) |
| Cu1–N1 | 2.034(3) | Cu1–O3 | 2.276(3) | O3–Cu1–N1 | 89.8(1) | O1–Cu1–N2 | 169.0(1) |
| Cu1–N2 | 1.933(3) | O3–Cu1–N2 | 104.0(1) | N1–Cu1–N3 | 159.7(1) | ||
| 2 | |||||||
| Cu1–O1 | 1.897(4) | Cu1–N3 | 2.024(5) | O1–Cu1–O3 | 88.5(2) | O3–Cu1–N3 | 93.1(2) |
| Cu1–N1 | 2.030(5) | Cu1–O3 | 2.291(5) | O3–Cu1–N1 | 94.0(2) | O1–Cu1–N2 | 173.4(2) |
| Cu1–N2 | 1.927(4) | O3–Cu1–N2 | 98.0(2) | N1–Cu1–N3 | 159.6(2) | ||
| 3 | |||||||
| Cu1–O1 | 1.932(2) | Cu1–N3 | 2.036(3) | O1–Cu1–O3 | 82.2(1) | O3–Cu1–N3 | 92.5(1) |
| Cu1–N1 | 2.027(3) | Cu1–O3 | 2.214(2) | O3–Cu1–N1 | 100.0(1) | O1–Cu1–N2 | 168.6(1) |
| Cu1–N2 | 1.951(2) | O3–Cu1–N2 | 108.6(1) | N1–Cu1–N3 | 158.5(1) | ||
| 4 | |||||||
| Cu1–O1 | 2.168(3) | Cu1–N3 | 2.035(4) | O1–Cu1–O3 | 89.9(1) | O3–Cu1–N3 | 95.4(2) |
| Cu1–N1 | 2.033(4) | Cu1–O3 | 1.938(3) | O3–Cu1–N1 | 102.2(2) | O1–Cu1–N2 | 107.3(1) |
| Cu1–N2 | 1.941(4) | O3–Cu1–N2 | 162.4(2) | N1–Cu1–N3 | 157.8(2) | ||
| 5 | |||||||
| Cu1–O1 | 1.928(3) | Cu1–N3 | 2.022(4) | O1–Cu1–O3 | 89.5(2) | O3–Cu1–N3 | 91.5(2) |
| Cu1–N1 | 2.027(4) | Cu1–O3 | 2.218(4) | O3–Cu1–N1 | 98.0(2) | O1–Cu1–N2 | 172.0(2) |
| Cu1–N2 | 1.937(4) | O3–Cu1–N2 | 98.5(2) | N1–Cu1–N3 | 158.7(2) | ||
| Cu2–O6 | 1.912(3) | Cu2–N6 | 2.015(4) | O6–Cu2–O8 | 86.7(1) | O8–Cu2–N6 | 100.9(2) |
| Cu2–N4 | 2.044(4) | Cu2–O8 | 2.301(4) | O8–Cu2–N4 | 100.9(2) | O6–Cu2–N5 | 173.3(2) |
| Cu2–N5 | 1.934(3) | O8–Cu2–N5 | 100.0(2) | N4–Cu2–N6 | 159.1(2) | ||
| 6 | |||||||
| Cu1–O1 | 1.922(3) | Cu1–N3 | 2.045(3) | O1–Cu1–O3 | 89.5(1) | O3–Cu1–N3 | 98.7(1) |
| Cu1–N1 | 2.045(3) | Cu1–O3 | 2.262(3) | O3–Cu1–N1 | 90.3(1) | O1–Cu1–N2 | 173.8(1) |
| Cu1–N2 | 1.949(3) | O3–Cu1–N2 | 96.2(1) | N1–Cu1–N3 | 158.2(1) | ||
The data base study was computed using the Cambridge Structural Database (CSD), version 5.28 (May 2007) containing 415246 entries.10 The only structures considered were those with no error, an R factor of less than 0.75 and 3-D coordinates determined. A ligand was considered planar tridentate when the dihedral angles between Cu–X–Y–Z was between 150 and 180° (where X, Y and Z represent the coordinated donor atoms). Also, only those producing five-membered rings were considered. As long as Cu(II) presents Jahn–Teller distortions, donor sets of 4 + 1, 4 + 2 and 5 + 1 are common. In this study we considered Cu(II)–L distances up to 3.0 Å as bonding distances, in this way the set with CN = 4 + 1 is included in CN = 5 and 4 + 2 and 5 + 1 are included in CN = 6.
3 Results
3.1 CSD study
A CSD study was done to evaluate the possible use of tridentate ligands to control the geometry of Cu(II) compounds, the main results are presented in Table 3. Firstly, we analyzed the frequency of different coordination numbers, CN, of Cu(II) compounds, finding that CN = 5 is the more common coordination number in the crystal structures reported in the CSD (64.6%). We then analyzed how frequent these coordination numbers were when tridentate ligands forming two five member rings were used, the pre-eminence of CN = 5 increases to 74.4%. An analysis of the stereochemistry of the complexes with CN = 5 using the model proposed by Kepert11 resulted in a considerable complication due to the large number of structures, but we found that a simple approach with complexes having one planar tridentate ligand and two monodentate ligands was possible. The angle between the two monodentate ligands is around 90° in a square pyramidal structure, SP, and 120° in a trigonal bypiramidal structure, TBP. A graphic including all the structures with CN = 5 and one tridentate ligand clearly indicates that the SP structure is favored, see Fig. 1. When complexes containing a tridentate planar ligand and monodentate ligands are used we found that CN = 5 is a lot more common that CN = 6, 91.7 and 8.3%, respectively, indicating that CN = 5 is clearly preferred.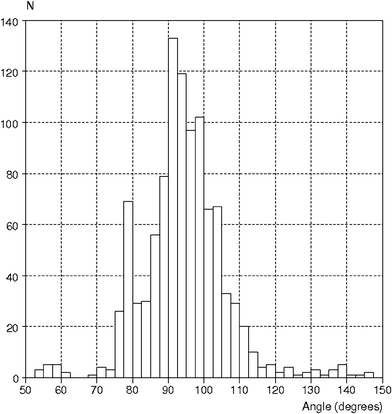 | ||
| Fig. 1 Histogram of the X–Cu–Y angle (X and Y monodentate ligands) in pentacoordinated Cu(II) compounds containing a tridentate ligand and two monodentate ligands. | ||
| Structure | Total number of structures | Number of structures with R < 0.075 |
|---|---|---|
| a Cu(II) complexes with any planar tridentate ligand. b Mono- and dicarboxylic acid. | ||
| Four-coordinate | 685 | 534 |
| Five-coordinate | 7266 | 6280 |
| Six-coordinate | 3252 | 2835 |
| Seven-coordinate | 28 | 22 |
| Eight-coordinate | 9 | 6 |
| Cu(planar tridentate)a, CN = 4 | 40 | 33 |
| Cu(planar tridentate), CN = 5 | 918 | 825 |
| Cu(planar tridentate), CN = 6 | 275 | 232 |
| Cu(planar tridentate), CN = 7 | 0 | 0 |
| Cu(planar tridentate), CN = 7 | 0 | 0 |
| Cu(planar tridentate) (monodentate)2, CN = 5 | 797 | 727 |
| Cu(planar tridentate) (monodentate)3, CN = 6 | 72 | 67 |
| Cu(planar tridentate)(H2O)(CAb) | 30 | 29 |
Considering the previous analysis, a series of six Cu(II) compounds with 2,2′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6′,2″-terpyridine as a planar tridentate ligand, carboxylic acids and one water molecule were obtained and their single crystal X-ray structures determined, Fig. 2.
6′,2″-terpyridine as a planar tridentate ligand, carboxylic acids and one water molecule were obtained and their single crystal X-ray structures determined, Fig. 2.
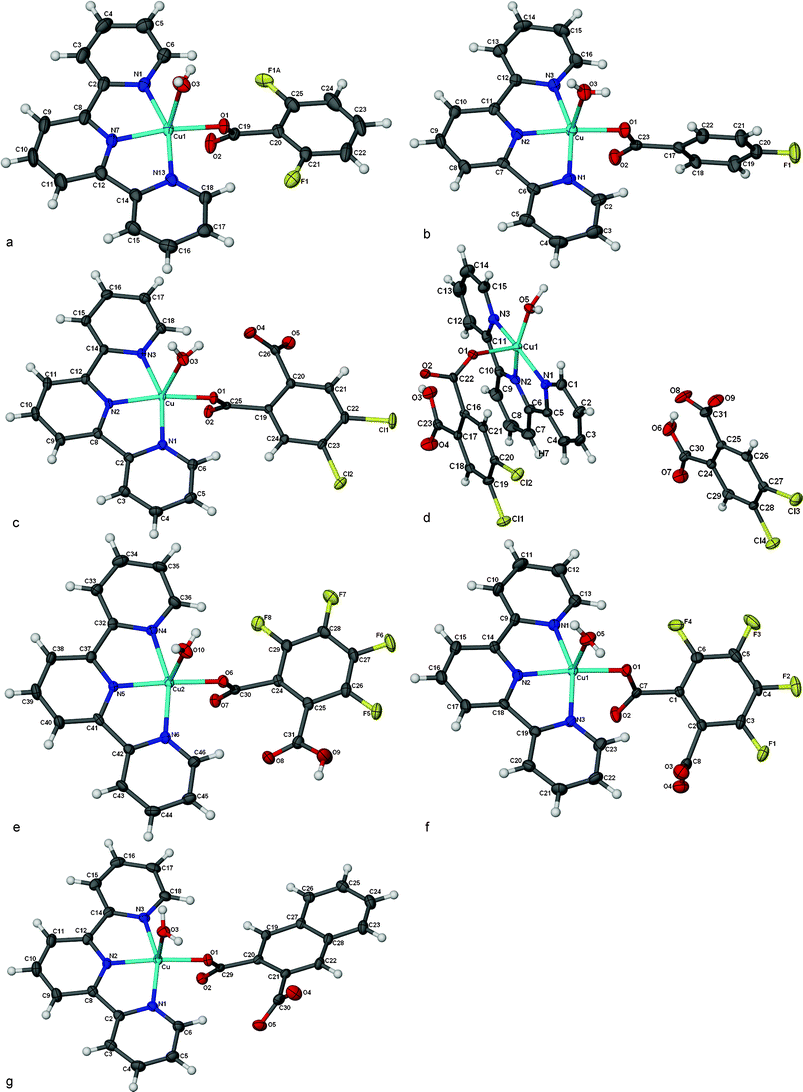 | ||
| Fig. 2 Molecular geometries, thermal ellipsoids, and numbering schemes for 1–6. | ||
3.2 Crystal structures
The crystal structure of 1 contains a [Cu(H2O)(trpy)(CA1)] cation and a disordered perchlorate anion, Fig. 2a. The CA1 is coordinated in basal position as well as the tridentate trpy ligand. The apical position is occupied by a water molecule. The complex cation presents a square pyramidal structure, τ = 0.16(1), as defined by Addison.12 The phenyl ring from the CA1 forms an angle of 12.1(2)° with respect to the mean plane of the central ring of the trpy. The coordinated water molecule forms an H-bond with the non-coordinated oxygen atom of the carboxylic acid of another molecule, generating a 1D H-bonded chain, O3⋯O2 (–1 + x, y, z), 2.756(4) Å, Fig. 3a. The phenyl rings of the CA1 ligand are always on the same side of the chain. There is also a π–π interaction between the N2/C7–C11 ring on one chain and the N3/C12–C15 (1 – x, –y, 1 – z) ring of another chain, with a centroid⋯centroid distance, Cg⋯Cg, of 3.833(2) Å.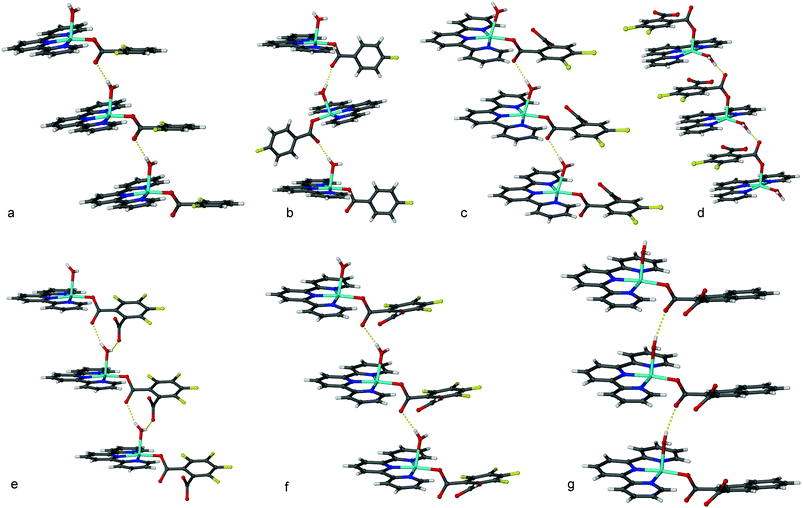 | ||
| Fig. 3 Hydrogen bonded chains observed in 1–6. | ||
The crystal structure of 2 shows a [Cu(H2O)(trpy)(CA2)]+ cation and a disordered perchlorate anion, Fig. 2b. The Cu(II) ion is pentacoordinated with a square pyramidal geometry, τ = 0.23(2). The trpy ligand is coordinated in a basal position with an oxygen atom of the CA2 in the fourth basal position. A water molecule is coordinated in an apical position. The phenyl ring of the CA2 is almost perpendicular to the trpy ligand. The coordinated water molecule forms a H-bond with the non-coordinated oxygen atom of the carboxylic acid of another complex cation producing a H-bonded 1D chain; O3⋯O2 (1/2 + x, ½ – y, ½ + z), 2.775(6) Å, the trpy and the CA2 alternate sides as shown in Fig. 3b. The 1D chains run parallel and interlock due to π–π interactions between the N1/C9–C13 ring with the N3/C19–C23 (1 + x, y, z) ring of another chain, with Cg⋯Cg, 3.630(4) Å. The phenyl ring of the CA2 (C17–C22) has also a π–π interaction with the CA2 of another chain, Cg⋯Cg (–x, 1 – y, –z), 3.804(4) Å.
The crystal structure of 3 contains the neutral molecule [Cu(H2O)(trpy)(DCA3)] and three water molecules. The metal complex is pentacoordinated with a square pyramidal geometry, τ = 0.17(1). The dianionic DCA3 coordinates as a monodentate. The trpy and the O atom from the phthalate coordinate in a basal position and the water molecule occupies the apical position, see Fig. 2c. The phenyl ring from the phthalate is almost parallel (1.6(2)°) with respect to the central ring of the trpy. One of the hydrogen atoms from the coordinated water molecule H-bonds with the oxygen atom of the coordinated carboxylate of another molecule, O3⋯O2 (–1 + x, y, z), 2.770(3) Å, forming the 1D chain shown in Fig. 3c. Parallel 1D structures pack through π–π interactions between N2/C8–C12 and N3/C13–C18 (1 – x, 1 – y, 2 – z), Cg⋯Cg = 3.695(2) Å, and N1/C2–C6 with C19–C24 (1 – x, 1 – y, 1 – z), Cg⋯Cg = 3.869(2) Å.
The asymmetric unit of 4 contains one [Cu(H2O)(trpy)(HDCA3)]+ cation, a HDCA3 anion and one solvent water molecule. The geometry of the Cu(II) cation is pentacoordinated with a square pyramidal geometry, τ = 0.08(2). The trpy is coordinated in a basal position as well as the water molecule and a mono-anionic phthalate coordinate in the apical position, see Fig. 2d. The coordinated water molecule H-bonds to the coordinated carboxylate generating 1D H-bonded chains, O5⋯O2 (–1 + x – 1, y, z), 2.602(5) Å, as shown in Fig. 3d. The solvate water molecule links the anions and chains together. There is an intramolecular π–π interaction between the HDCA3 (C16–C21) and one of the trpy rings (N1/C1–C5), Cg⋯Cg (x, –y, –z), 3.703(3) Å. The non-coordinated anionic HDCA3 stack through π–π interactions, Cg⋯Cg (–x, –y, 2 – z), 3.675(3) Å.
The asymmetric unit of 5 contains two different coordination compounds, a monocationic [Cu(H2O)(trpy)(HDCA4)]+ and the neutral [Cu(H2O)(trpy)(DCA4)]. One perchlorate anion and four water molecules are also present. In both complexes the coordination number is five with a square pyramidal geometry, with the trpy ligand and one O atom of the phthalic acid in a basal position and a water molecule in the apical position, τ(Cu1) = 0.22(2) and τ(Cu2) = 0.24(2). The phenyl ring from the DCA4 forms an angle of 11.4(2)° in the cationic complex, as shown in Fig. 2f and 21.8(2)° in the neutral complex, see Fig. 2e, with respect to the central ring of the trpy. Each complex forms a 1D H-bonded chain. In the cationic chain, see Fig. 3f, the H atom of the HDCA4 is H-bonded to a water molecule, O10⋯O7 (–1 + x, y, z), 2.827(4) Å, and this water molecule is also H-bonded to the chain formed by the neutral [Cu(trpy)(DCA4)(H2O)] complexes. In the neutral chain, Fig. 3e, the coordinated water molecule forms H-bonds to O atoms of both carboxylates of the DCA4 of another molecule generating a 1D H-bonded chain, O5⋯O2 (–1 + x, y, z), 2.858(5) Å. Alternating neutral–anionic molecules stack through π–π interactions, N4/C33–C36⋯N2/C14–C18 (1 – x, ½ + y, 1 – z), 3.670(3) Å, and N3/C19–C23 with N5/C37–C41 (–1 – x, ½ + y, 1 – z), Cg⋯Cg = 3.714(3) Å.
The asymmetric unit of the crystal structure of 6 contains a neutral coordination compound [Cu(trpy)(DCA5)(H2O)], one quarter of a methanol molecule and three water molecules, Fig. 2g. The metal complex is pentacoordinated with a square pyramidal geometry, τ = 0.26(2), Table 2. The dianionic 2,3-naphthalene-dicarboxylato coordinates as a monodentate. The trpy and an O atom from the carboxylate coordinate in basal position and the water molecule occupies the apical position. One of the hydrogen atoms from the coordinated water molecule H-bonds with the oxygen atom of the coordinated carboxylate of another complex forming a 1D chain shown in Fig. 3g, O3⋯O2 (1 + x, y, z), 2.791(4) Å. Two parallel 1D chains pack through π–π interactions, N1/C2–C6 with N1/C2–C6 (–x, 1 – y, 2 – z), Cg⋯Cg = 3.670(3) Å, and N3/C14–C18 with C19–C20–C21–C22–C28–C27 (1 – x, 1 – y, 1 – z), Cg⋯Cg, = 3.673(2) Å.
4 Discussion
The results from the CSD analysis clearly indicate that a pentacoordinated square pyramid is the more probable geometry in Cu(II) compounds with a planar tridentate ligand and two monodentate ligands. The compounds reported here, as well as the two structures reported in the CSD containing trpy, a carboxylic acid and a coordinated water molecule (JEDOIQ13 and NATHAR14), have a square pyramidal geometry as indicated by both the τ parameter 12 (τ = 0 for a perfect square pyramid, τ = 1 for a perfect trigonal bipyramid) and the Kepert's model.11 When this analysis is extended to Cu(II) compounds with any planar tridentate ligand and two monodentate ligands (28 more structures) the square pyramid is still the preferred geometry as shown in Fig. 4, see ESI for details.‡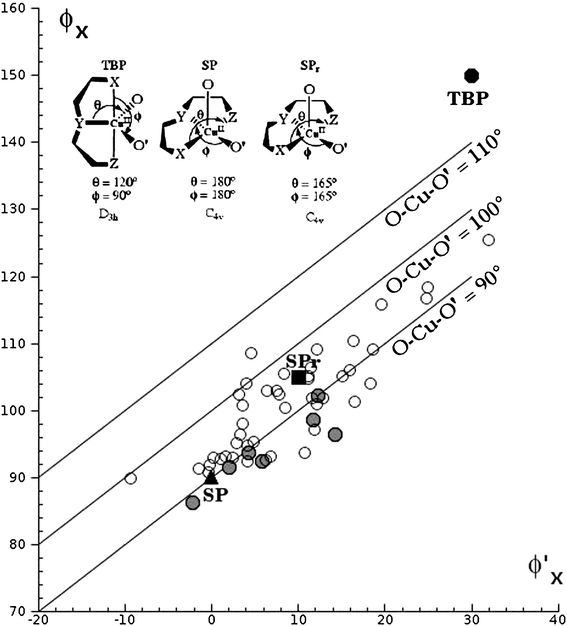 | ||
| Fig. 4 Variation of the angular coordinates φx and φ′x for the ideal geometries, structures 1–6 (gray circles) and structures reported in the CSD having a planar tridentate ligand, a carboxylate and a water molecule. | ||
In structures 1–2, 4–6 as well as in JEDOIQ and NATHAR the trpy ligand is always in a basal position, the carboxylate is coordinated as monodentate, also in a basal position, and in the apical position there is a water molecule, the only exception being structure 3 where the water molecule is in a basal and the carboxylate in an apical position. These results indicate that an important control on the molecular geometry of the compounds is obtained with the trpy ligand.
From a supramolecular point of view a common feature in all structures 1–6 is that there is an O–H⋯O hydrogen bond between the coordinated water molecule and the non-coordinated O atom of the carboxylate, generating the hydrogen bonded chains shown in Fig. 3. This same chains are observed in JEDOIQ and NATHAR. An analysis of the other 28 structures reported in the CSD, Table 3, shows that in eight of them similar H-bonded 1D chains are present. This indicates that in 53.3% of the structures (the six reported here plus ten of the 28 reported in the CSD) the 1D chains are present (supramolecular yield of 53.3%), but with trpy the supramolecular yield is 100%.
Due to the presence of polarized aromatic rings in the trpy ligands π–π stacking interactions actively participate in the organization of the molecules in the crystal. This is interesting given that from the beginning of crystal engineering there has been an interest in understanding the packing of aromatic tectones.15 However, little attention has been paid until recently by inorganic coordination and organometallic chemists to the use of π–π interactions as a tool in the design and synthesis of crystal structures.16 In the present compounds, π–π interactions are important in the organization of the molecules in all 1–6 structures.
5 Conclusion
We have shown that it is possible to control the geometry around the Cu(II) centers using tridentate amines and two monodentate ligands, obtaining reliable tectons that can be used to obtain predictable supramolecular structures. We have also found that it is possible to incorporate water molecules into a tecton and use them as hydrogen bonding donors obtaining in the present cases 1D H-bonded chains. The molecular and supramolecular control is preserved even in the presence of carboxylic acids, anions and solvent water molecules that participate actively in the H-bond networks. Studies with more flexible tridentate amines are in progress and will be published elsewhere.Acknowledgements
S. M-V would like to thank CONACYT for a Ph.D Scholarship. Gabriela Salcedo is thanked for technical assistance. The authors are grateful to CONACYT (40332-Q) and DGAPA-UNAM (IN216806) for financial support, and to the CSCI, Spain, for a license to use the Cambridge Structural Database.References
- M. Simard, D. Su and J. D. Wuest, J. Am. Chem. Soc., 1991, 113, 4696 CrossRef.
- M. Henry and M. W. Hosseini, New J. Chem., 2004, 28, 897 RSC.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- C. B. Aakeröy, A. M. Beatty, J. Desper, M. O'Shea and J. Valdés-Martínez, Dalton Trans., 2003, 3956 RSC.
- (a) D. López, R. Costa, F. Illas, E. Molins and E. Espinosa, Inorg. Chem., 2000, 39, 4560 CrossRef CAS; (b) R. Costa, A. García, J. Ribas, T. Mallah, Y. Journaux, J. Slatten, X. Solans and V. Rodriguez, Inorg. Chem., 1993, 32, 3733 CrossRef; (c) T. R. Felthouse, E. J. Laskowsky and D. N. Hendrickson, Inorg. Chem., 1977, 16, 1077 CrossRef CAS.
- SMART (Version 6.625), SAINT-Plus (Version 6.23C), SADABS and XPREP, Bruker AXS Inc., Madison, Wisconsin, USA.
- G. M. Sheldrick, SHELXS-97, Program for Structure Solution, Acta Crystallogr., Sect. A, 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1998 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- (a) F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef; (b) F. H. Allen and W. D. S. Motherwell, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 407 CrossRef.
- D. L. Kepert, E. S. Kucharski and A. H. White, J. Chem. Soc., Dalton Trans., 1980, 1932 RSC.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- J. V. Folgado, E. Escriva, A. Beltran-Porter, D. Beltran-Porter, A. Fuertes and C. Miravitlles, Polyhedron, 1987, 6, 1533 CrossRef CAS.
- J. Cano, G. De Munno, J. L. Sanz, R. Ruiz, J. Faus, F. Lloret, M. Julve and A. Caneschi, J. Chem. Soc., Dalton Trans., 1997, 1915 RSC.
- G. R. Desiraju and A. Gavezzotti, J. Chem. Soc., Chem. Commun., 1989, 621 RSC.
- See for example: (a) C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885 RSC; (b) H. W. Roesky and M. Andruh, Coord. Chem. Rev., 2003, 236, 91 CrossRef CAS; (c) J. McMurtrie and I. Dance, CrystEngComm, 2005, 7, 216 RSC; (d) I. Dance, CrystEngComm, 2003, 5, 208 RSC; (e) D. Ghoshal, A. K. Ghosh, J. Ribas, G. Mostafa and N. Ray Chaudhuri, CrystEngComm, 2005, 7, 61 Search PubMed; (f) D. Ghoshal, A. K. Ghosh, J. Ribas, E. Zangrando, G. Mostafa, T. K. Maji and N. Ray Chaudhuri, Cryst. Growth Des., 2005, 5, 941 CrossRef CAS; (g) N. S. Oxtoby, N. R. Champness and C. Wilson, CrystEngComm, 2005, 284 RSC; (h) S. Banerjee, B. Wu, P.-G. Lassahn, C. Janiak and A. Ghosh, Inorg. Chim. Acta, 2005, 358, 535 CrossRef CAS; (i) S. Banerjee, A. Ghosh, B. Wu, P.-G. Lassahn and C. Janiak, Polyhedron, 2005, 24, 593 CrossRef CAS; (j) K. Abu-Shandi, H. Winkler, H. Paulsen, R. Glaum, B. Wu and C. Janiak, Z. Anorg. Allg. Chem., 2005, 631, 2705 CrossRef; (k) A. M. Madalan, N. Avarvari and M. Andruh, Cryst. Growth Des., 2006, 6, 1671 CrossRef CAS; (l) R. Carballo, B. Covelo, C. Lodeiro and E. M. Vázquez-López, CrystEngComm, 2005, 7, 294 RSC.
Footnotes |
| † CCDC reference numbers 649820–649825. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b708585k |
| ‡ Electronic supplementary information (ESI) available: hydrogen bonds (Table S1); analysis of short ring interactions (Table S2); distortion parameter τ and angular coordinates for structures reported on CSD (Table S3); intermolecular interactions, hydrogen bonds geometries (Å, °) for structures reported on CSD (Table S4). See DOI: 10.1039/b708585k |
| This journal is © The Royal Society of Chemistry 2008 |
