The characteristic red chemiluminescence from reactions with acidic potassium permanganate: further spectroscopic evidence for a manganese(II) emitter
Jacqui L.
Adcock
*a,
Paul S.
Francis
a,
Trevor A.
Smith
b and
Neil W.
Barnett
a
aSchool of Life and Environmental Sciences, Deakin University, Geelong, Victoria, 3217, Australia. E-mail: jladcock@gmail.com; Fax: +61 3 5227 1040; Tel: +61 3 5227 1294
bSchool of Chemistry, University of Melbourne, Parkville, Victoria, 3010, Australia
First published on 21st November 2007
Abstract
A direct comparison of the laser-induced photoluminescence of manganese(II) with the chemiluminescence from the reaction between acidic potassium permanganate and sodium borohydride was used to confirm that the characteristic red emission from this widely used chemiluminescence reagent emanates from an electronically excited manganese(II) species.
There have been over two hundred and seventy papers describing analytical applications of chemiluminescence reactions with acidic potassium permanganate published in the open scientific literature.1,2 In spite of the extensive use of this reagent, the species responsible for the emission of light is yet to be fully elucidated. Many researchers have reported emission spectra consisting of a single broadly distributed band with an apparent maximum intensity between 610 nm and 750 nm.3–22 This red emission has often been attributed to the production of singlet oxygen,3,9–18,23–25 but the spectral distribution for singlet oxygen (bands at 633, 703 and 1268 nm)26,27 is unlike that observed for acidic potassium permanganate chemiluminescence.28
Many other researchers have suggested that the chemiluminescence emanates from a manganese(II) species.5,6,8,29–38 Some evidence to support this proposal has been presented: the spectral distribution of the chemiluminescence from reactions with acidic potassium permanganate [manganese(VII)] matches that from reactions with manganese(IV) or manganese(III), and the emission occurs in a similar spectral region to the phosphorescence (4T1 → 6A1 transition) of manganese(II) in phosphate glass and in solution at 77 K.28 However, a direct comparison between acidic potassium permanganate chemiluminescence and the photoluminescence of manganese(II) in simple solution at room temperature has been elusive, due to the forbidden character of the transitions in the d5 high-spin configuration.39,40 In contrast to solutions of potassium permanganate [manganese(VII)], which are intensely coloured, solutions of manganese(II) salts are almost colourless.39,40 The absorbance spectrum† of manganese(II) chloride (2 M) in aqueous solution exhibits numerous weak bands over the ultraviolet and visible regions (Fig. 1).
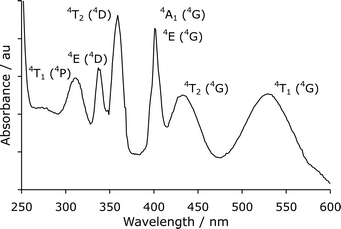 | ||
| Fig. 1 UV-visible absorbance spectrum of manganese(II) chloride (transitions assigned as in text by Blasse and Grabmaier39). | ||
Sveshnikova and Stroganov reported that the laser-induced luminescence of manganese(II) halides in various solvents at 295 K (using a nitrogen laser to excite the sample at 337 nm) was sufficiently intense to collect emission spectra and examine the mechanism of luminescence quenching.41 In a similar manner, we have collected the photoluminescence emission spectrum of manganese(II) chloride (2 M) in aqueous solution at room temperature (Fig. 2a) using a Nd:YAG laser (λex = 355 nm) and a 0.3 m imaging spectrometer with ICCD detector.‡ The spectrum consisted of a broad band with a maximum intensity at 710 ± 5 nm, corresponding to the 4T1 → 6A1 transition of manganese(II).39 In our previous publication,28 we failed to observe photoluminescence from manganese(II) in simple aqueous solutions using a commercial fluorimeter with a relatively weak pulsed lamp as the excitation source and a conventional photomultiplier tube as the detector. In the current investigation, using a milliJoule, nanosecond laser as the excitation source and a sensitive, intensified cooled CCD camera (ICCD) as the detection system, the photoluminescence was easily detected.
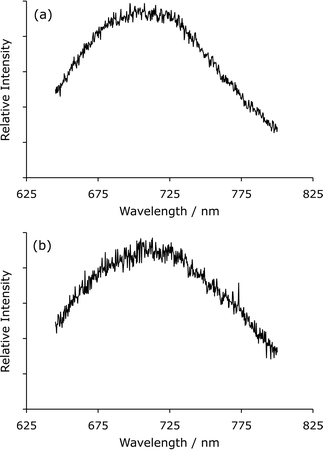 | ||
| Fig. 2 (a) Laser-induced photoluminescence spectrum of manganese(II) chloride. (b) Chemiluminescence spectrum for the reaction of acidic potassium permanganate with sodium borohydride (using the same spectrometer). | ||
To compare the spectral distribution of the photoluminescence with that of the red chemiluminescence elicited in reactions with acidic potassium permanganate, the reagent was continuously merged with a strong reducing agent (sodium borohydride) in a spiral flow cell§ mounted against the entrance slit of the same imaging spectrometer. The chemiluminescence (Fig. 2b) had a similar spectral distribution to the photoluminescence of manganese(II) (Fig. 2a).
In order to obtain a chemiluminescence spectrum that was independent of the relative sensitivity of the detector response and monochromator transmission at different wavelengths, the same solutions were merged in a spiral flow cell mounted in front of the emission window of a Cary Eclipse spectrofluorometer.¶ The chemiluminescence spectrum was then corrected using a multiplication factor that had previously been established with a standard light source. After correction (Fig. 3), the wavelength of maximum intensity (∼735 nm) was consistent with that reported for a wide range of chemiluminescence reactions with either potassium permanganate [manganese(VII)], manganese(IV) or manganese(III) in acidic solution.28,42
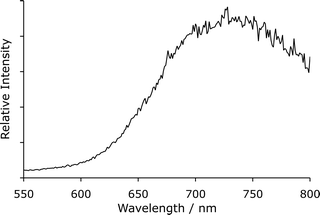 | ||
| Fig. 3 Corrected chemiluminescence spectrum for the reaction of acidic potassium permanganate with sodium borohydride, collected using a Cary Eclipse spectrofluorometer. | ||
These findings confirm that the characteristic red luminescence produced in many reactions with manganese oxidants (including potassium permanganate) in acidic solution corresponds to the 4T1 → 6A1 transition of manganese(II).
This study was financially supported in part by the Institute of Biotechnology (BioDeakin), Deakin University. The authors thank Donna Edwards (Deakin University) for the image used in the illustrated contents entry.
Notes and references
- J. L. Adcock, P. S. Francis and N. W. Barnett, Anal. Chim. Acta, 2007, 601, 36–67 CrossRef CAS.
- B. J. Hindson and N. W. Barnett, Anal. Chim. Acta, 2001, 445, 1–19 CrossRef CAS.
- G. N. Chen, F. X. Huang, X. P. Wu, Z. F. Zhao and J. P. Duan, Anal. Bioanal. Chem., 2003, 376, 873–878 CAS.
- I. B. Agater, R. A. Jewsbury and K. Williams, Anal. Commun., 1996, 33, 367–369 RSC.
- L. Wang, Chem. Anal. (Warsaw), 2006, 51, 211–219 CAS.
- T. Slezak, P. S. Francis, N. Anastos and N. W. Barnett, Anal. Chim. Acta, 2007, 593, 98–102 CrossRef CAS.
- L. N. Li, N. B. Li and H. Q. Luo, Anal. Sci., 2005, 21, 963–966 CrossRef CAS.
- Z. Zhang, H. Cui and M. Shi, Phys. Chem. Chem. Phys., 2006, 8, 1017–1021 RSC.
- Y. Sun, Y. Tang, H. Yao and Y. Li, Anal. Sci., 2005, 21, 457–460 CrossRef CAS.
- H. Qi, M. Yang, M. Feng and J. Lu, Chem. Res. Chin. Univ., 1997, 13, 229–234 Search PubMed.
- M. Liu, Y. He and J. Lu, Fenxi Huaxue, 2005, 33, 535–537 CAS.
- H. Wei and E. Liu, J. Chin. Chem. Soc. (Taipei), 2005, 52, 1043–1048 CAS.
- W. Cao, X. Mu, J. Yang, W. Shi and Y. Zheng, Spectrochim. Acta, Part A, 2007, 66A, 58–62 CrossRef CAS.
- Y. He, Y. Xue, M. Feng and J. Lu, Fenxi Huaxue, 1998, 26, 1136–1138 CAS.
- S. Liao, X. Wu and Z. Xie, Anal. Chim. Acta, 2005, 537, 189–195 CrossRef CAS.
- J. Du, Y. Li, Y. Tang and J. Lu, Anal. Lett., 2002, 35, 463–472 CrossRef CAS.
- J. Pan and Y. Huang, Anal. Lett., 2004, 37, 2321–2335 CrossRef CAS.
- Y. Wei, Z. Zhang, Y. Zhang and Y. Sun, J. Chromatogr., B, 2007, 854, 239–244 CrossRef CAS.
- I. B. Agater and R. A. Jewsbury, Anal. Chim. Acta, 1997, 356, 289–294 CrossRef CAS.
- N. W. Barnett, B. J. Hindson and S. W. Lewis, Anal. Chim. Acta, 1998, 362, 131–140 CrossRef CAS.
- L. Zhu, M. Feng, X. Wan and J. Lu, Gaodeng Xuexiao Huaxue Xuebao, 1996, 17, 1693–1696 CAS.
- V. S. Lebedev and V. A. Veselovskii, Biol. Nauki (Moscow), 1978, 44–48 Search PubMed.
- Y. Sun, Y. Tang, H. Yao and X. Zheng, Talanta, 2004, 64, 156–159 CrossRef CAS.
- X. Wu, J. Duan, H. Chen, D. Chen, F. Huang and G. Chen, Guangpu Shiyanshi, 2003, 20, 781–786 Search PubMed.
- C. Thongpoon, B. Liawruangrath, S. Liawruangrath, R. A. Wheatley and A. Townshend, J. Pharm. Biomed. Anal., 2006, 42, 277–282 CrossRef CAS.
- A. U. Khan and M. Kasha, J. Chem. Phys., 1963, 39, 2105–2106 CrossRef CAS.
- A. U. Khan, J. Biolumin. Chemilumin., 1989, 4, 200–207 CrossRef CAS.
- N. W. Barnett, B. J. Hindson, P. Jones and T. A. Smith, Anal. Chim. Acta, 2002, 451, 181–188 CrossRef CAS.
- R. Su, J. Lin, F. Qu, Z. Chen, Y. Gao and M. Yamada, Anal. Chim. Acta, 2004, 508, 11–15 CrossRef CAS.
- H. Paseková and M. Polášek, Talanta, 2000, 52, 67–75 CrossRef CAS.
- N. W. Barnett, D. G. Rolfe, T. A. Bowser and T. W. Paton, Anal. Chim. Acta, 1993, 282, 551–557 CrossRef CAS.
- B. A. Gorman, N. W. Barnett and R. Bos, Anal. Chim. Acta, 2004, 541, 119–124.
- Y. B. Tsaplev, Russ. J. Phys. Chem., 1991, 65, 420–422.
- A. D. Karavaev, A. I. Voloshin and V. P. Kazakov, Theor. Exp. Chem., 1989, 25, 174–179 CrossRef.
- K. M. Agg, A. F. Craddock, R. Bos, P. S. Francis, S. W. Lewis and N. W. Barnett, J. Forensic Sci., 2006, 51, 1080–1084 CrossRef CAS.
- D. Zhang, Y. Ma, M. Zhou, L. Li and H. Chen, Anal. Sci., 2006, 22, 183–186 CrossRef CAS.
- A. Campiglio, Analyst, 1998, 123, 1053–1056 RSC.
- E. J. Llorent-Martínez, P. Ortega-Barrales and A. Molina-Díaz, Anal. Chim. Acta, 2006, 580, 149–154 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, Berlin, 1994 Search PubMed.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, John Wiley & Sons, New York, 6th edn, 1999 Search PubMed.
- E. B. Sveshnikova and A. A. Stroganov, Opt. Spektrosk., 1986, 60, 521–527 ( Opt. Spectrosc. (USSR) , 1986 , 60 , 320–324 ) Search PubMed English translation.
- N. W. Barnett, B. J. Hindson, S. W. Lewis, P. Jones and P. J. Worsfold, Analyst, 2001, 126, 1636–1639 RSC.
Footnotes |
| † The absorption spectrum of the manganese(II) chloride solution was obtained using a Cary 300 Bio UV-visible spectrophotometer (Varian, Mulgrave, Vic., Australia). |
| ‡ The sample (in a standard 1 cm quartz cuvette) was excited by the third harmonic of a Nd:YAG laser (355 nm) (NY-61; Continuum, Santa Clara, CA, USA), using ∼10 ns pulses at 10 Hz and ∼10 mJ per pulse. The photoluminescence was measured at a right angle to the excitation beam. The emission was focussed onto the entrance slit (∼2 mm) of a 0.3 m imaging spectrometer (SpectroPro 300i; Acton Research Corporation, Acton, MA, USA), with grating blazed at 500 nm and 300 grooves mm–1. A longpass filter (GG435) was placed in front of the entrance slit. The spectrum was collected with a gated intensified CCD camera (ICCD Max; Roper Scientific/Princeton Instruments, Trenton, NJ, USA), using a gate width of 150 ns and a delay of 150 ns (following the arrival of the excitation pulse) in order to minimise any residual scattered light. |
| § Acidic potassium permanganate (1 mM in 1 M sulfuric acid) and sodium borohydride (0.5 g L–1) were continuously mixed using a flow manifold consisting of a peristaltic pump (Gilson Minipuls 3; John Morris Scientific, Balwyn, Vic., Australia) with bridged PVC tubing (1 mm id; DKSH, Caboolture, Qld, Australia), PTFE manifold tubing (0.8 mm id; DKSH) and an integrated glass T-piece and spiral flow cell (0.5 mm id, 90 µL volume; Embell Scientific, Murwillumbah, NSW, Australia). Solutions were pumped at a flow rate of approximately 3 mL min–1 (per line). |
| ¶ The Cary Eclipse spectrofluorometer (Varian), fitted with an R928 photomultiplier tube (Hamamatsu, Hamamatsu City, Shizuoka Prefecture, Japan), was used in ‘Bio/Chemiluminescence’ mode (i.e. the excitation source of the spectrofluorometer was turned off). The spectrum (an average of ten scans using 1000 ms gate time, 1 nm data interval and 20 nm band pass) was corrected as previously described.28 |
| This journal is © The Royal Society of Chemistry 2008 |
