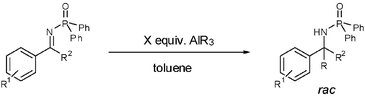
1,2-Addition of trialkylaluminium reagents to N-diphenylphosphinoylketimines in the absence of any additional reagents†
Rüdiger
Reingruber
and
Stefan
Bräse
*
Institut für Organische Chemie, Universität Karlsruhe (TH), Fritz-Haber-Weg 6, D-76131, Karlsruhe, Germany. E-mail: braese@ioc.uka.de; Fax: +49 721 608 8581; Tel: +49 721 608 2902
First published on 23rd October 2007
Abstract
Using reactive trialkylaluminium reagents for the 1,2-addition on acetophenone- and benzophenone-derived ketimines, α-trisubstituted amines were obtained in excellent yields up to 99%.
The synthesis of α-trisubstituted amines is of high interest in medicinal chemistry as these compounds inhibit particular biological activity. Some examples for such substrates are the alkaloid Hemiargin D (1), the aminosesquiterpene Aminobisabolenol (2) as well as the NMDA receptor blocker Dizocilpine (3) (Fig. 1).1–3
 | ||
| Fig. 1 Biologically active α-trisubstituted amines. | ||
While a variety of α-disubstituted amines can be prepared by addition reactions to aldimines,4 the synthesis of α-trisubstituted amines from ketimines is not well established so far (Fig. 2).
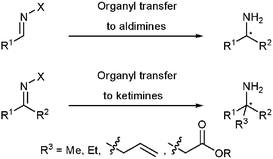 | ||
| Fig. 2 Synthesis of α-branched amines. | ||
Addition of nucleophiles to ketimines is one of the most simple routes for the preparation of α-trisubstituted amines. The synthesis of α-branched amines by 1,2-addition of organometallic reagents is one of the most important reactions in homogeneous catalysis.5 In contrast to the well established 1,2-addition of zinc organyls to aromatic ketones,6 the application of ketimines as alkyl or aryl group acceptors under similar conditions has proven much more challenging. Recently, Shibasaki and co-workers were able to apply N-diphenylphosphinoylketimines in the asymmetric allylation and Mannich-type reaction to obtain α-chiral amines.7
The low reactivity of ketimines as well as their rapid isomerization to an unreactive enamine under basic conditions are two important difficulties, which have to be overcome to establish efficient processes for the addition of organometallic reagents.
Trialkylaluminium reagents are of high interest for alkylation since they are commercially available and are used on industrial scale.8 Therefore the alkylation of imines by AlR3 should have great potential for practical applications. Although triethylaluminium is a cheap and powerful ethylation reagent for carbon–carbon and carbon–heteroatom double bonds,9 it fails to affect imines under standard laboratory conditions.10
In this report, we explored the 1,2-addition of trialkylaluminium reagents to several acetophenone- or benzophenone-derived N-diphenylphosphinoylketimines in the absence of any additional reagents (Fig. 3).11
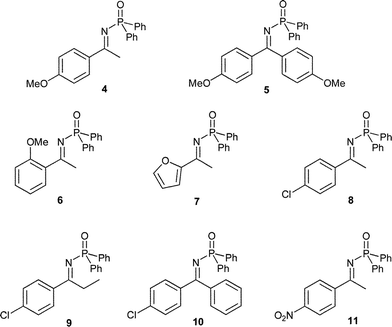 | ||
| Fig. 3 N-Diphenylphosphinoylketimines used. | ||
We examined both triethyl- and trimethylaluminium under several reaction conditions.‡ In the presence of AlEt3 or AlMe3, electron-neutral and electron-rich imines undergo 1,2-addition in almost quantitative yields. At room temperature the reaction proceeds without the use of any additional reagents. Thus alkylation of a variety of ketimines could be accomplished at room temperature.
First, we investigated the necessary amount of trialkylaluminium reagent to achieve good yields for the activated model compound 8 (Table 1, entries 1a–c). We found that at least 3 to 4 equiv. of the aluminium reagent are needed to obtain yields up to 88%. However to obtain a quantitative yield within short reaction times, 5 equiv. of the corresponding aluminium organyl are required. Lowering the nucleophile loading to 3 equiv. required prolonged reaction times (20 h) to achieve full conversion with quantitative yield (Table 1, entry 2).
Since the temperature is an important factor for the selectivity of a chemical process we investigated the temperature limit for this model reaction (Table 1, entries 3a and 3b).
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | Reagent | R1 | R2 | Metal organyl | T/°C | Time/h | Yield (%) |
| a Addition of 0.1 equiv. (Rp,S)-paracyclophane-ligand in n-hexane, b Addition of 1.0 equiv. N,N-dimethylethanolamine in toluene. | |||||||
| 1a | 8 | 4-ClC6H4 | CH3 | 3 equiv. AlEt3 | 25 | 0.5 | 74 |
| 1b | 8 | 4-ClC6H4 | CH3 | 4 equiv. AlEt3 | 25 | 0.5 | 88 |
| 1c | 8 | 4-ClC6H4 | CH3 | 5 equiv. AlEt3 | 25 | 0.5 | 98 |
| 2 | 4 | 4-MeOC6H4 | CH3 | 3 equiv. AlEt3 | 25 | 20 | 99 |
| 3a | 8 | 4-ClC6H4 | CH3 | 4 equiv. AlEt3 | 0 | 1 | 65 |
| 3b | 8 | 4-ClC6H4 | CH3 | 4 equiv. AlEt3 | −25 | 1 | 7 |
| 4a | 8 | 4-ClC6H4 | CH3 | 4 equiv. ZnEt2a | 0 | 16 | — |
| 4b | 11 | 4-O2NC6H4 | CH3 | 4 equiv. ZnEt2b | −25 | 16 | — |
| 5 | 6 | 2-MeOC6H4 | CH3 | 4 equiv. AlEt3 | 25 | 72 | — |
| 6 | 7 | Furyl | CH3 | 4 equiv. AlEt3 | 25 | 16 | 99 |
| 7 | 8 | 4-ClC6H4 | CH3 | 4 equiv. AlMe3 | 25 | 24 | 92 |
| 8 | 9 | 4-ClC6H4 | CH2CH3 | 6 equiv. AlMe3 | 25 | 20 | 63 |
| 9 | 10 | 4-ClC6H4 | C6H5 | 4 equiv. AlMe3 | 25 | 20 | 84 |
| 10 | 5 | 4-MeOC6H4 | 4-MeOC6H4 | 4 equiv. AlEt3 | 25 | 16 | 87 |
| 11 | 10 | 4-ClC6H4 | C6H5 | 4 equiv. AlEt3 | 25 | 20 | 62 |
When the reaction was performed at −25 °C or below (see ESI†) the starting material was recovered in almost (near to) quantitative yield. The choice of solvents also has to be considered, since there is no reaction taking place in coordinating solventse.g. Et2O or THF at temperatures around 0 °C or slightly below. Additionally the considered imines are unreactive towards zinc organyls as we tried to convert them with diethylzinc and different N,O-additives (Table 1, entries 4a and 4b).
The scope of the optimized reaction is shown in Table 1, entries 5–11. The 1,2-addition to electron-rich ketimines such as 6 (entry 5), the furyl-derivative 7 (entry 6) as well as 5 (entry 10) proceed in good to excellent yields up to 99%, although longer reaction times are necessary (16–20 h). The 1,2-addition to the electron-poor ketimines 8, 9 and 10 (Table 1, entries 7–9) initiated by trimethylaluminium proceed in up to 92% yield. Due to the poor reactivity of trimethylaluminium the reaction needs to be run 24 h for completion.
It is worth mentioning that all products yielded by 1,2-addition are of high purity without further purifications as flash-chromatography or recrystallization. We explored this favourable circumstance by a modified quenching procedure by saturated K/Na-tartrate solution.
In summary we explored the first 1,2-addition of trialkylaluminium organyls to N-diphenylphosphinoylketimines to obtain α-trisubstituted amines in high yields up to 99%.
In our ongoing studies we are exploring this reaction to be carried out in an asymmetric fashion to achieve enantiomerically pure products.
This work has been supported by the BMBF (WING program) and the Landesgraduiertenförderung (fellowship to R. R.).
Notes and references
- W.-H. Lin, H.-Z. Fu, J. Li, G. Cheng and R. A. Barnes, J. Chin. Pharm. Sci., 2003, 12, 117 Search PubMed.
- I. Kitagawa, N. Yoshioka, C. Kamba, M. Yoshikawa and Y. Hamamoto, Chem. Pharm. Bull., 1987, 35, 928 CAS.
- G. X. Ayala and R. Tapia, Eur. J. Neurosci., 2005, 22, 3067 CrossRef.
- S. Bräse, T. Baumann, S. Dahmen and H. Vogt, Chem. Commun., 2007, 1881 RSC.
- (a) D. Enders and U. Reinhold, Tetrahedron: Asymmetry, 1997, 8, 1895 CrossRef CAS; (b) R. Bloch, Chem. Rev., 1998, 98, 1407 CrossRef CAS; (c) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS.
- (a) P. I. Dosa and G. C. Fu, J. Am. Chem. Soc., 1998, 120, 445 CrossRef CAS; (b) C. Garcia, L. K. LaRochelle and P. J. Walsh, J. Am. Chem. Soc., 2002, 124, 10970 CrossRef CAS.
- (a) R. Wada, T. Shibuguchi, S. Makino, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 7687 CrossRef CAS; (b) Y. Suto, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 500 CrossRef CAS.
- F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, Wiley, New York, 4th edn, 1980, p. 342 Search PubMed.
- J. J. Eisch, in Comprehensive Organometallic Chemistry: Vol. 1, ed. G. Wilkinson, F. G. A. Stone and E. N. Abel, Pergamon Press, Oxford UK, 1982, ch. 6 Search PubMed, and references therein.
- (a) A. Alberola, F. A. Cermeño and A. Anton, An. Quim., 1977, 73, 886 CAS; (b) H. Schumann, J. Kaufmann, S. Dechert and H.-G. Schmalz, Tetrahedron Lett., 2002, 43, 3507 CrossRef CAS.
- (a) Y. Chu, Z. Shan, D. Liu and N. Sun, J. Org. Chem., 2006, 71, 3998 CrossRef CAS; (b) S. Masumoto, H. Usuda, M. Suzuki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2003, 125, 5634 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section and spectroscopic data. See DOI: 10.1039/b714161k |
| ‡ General procedure for the addition of trialkylaluminium reagents to N-diphenylphosphinoylketimines: Under a dry argon atmosphere, the corresponding imine (0.5 mmol) was dissolved in 1.0 mL of dry toluene and stirred for 0.5 h at room temperature to give a homogeneous solution. Then AlEt3 or AlMe3 (4.00 mmol), used as a 1 M solution in toluene or n-hexane, was added under argon and stirred for the indicated time. After all of the starting material was consumed, the mixture was quenched by addition of methanol and saturated solution of K/Na-tartrate. The aqueous layer was extracted with ethyl acetate (3 × 5 mL), dried over MgSO4 and filtered. The filtrate was concentrated under reduced pressure. The products were obtained in high purity without further purification. |
| This journal is © The Royal Society of Chemistry 2008 |