Characterisation of organometallic and coordination compounds by solvent-free matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry
Mark F.
Wyatt
*,
Bridget K.
Stein
and
A. Gareth
Brenton
EPSRC National Mass Spectrometry Service Centre (NMSSC), School of Medicine, Swansea University, Singleton Park, Swansea, UK SA2 8PP. E-mail: m.f.wyatt@swansea.ac.uk; Fax: +44 (0) 1792 295554; Tel: +44 (0) 1792 295653
First published on 19th October 2007
Abstract
Insoluble or low solubility organometallic and coordination compounds have been characterised by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry, with solvent-free sample preparation being the key step toward successful analysis.
Matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOFMS) is an important and successful analytical technique for a wide variety of compounds. A traditional dried-droplet sample preparation technique relies on sample and matrix combinations that are soluble in the same volatile solvent or miscible solvents. However, many samples submitted to our Centre are insoluble or only soluble in less volatile solvents, e.g.dimethyl sulfoxide. Development of a solvent-free MALDI method complements other potentially solvent-free ionisation techniques, such as electron ionisation (EI), offering a softer method of ionisation. In addition, the method facilitates the analysis of compounds not suited to these other techniques.
Previous work in this area has focused mainly on synthetic polymers,1,2 but has also been applied to biochemical samples.3,4 Preliminary investigations into the analysis of various first-row transition metal acetylacetonate complexes using assorted literature and NMSSC solvent-free methods were summarised in a recent conference poster.5 The preparation method of ball-milling the sample and matrix mixture, followed by ‘smearing’ onto the target plate,6 including our own variations,5 is evaluated here for vanadium(III) acetylacetonate [V(acac)3], in order to establish a reliable protocol. V(acac)3 is partially soluble in dichloromethane and 1,1,1,3,3,3-hexafluoroisopropanol, and, together with related complexes, was the subject of a recent MALDI-TOF investigation within this laboratory.7 Intense positive radical ions were observed using the 2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile (DCTB) matrix.
The positive ion MALDI-TOFMS data for the various preparations† of V(acac)3 are given in Table 1. Radical ions for the sample (m/z 348.1) were observed for all preparations, showing that for a range of sample loadings, any milling/mixing method and method of sampling each mixture for analysis may be viable. The relative intensities of ions were moderately consistent between preparations with identical sample amounts. However, the total ion count fluctuated randomly between all preparations, despite every acquisition parameter remaining constant, and taking ‘hot spots’ into consideration. Re-analysis of the prepared plate revealed that a large increase in ion count and improvement in signal-to-noise ratio was observed when the laser was aimed at more translucent areas of the preparation, compared to opaque, powdery areas. This is a strange result, as translucent areas for a dried-droplet preparation usually indicate a low sample-to-matrix ratio. It is clear from these data that the smearing stage of the preparation is more critical than the milling/mixing stage.
| Preparation method | Sample/mg | Relative intensity (%) | |
|---|---|---|---|
| V(acac)3 (M+˙) | DCTB (M+˙) | ||
| A | 0.1 | 16 | 100 |
| 0.2 | 46 | 100 | |
| 0.5 | 100 | 43 | |
| 1.0 | 100 | 9 | |
| B | 0.1 | 30 | 100 |
| 0.2 | 45 | 100 | |
| 0.5 | 84 | 37 | |
| 1.0 | 100 | 27 | |
| C | 0.1 | 6 | 100 |
| 0.2 | 23 | 100 | |
| 0.5 | 92 | 100 | |
| 1.0 | 100 | 40 | |
In addition, two insoluble samples submitted to the NMSSC were examined as case studies. Compound 1 was ferrocene fully substituted with mercury acetate groups, while compound 2 was an erbium complex with three bis(pentafluorophenyl)phosphinate ligands (ErL3); structures are given in Fig. 1.
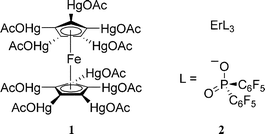 | ||
| Fig. 1 Chemical structures of 1 and 2. | ||
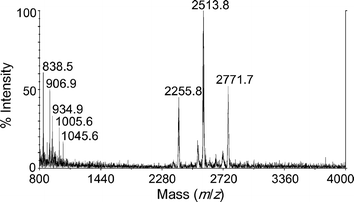
The ions for 1, given in Fig. 2, are very low intensity, but are sufficient for the major species to be identified. Positive radical ions for the fully substituted compound were observed at m/z 2771.7. The species at m/z 2255.8 and 2513.8 correspond to radical ions for compounds containing eight and nine mercury acetate groups respectively. These are not fragments, as the extra hydrogen is accounted for in the isotope patterns. The low mass species do not contain iron or mercury isotopes. Direct analysis by laser desorption was also attempted, by simply smearing some sample onto the target plate; no ions were observed.
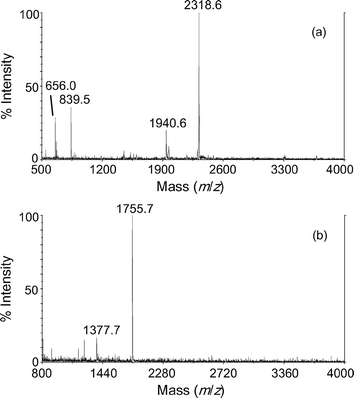
Compound 2 was considered to be sparingly soluble in methanol, but negative ions for the ligand were the only sample-related species observed by the dried-droplet method with DCTB. However, a multitude of positive and negative species were observed up to m/z 6000 with the DCTB solvent-free method, suggesting oligomeric complexes. Two fluorinated matrices, 2,3,4,5,6-pentafluorobenzoic acid (PFBA) and 2,3,4,5,6-pentafluorocinnamic acid (PFCA), were used also, as they are more miscible with fluorinated samples.8Spectra acquired with PFBA (see Fig. 3) are much simpler to interpret, with an [Er2L5]+ species at m/z 2318.6 and an anticipated [ErL4]– species being observed. Similar results were observed with the PFCA matrix. Characterisation of 2 as a solid was also attempted by positive ion EI and negative ion chemical ionisation (CI), but only low mass species (<m/z 500) were observed.
Here we demonstrate that useful data can be acquired for insoluble organometallic and coordination complexes by MALDI-TOFMS via solvent-free preparation methods. The smearing technique of Hanton and Parees,6 or more generally the application of mixture to the target, appears to be the critical step of such methods, and successful analysis is independent of the solids milling/mixing route (A, B, or C).
We wish to thank Dr I. R. Butler, University of Wales Bangor, UK, and Dr P. B. Wyatt, Queen Mary, University of London, UK, for permission to reproduce data pertaining to their compounds.
Notes and references
- R. Skelton, F. Dubois and R. Zenobi, Anal. Chem., 2000, 72, 1707–1710 CrossRef CAS.
- A. Marie, F. Fournier and J. C. Tabet, Anal. Chem., 2000, 72, 5106–5114 CrossRef CAS.
- M. Z. Wang and M. C. Fitzgerald, Anal. Chem., 2001, 73, 625–631 CrossRef CAS.
- S. Trimpin and M. L. Deinzer, J. Am. Soc. Mass Spectrom., 2005, 16, 542–547 CrossRef CAS.
- M. F. Wyatt, S. Havard, B. K. Stein and A. G. Brenton, Proceedings of 17th International Mass Spectrometry Conference (IMSC) in Prague, Czech Republic, http://www.swan.ac.uk/nmssc/Documents/2006-Solvent-free-prep.pdf, January 2007 (accessed 11 October 2007).
- S. D. Hanton and D. M. Parees, J. Am. Soc. Mass Spectrom., 2005, 16, 90–93 CrossRef CAS.
- M. F. Wyatt, S. Havard, B. K. Stein and A. G. Brenton, Rapid Commun. Mass Spectrom., submitted Search PubMed.
- A. Marie, S. Alves, F. Fournier and J. C. Tabet, Anal. Chem., 2003, 75, 1294–1299 CrossRef CAS.
Footnote |
| † For V(acac)3, 0.1, 0.2, 0.5, and 1.0 mg were added to both tapered plastic and flat-bottomed glass sample vials. 10 mg of DCTB matrix was added to each vial. One 3 mm steel ball was added to the plastic vials (Method A), and two balls to the glass vials (Method B). Each vial was agitated for 4 × 15 s to ensure complete homogenisation, using a vortex mixer. After each round of mixing, material was dislodged from vial crevices with a thin, stiff object, i.e. a microspatula or straightened paperclip. A small amount of loose material from each vial was then placed onto a stainless steel sample plate and smeared with the flat side of a microspatula. Material stuck to the ball from the plastic vials (Method C) was dabbed onto the plate and then smeared. Gentle pressure and a circular motion was used to smear until no loose powder remained. For 1, 1 mg was mixed with 10 mg of DCTB matrix. For 2, 0.3 mg was mixed with 10 mg each of DCTB, PFBA, and PFCA matrices. Method B was followed for all case study preparations. MALDI-TOFMS spectra were acquired using an Applied Biosystems Voyager DE-STR spectrometer, which is equipped with a nitrogen laser (λ = 337 nm). The instrument was operated in positive or negative ion, reflectron mode. The accelerating voltage was 20 kV, while the grid voltage was maintained at 65.5%. The delay time and laser fluence were optimised for each sample, but were kept constant for all data acquisitions for the V(acac)3 complex. |
| This journal is © The Royal Society of Chemistry 2008 |