Preparation and photosensitizing property of novel Cd10S16 molecular cluster dendrimer†
Takaaki
Tsuboi
,
Yutaka
Takaguchi
* and
Sadao
Tsuboi
Graduate School of Environmental Science, Okayama University, Tsushima-Naka 3-1-1, Okayama 700-8530, Japan. E-mail: yutaka@cc.okayama-u.ac.jp; Fax: +81-86251-8903; Tel: +81-86251-8903.
First published on 21st November 2007
Abstract
A new Cd10S16 molecular cluster dendrimer has been prepared and characterized; photooxygenation reaction using the molecular cluster dendrimer as a photosensitizer was successful.
Semiconductor nanoparticles of cadmium chalcogenides (CdS, CdSe, CdTe) have attracted attention because of their optical and electrochemical properties.1 In particular, semiconductive molecular clusters of cadmium chalcogenides stabilized by organic ligands are useful for optical and electrochemical devices since they have uniform structure, molecular size and characteristics.2 Although many studies on an ammonium salt of CdS molecular cluster anion have been reported,2 few applications of neutral CdS molecular clusters have been reported due to their low solubility. Furthermore, photosensitizing activity of a neutral CdS cluster to generate singlet oxygen has never been reported. Recently, Konishi and co-workers have reported the synthesis of a water-soluble Cd10S16 molecular cluster having oligo(ethyleneglycol) units by the ligand-exchange reaction of Cd10S16 cluster with thiol ,3 and its luminescence sensing of copper ions.3c Meanwhile, we have reported the syntheses of dendron-functionalized fullerenes (fullerodendrons), and found that dendritic substituents were effective to make fullerene , a poorly soluble organic semiconductor , soluble in various organic solvent and water.4a–d Because of high solubility of the fullerodendrons, they can act as photosensitizers to generate singlet oxygen in various solution systems.4a,d,e In this connection, cadmium chalcogenides having dendritic wedges are of interest. Although size-controlled CdX (X = S, Se) nanoparticles stabilized by dendrons were reported,5 a structure-defined cadmium chalcogenide molecular cluster having dendritic ligands has never been reported. In this paper, we describe the synthesis of a novel Cd10S16 molecular cluster dendrimer 1 having twelve dendritic thiolate ligands. Furthermore, photooxygenation reactions of various organic sulfides using the dendrimer 1 as a photosensitizer are also reported.
Cd10S16 molecular cluster dendrimer 1 was synthesized using the ligand-exchange reaction, as reported by Konishi.3Scheme 1 shows the synthesis of Cd10S16 molecular cluster dendrimer 1. A neutral cluster Cd10S4(SPh)12 (2)2 (104 mg, 0.041 mmol) was added to a solution of dendron thiol 3 (360 mg, 0.98 mmol) in MeCN (4 mL), and the mixture was stirred at 45 °C for 2 days under Ar atmosphere. After filtration of the solution, the filtrate was concentrated. The residue was washed copiously with ether, then reprecipitated from a chloroform–methanol solution to remove incomplete ligand exchange products, i.e. Cd10S16 clusters containing PhS ligands, and decomposed clusters. Further reprecipitation from a chloroform–ether solution afford the Cd10S16 molecular cluster dendrimer 1 (168 mg, 73%) as a yellow solid; mp 86–90 °C (decomp.). Interestingly, molecular cluster dendrimer 1 was soluble in organic solvents such as acetonitrile (solubility ca. 2.9 g mL−1) and chloroform (solubility ca. 3.1 g mL−1), in contrast to the original cluster 2 (solubility ca. 2.7 × 10−6 g mL−1 in chloroform).
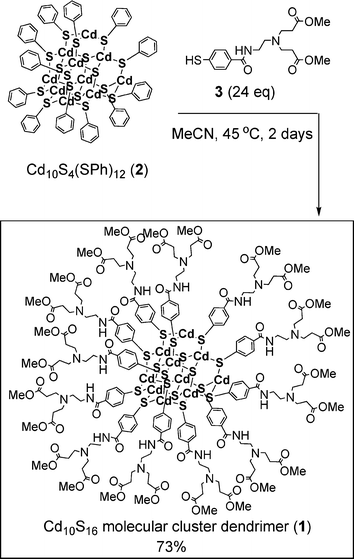 | ||
| Scheme 1 Synthesis of C10S16 molecular cluster dendrimer 1. | ||
The structure of molecular cluster dendrimer 1 was confirmed by 1H NMR spectroscopy, elemental analysis, and ICP-AES spectroscopy. The 1H NMR spectrum of Cd10S16 molecular cluster dendrimer 1 showed broad signals at 2.3–3.8 ppm (aliphatic protons) and 6.9–8.0 ppm (amide and aromatic protons) (Fig. 1a). Usually such broadening of NMR signals of nanoparticles is due to slow tumbling which stops the averaging of anisotropic dipole–dipole interactions . However, in very small clusters, such as Cd10S16, the tumbling of the whole particle is presumably fast enough so that this effect is not noticeable. So we can conclude that the main reason for the large linewidths of the NMR peaks is the heterogeneity of the environment around the protons, due to many different slowly-interconverting conformations as reported by Konishi et. al.3a In order to remove such an heterogeneous environment, we measured the 1H NMR of 1 in the presence of 20 equiv. of cetyltrimethylalkylammonium bromide (Fig. 1b). Complexation with ammonium ions to anionic sulfur atom of 1 makes the structure more rigid and uniform. Hence the dendron group signals at 2.3–3.8 ppm were isolated to four signals of methylene protons (δ 2.45, 2.64, 2.77, and 3.45) and one signal of methyl ester protons (δ 3.61). The signals at 6.9–8.0 ppm were changed into one triplet of amide proton (δ 7.21) and four doublets of aromatic protons (δ 7.29, 7.48, 7.59, and 7.75). It is notable that phenyl protons (δ 6.5–7.0)3a of original cluster 2 have completely disappeared and the new four doublets are different from dendron thiol 3 (δ 7.28 and 7.77) and the corresponding disulfide (δ 7.51 and 7.83).6 The peak integration ratio of δ 7.00–7.95 (aromatic and amide protons) and δ 2.28–2.90 (methylene protons of dendritic wedge) is 5 : 10, which is consistent with the structure of 1 (see Supporting Information† ). Furthermore, elemental analysis confirmed the molecular formula (Found: C, 43.1; H, 4.90; N, 5.69; S, 9.05%. Calc. for C204H276Cd10N24O60S16: C, 43.3; H, 4.91; N, 5.94; S, 9.06%). Cd content of cluster-core dendrimer 1 was 20.1% (calc. for dendrimer 1: 19.9%) determined by ICP-AES after destruction of the cluster with analytical grade HNO3. Meanwhile, LD-TOF-mass spectrum of molecular cluster dendrimer 1 showed a broad peak centered at ca. 5200 (see Supporting Information).
 | ||
| Fig. 1 1H NMR spectra of a) C10S16 molecular cluster dendrimer 1 (0.91 mM) and b) 1/CTAB (0.91/18.2 mM) in CDCl3. Triangles indicate C10S16 molecular cluster dendrimer 1. Asterisks, daggers, and double dagger indicate chloroform, CTAB, and water, respectively. | ||
Fig. 2 shows emission spectra of molecular clusters 1 and 2. The emission spectrum of molecular cluster dendrimer 1 in chloroform showed a broad peak at 570 nm. Meanwhile, the emission spectrum of neutral molecular cluster Cd10S4(SPh)12 (2) was difficult to observe because of its low solubility. The chloroform solution of complex 2/TOAB (tetraoctylammonium bromide)3a showed weaker fluorescence than molecular cluster dendrimer 1. Hamity and co-workers reported that addition of tetraalkylammonium salts induces quenching of fluorescence.7 This result indicates that dendritic ligands are effective for dissolving CdS clusters in organic solvents without changing their photochemical properties. The absorption spectrum of molecular cluster dendrimer 1 shows a maximum at 272 nm, which is due to the absorption of dendron groups (Fig. 3). However, excitation spectra of molecular clusters 1 and 2 were similar in shape with a maximum at 360 nm (Fig. 4). These results also suggest that the photochemical properties of the CdS cluster core are not changed by any correlation with dendron groups.
 | ||
| Fig. 2 Emission spectra (λex = 360 nm) of (a) C10S16 molecular cluster dendrimer 1 (7.8 µM) and (b) 2/TOAB (7.8/6.22 µM) in chloroform. | ||
 | ||
| Fig. 3 Absorption spectra of (a) C10S16 molecular cluster dendrimer 1 (7.8 µM) and (b) 2/TOAB (6.7/26.8 µM) in chloroform. | ||
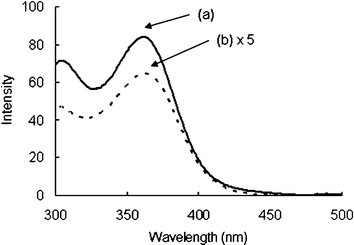 | ||
| Fig. 4 Excitation spectra (λem = 570 nm) of (a) C10S16 molecular cluster dendrimer 1 (7.8 µM) and (b) 2/TOAB (7.8/6.22 µM) in chloroform. | ||
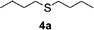
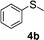
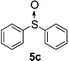
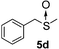
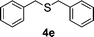
To clarify the utility of molecular cluster dendrimer 1 as a photosensitizer , we subjected a series of sulfides to photooxygenation sensitized by molecular cluster dendrimer 1 (Table 1). In a typical run, a mixture of di-n-butyl sulfide (4a) (26 mg, 0.18 mmol) and 10 mol% of Cd10S4 molecular cluster dendrimer 1 (100 mg, 0.018 mmol) in chloroform–methanol (9 : 1, 4.3 mL) was irradiated for 2.5 h with a high-pressure mercury lamp (λ > 300 nm) through a Pyrex filter at room temperature, while oxygen was passed through the reaction mixture. The resultant mixture was analyzed by GC ; n-decane was used as the internal standard. Di-n-butyl sulfoxide (5a) was detected in 98% yield (entry 1). A control experiment, which was conducted without cluster-core dendrimer 1, did not afford photooxygenation products. Oxidation reactions of aromatic and benzylic sulfides were also examined. Photooxygenation of thioanisole (4b) in the presence of molecular cluster dendrimer 1 (10 mol %) gave methyl phenyl sulfoxide (5b) in 62% yield (entry 2). Benzyl methyl sulfide (4d) and dibenzyl sulfide (4e) were also converted to benzyl methyl sulfoxide (5d) and dibenzyl sulfoxide (5e) in 99% and 72% yield (entry 4 and 5), respectively. The photooxygenation of diphenyl sulfide (4c), which has low reactivity upon oxidation, gave diphenyl sulfoxide (5c) and unchanged diphenyl sulfide (4c) in 17% and 65% yields (entry 3), respectively. To our knowledge, these are the first examples of photooxygenation reaction using the Cd10S16 molecular cluster as a photosensitizer .
|
|
|||||
|---|---|---|---|---|---|
| Entry | Sulfide | Solvent | Time | Product | Yield |
| a Reaction condition: Cd10S16 molecular cluster dendrimer 1 (15 mol%). b GC yield. c NMR yield. | |||||
| 1 |
|
CHCl3/MeOH (9 : 1) | 2.5 h |
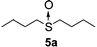
|
98%b |
| 2 |
|
CHCl3/MeOH (9 : 1) | 4 h |

|
62%b |
| 3a |

|
CHCl3/MeOH (9 : 1) | 4 h |
|
17%b |
| (Starting sulfide 4c was recovered in 65% yield)b | |||||
| 4 |

|
CDCl3/CD3OD (9 : 1) | 3 h |
|
99%c |
| 5 |
|
CDCl3/CD3OD (1 : 5) | 2 h |
|
72%c |
In conclusion, we have synthesized the novel Cd10S16 molecular cluster having twelve dendritic thiolate ligands. By incorporation of the dendrons, the Cd10S16 molecular cluster can acquire high solubility in organic solvents, and exhibit fluorescence in chloroform solution. We have also shown the photosensitizing activity of the CdS molecular cluster dendrimer 1 to generate singlet oxygen. Future work is in progress to explore applications and advantages of the molecular cluster dendrimer as a photosensitizer , as a semiconductor , and as a photoluminescence reagent.
We thank Prof. M. Oshima for ICP-AES measurement. This work was partly supported by a Grant-in-Aid for Scientific Research (19010105) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Notes and references
- (a) A. Henglein, Chem. Rev., 1989, 89, 1861 CrossRef CAS; (b) A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS; (c) M. Nirmal and L. Brus, Acc. Chem. Res., 1999, 32, 407 CrossRef CAS; (d) K. Sooklal, L. H. Hanus, H. J. Ploehn and C. J. Murphy, Adv. Mater., 1998, 10, 1083 CrossRef CAS.
- (a) I. G. Dance, A. Choy and M. L. Scudder, J. Am. Chem. Soc., 1984, 106, 6285 CrossRef CAS; (b) W. E. Farneth, N. Herron and Y. Wang, Chem. Mater., 1992, 4, 916 CrossRef CAS; (c) T. Løver, W. Henderson, G. A. Bowmaker, J. M. Seakins and R. P. Cooney, Inorg. Chem., 1997, 36, 3711 CrossRef CAS; (d) R. D. Adams, B. Zhang, C. J. Murphy and L. K. Yeung, Chem. Commun., 1999, 383 RSC; (e) F. E. Osterloh and D. P. Hewitt, Chem. Commun., 2003, 1700 RSC; (f) X. Zhang, Y. Tian, F. Jin, J. Wu, Y. Xie, X. Tao and M. Jiang, Cryst. Growth Des., 2005, 5, 565 CrossRef CAS.
- (a) T. Hiratani and K. Konishi, Angew. Chem., Int. Ed., 2004, 43, 5943 CrossRef CAS; (b) K. Konishi and T. Hiratani, Chem. Lett., 2006, 35, 184 CrossRef CAS; (c) K. Konishi and T. Hiratani, Angew. Chem., Int. Ed., 2006, 45, 5191 CrossRef CAS.
- (a) Y. Takaguchi, T. Tajima, K. Ohta, J. Motoyoshiya, H. Aoyama, T. Wakahara, T. Akasaka, M. Fujitsuka and O. Ito, Angew. Chem., Int. Ed., 2002, 41, 817 CrossRef CAS; (b) Y. Takaguchi, Y. Sako, Y. Yanagimoto, S. Tsuboi, J. Motoyoshiya, H. Aoyama, T. Wakahara and T. Akasaka, Tetrahedron Lett., 2003, 44, 5777 CrossRef CAS; (c) C. Hirano, T. Imae, S. Fujima, Y. Yanagimoto and Y. Takaguchi, Langmuir, 2005, 21, 272 CrossRef CAS; (d) Y. Takaguchi, Y. Yanagimoto, S. Fujima and S. Tsuboi, Chem. Lett., 2004, 33, 1142 CrossRef CAS; (e) B. Talukdar, Y. Takaguchi, Y. Yanagimoto, S. Tsuboi, M. Ichihara and K. Ohta, Bull. Chem. Soc. Jpn., 2006, 79, 1983 CrossRef CAS.
- (a) M. Kawa, S. Saita and Y. Saito, Polym. Mater. Sci. Eng., 2001, 84, 736 CAS; (b) A. C. Wisher, I. Bronstein and V. Chechik, Chem. Commun., 2006, 1637 RSC; (c) S.-H. Hwang, C. N. Moorefield, P. Wang, K.-U. Jeong, S. Z. D. Cheng, K. K. Kotta and G. R. Newkome, J. Am. Chem. Soc., 2006, 128, 7505 CrossRef CAS.
- Y. Takaguchi, K. Saito, S. Suzuki, K. Hamada, K. Ohta, J. Motoyoshiya and H. Aoyama, Bull. Chem. Soc. Jpn., 2002, 75, 1347 CrossRef CAS.
- M. Hamity, R. H. Lema and C. A. Suchetti, J. Photochem. Photobiol., A, 1998, 115, 163 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and LD-TOF-mass spectrum of Cd10S16 molecular cluster dendrimer 1. See DOI: 10.1039/b713680c |
| This journal is © The Royal Society of Chemistry 2008 |

