Continuous catalytic Friedel–Crafts acylation in the biphasic medium of an ionic liquid and supercritical carbon dioxide†
Firas
Zayed
a,
Lasse
Greiner
a,
Peter S.
Schulz‡
a,
Alexei
Lapkin
b and
Walter
Leitner
*ac
aInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringer Weg 1, D-52074 Aachen, Germany. E-mail: leitner@itmc.rwth-aachen.de; Fax: +49 241 80 22177
bDepartment of Chemical Engineering, University of Bath, Bath, UK BA2 7AY
cMax-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, Germany
First published on 13th November 2007
Abstract
Immobilisation of catalytically-active metal salts in ionic liquids, with extraction by supercritical carbon dioxide, affords continuous Friedel–Crafts acylation, with in situ-recycling of the catalyst.
Friedel–Crafts acylation (FCA) is widely used to functionalise aromatic compounds. Commonly, however, stoichiometric amounts of a Lewis acid , such as aluminium chloride, are employed.1 The strongly-bound product complex prevents the recycling of the catalyst, and successive aqueous downstream processes to achieve its cleavage inevitably lead to the formation of HCl and aluminium hydroxide.
A promising class of catalysts for FCA are highly oxophilic Lewis acids ,2 especially of the late transition metals. These relatively soft metal centres balance the need for catalytic interaction with the hard carbonyl oxygen atoms of the reactants and release of the products.3 A range of early transition metal triflates and lanthanide salts have been shown as promising FCA catalysts,4 most recently in combination with ionic liquids (ILs) as solvents.5 Nonetheless, the majority of previous examples either need a stoichiometric co-catalyst for their application or are carried out in organic solvents, rendering recycling and continuous operation difficult.
ILs, in combination with supercritical fluids (SCFs ), have been shown to be a versatile method for the immobilisation and continuous recycling of homogeneous catalysts.6Supercritical carbon dioxide (sc CO2) is the first choice SCF . Its inherent properties include non-flammability, mild critical conditions, tuneable solubility near to the critical point and very low environmental impact. In previous studies, we have demonstrated that cationic metal complexes are effectively activated, tuned and immobilised utilising these biphasic conditions.7
Based on this experience, we aimed to employ an IL as the stationary reactive phase, retaining metal triflates as catalysts and sc CO2 as the mobile (non-reactive) phase. As a model reaction, the FCA of anisole with acetic anhydride was chosen (eqn. 1). From a preliminary screening, 1-butyl-4-methylpyridinium bis(trifluoromethylsulfonyl)imide ([4-MBP][NTf2]) was identified as a suitable IL as it showed negligible mass loss when extracted with sc CO2 and apparently no negative influence on catalytic activity. Furthermore, it gave comparably good solubilities to the metal salts employed as catalysts.
 | (1) |
At first, reactions in the IL, with In(OTf)3 as the catalyst, were carried out in batch mode. The reactions were carried out under ambient pressure with successive extraction by toluene. The IL was reused three times. The reaction was also carried out under CO2 pressure in batch mode, with successive extraction in a continuous carbon dioxide stream. The IL/catalyst mixture was reused three times (Table 1). In all cases, the yields under biphasic conditions in the presence of compressed CO2 were significantly higher (4–17%) compared to monophasic conditions. This may be associated, at least partly, with a favourable partitioning of potentially inhibitory reactants into the non-reactive sc CO2-phase, combined with good mass transport between the two phases. Irrespective of the exact reasons, these results indicate that the IL/sc CO2 system should be beneficial to continuous operation.
| Cycle | Monophasic yield (%) | Biphasic yield (%) |
|---|---|---|
| a 60 °C, 0.1 mmol In(OTf)3, 4 mmol anisole and 2 mmol acetic anhydride in 10 mmol (3 mL) IL. Monophasic: 5 h. Biphasic: 30 MPa sc CO2 for 5 h and successive extraction for 16 h at a flow rate of 100 g h−1. | ||
| 0 (fresh IL) | 45 | 49 |
| 1 | 44 | 55 |
| 2 | 36 | 53 |
| 3 | 24 | 36 |
In the flow system, the FCA was carried out continuously using In(OTf)3 immobilised in the IL as the catalyst and sc CO2 as the continuous extraction phase. The apparatus used for these experiments was designed for conditions up to 35 MPa (see Fig. 1 and the ESI† ). CO2 was dosed into the system by means of a mass flow controller from a high pressure reservoir. The substrate was injected by a high pressure piston pump into the CO2 stream just before it entered the reaction chamber. The pressure was held constant by means of a back pressure regulator, and the reactants and products were removed from the gaseous components by the combination of a cooling trap and a gas washer leading to a closed mass balance. The reactor was stirred with a magnetic stirring bar and operated as a continuously stirred tank reactor. The dimension-less time or mean residence time, τ, was calculated according to the SCF volume, VR, as a function of the sc CO2 density, δ, and mass flow, ṁsc CO2:
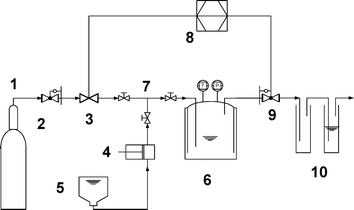 | ||
| Fig. 1 Reactor for batch and continuous flow FCA. (1) CO2 reservoir, (2) pressure reducer, (3) mass flow controller, (4) high pressure piston pump, (5) substrate reservoir, (6) stirred tank reactor with pressure temperature transducer, (7) valves, (8) analog/digital interface unit, (9) back pressure regulator, (10) cooling trap and gas washer (see ESI for further details† ). | ||
Turnover numbers (TON ) based on the amount of indium in the reactor, as a function of the residence time, for two different pressures are shown in Fig. 2. Assuming first order reaction kinetics, which has been shown to be the case for metal triflate catalysts in ILs,4b the half-life§ of the catalyst activity increased by more than 40%, from 28 to 45 h, by increasing the sc CO2 pressure from 25 to 30 MPa. One probable explanation is the higher solubility of the reactants and products in sc CO2 at higher pressures. Metal loss was shown by ICP-MS to be negligible on the basis of measurements of indium in the IL prior to and following the reaction. Even though the initial turnover frequency (TOFmax ) was higher in the case of 25 MPa CO2 pressure, the catalyst utilisation and total TON was better at higher pressure, as the reaction maintained a higher level of conversion (Table 2).
 | ||
| Fig. 2 TON of In(OTf)3 in continuous biphasic FCA at 300 bar (●) and 25 MPa (◇) as a function of time (3 mL IL, 0.1 mmol In(OTf)3, temperature 60 °C, VR = 10 mL, anisole/acetic anhydride (mol/mol) = 3 : 1, residence time τ = 2.3 h). | ||
Encouraged by these promising results, other metal triflate salts were tested under continuous reaction conditions using high pressure conditions (Fig. 3). In this screening, we included 10 triflate salts for their activity and stability towards FCA, aiming primarily for better catalyst robustness with reasonable activity. Of the tested salts, yttrium triflate showed a significantly higher half-life with a promising activity (Table 3). Thus, among the tested catalysts, yttrium triflate possesses the best balance between sufficient acidity for catalytic activity and softness to release the product. Scandium, gadolinium, ytterbium and bismuth showed a comparable half-life of catalyst activity to indium. It is noteworthy that a hafnium-based catalyst showed one of the highest initial activities, but proved not to be well-suited for continuous operation since the half-life was among the lowest in the tested range. This clearly demonstrates the need for screening under continuous conditions to reveal the stability under process conditions.8
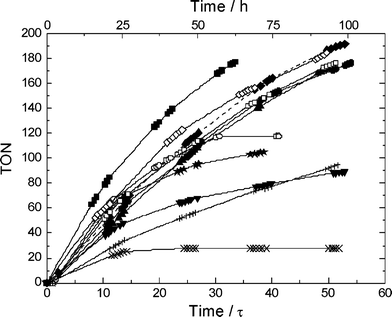 | ||
| Fig. 3 TON as a function of time for the continuous FCA of anisole in the biphasic system IL/sc CO2 for various metal triflates: In (■), Sc (●), Y (▲), Hf (★), Gd (◆), Yb (□), polyoxywolframate (○), Bi (◇), Ce (+), Cu (×), Zn (▼) (3 mL IL, 0.1 mmol metal triflate, 60 °C, 30 MPa, VR = 10 mL, anisole/acetic anhydride (mol/mol) = 3 : 1, ṁ(sc CO2) = 4 g h−1, residence time ô = 1.9 h). | ||
| Catalyst | Atomic number | Half-life/h | Maximum conversion (%) | TON | TOFmax /h−1 |
|---|---|---|---|---|---|
| a 3 mL IL, 0.1 mmol triflate salt, 60 °C, VR = 10 mL, anisole/acetic anhydride (mol/mol) = 10 : 1, ṁ(sc CO2) = 4.9 g h−1. b ṁ(sc CO2) = 4 g h−1. | |||||
| Sc(OTf)3 | 21 | 48 | 58 | 176 | 3.0 |
| Cu(OTf)2 | 29 | 11 | 21 | 28 | 1.1 |
| Zn(OTf)2 | 30 | 27 | 39 | 89 | 2.0 |
| Y(OTf)3 | 39 | 71 | 53 | 172 | 2.7 |
| In(OTf)3 b | 49 | 52 | 66 | 177 | 3.3 |
| Ce(OTf)4 | 58 | 23 | 29 | 94 | 1.5 |
| Gd(OTf)3 | 64 | 43 | 54 | 192 | 2.8 |
| Yb(OTf)3 | 70 | 50 | 60 | 177 | 3.0 |
| Hf(OTf)4 | 72 | 17 | 64 | 105 | 3.2 |
| Bi(OTf)3 | 83 | 47 | 65 | 184 | 3.3 |
| W-HPA b | 74 | 23 | 56 | 117 | 2.8 |
To compare the metal triflate system with other potential FCA catalysts under continuous SCF conditions, we also tested tungstophosphoric acidH3[P(W3O10)4]aq (W-HPA) suspended in the IL and supported on mesoporous carbon as an alternative heterogeneous catalyst . Neat and supported W-HPA has been reported as a very active catalyst for the acylation of anisole in solvent-less conditions, exhibiting a reversible deactivation mechanism.9 In the case of neat W-HPA dispersed in our IL, the half-life and activity were of similar orders to the cases of the metal triflates (Table 3). However, the continuous reaction over carbon-supported W-HPA in solvent-less conditions (Fig. 4) showed a very rapid deactivation that was characteristic of deactivation by complexation with the product. These data show that, in the case of solvent-less reactions using a very active W-HPA catalyst, operating under a continuous flow regime does not prevent deactivation by product inhibition. Similar observations of rapid catalyst deactivation in a continuous acylation were obtained recently.10 Previously, due to ion exchange between the IL and the zeolite, forming an active homogeneous catalyst, rapid deactivation of zeolite catalysts in a continuous acylation with ILs as solvents was shown.11 Thus acylation with FCA catalysts immobilised in ILs with continuous extraction of the product using sc CO2 shows comparably higher stability.
 | ||
| Fig. 4 Continuous acylation of anisole by a W-HPA/carbon catalyst under solvent-less conditions. Reaction conditions: 110 °C, anisole/acetic anhydride (mol/mol) = 10 : 1, flow rate 1 mL min−1. | ||
In conclusion, biphasic IL/sc CO2 conditions are favourable for continuous FCA, allowing recycling of the homogeneous soluble catalysts. Good catalyst usage, with TONs of up to 190, can be achieved. For the model reaction, this compares favourably with previously reported TONs for indium triflate of 100 in nitromethane as the solvent and lithium perchlorate as the additive,2 and for copper triflate of 5 in the IL [BMIM]BF4.4c
We thank Markus Kaever, Horst Kronenberg and Ralph Thelen (RWTH Aachen University) for technical support and discussion. We are indebted to Professor Arno Behr (University of Dortmund) for ICP-MS measurements. Financial support by the Deutsche Forschungsgemeinschaft (SPP 1191), the Federal Ministry of Education and Research (NMT/03C0342F), and the EPSRC (Industrial CASE to A. L.) is acknowledged.
Notes and references
- H. Yamamoto, Lewis Acids in Organic Synthesis, Wiley-VCH, Weinheim, 2000 Search PubMed.
- C. J. Chapman, C. G. Frost, J. P. Hartley and A. J. Whittle, Tetrahedron Lett., 2001, 42, 773 CrossRef CAS.
- A. Fürstner, D. Voigtländer, W. Schrader, D. Giebel and M. T. Reetz, Org. Lett., 2001, 3, 417 CrossRef CAS.
- For example, see: (a) I. Komoto, J. Matsuo and S. Kobayashi, Top. Catal., 2002, 19, 43 CrossRef CAS; (b) A. Kawada, S. Mitamura, J. Matsuo, T. Tsuchiya and S. Kobayashi, Bull. Chem. Soc. Jpn., 2000, 73, 2325 CrossRef CAS; (c) J. Ross and J. Xiao, Green Chem., 2002, 4, 129 RSC; (d) P. Goodrich, C. Hardacre, H. Mehdi, P. Nancarrow, D. W. Rooney and J. M. Thompson, Ind. Eng. Chem. Res., 2006, 45, 6640 CrossRef CAS; (e) M. Y. Yoon, J. H. Kim, D. S. Choi, U. S. S. Shin and J. Y. Lee and; (f) C. E. Song, Adv. Synth. Catal., 2007, 349, 1725 CrossRef.
- For recent reviews, see: V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615 Search PubMed; K. Binnemans, Chem. Rev., 2007, 107, 2592 CrossRef CAS.
- For recent reviews, see: D. J. Cole-Hamilton, Adv. Synth. Catal., 2006, 348, 1341 Search PubMed; W. Leitner, in Multiphase Homogeneous Catalysis, ed. B. Cornils, W. A. Herrmann, I. T. Horvath, W. Leitner, S. Mecking, H. Olivier-Bourbigou and D. Vogt, Wiley-VCH, Weinheim, 2005, pp. 605–750 CrossRef CAS.
- A. Bösmann, G. Franciò, E. Janssen, M. Solinas, W. Leitner and P. Wasserscheid, Angew. Chem., Int. Ed., 2001, 40, 2697 CrossRef; M. Solinas, A. Pfaltz, P. G. Cozzi and W. Leitner, J. Am. Chem. Soc., 2004, 126, 16142 CrossRef CAS; A. Scurto and W. Leitner, Chem. Commun., 2006, 3681 RSC.
- D. Degenring, I. Schröder, C. Wandrey, A. Liese and L. Greiner, Org. Process Res. Dev., 2004, 8, 213 Search PubMed; S. Laue, L. Greiner, J. Wöltinger and A. Liese, Adv. Synth. Catal., 2001, 343, 711 CrossRef CAS; L. Greiner, S. Laue, A. Liese and C. Wandrey, Chem. Eur. J., 2006, 12, 1818 CrossRef CAS.
- J. Kaur, K. Griffin, B. Harrison and I. V. Kozhevnikov, J. Catal., 2006, 208, 448.
- V. R. Sarsani, C. J. Lyon, K. W. Hutchenson, M. A. Harmer and B. Subramaniam, J. Catal., 2007, 245, 184 CrossRef CAS.
- C. Hardacre, S. P. Katdare, D. Milroy, P. Nancarrow, D. W. Rooney and J. M. Thompson, J. Catal., 2004, 227, 44 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed description of the apparatus in Fig. 1. See DOI: 10.1039/b712865g |
| ‡ Current address: Lehrstuhl für Chemische Reaktionstechnik, University of Erlangen-Nuremberg, Egerlandstraße 3, 91058 Erlangen, Germany. |
| § Half lives were estimated from a first order decay over a 30 to 10% conversion range, assuming a linear dependency of the conversion on catalyst activity. |
| This journal is © The Royal Society of Chemistry 2008 |

