Evaporation induced micellization of poly(2-oxazoline) multiblock copolymers on surfaces
Charles-André
Fustin
a,
Haiying
Huang
a,
Richard
Hoogenboom
b,
Frank
Wiesbrock
b,
Alain M.
Jonas
c,
Ulrich S.
Schubert
b and
Jean-François
Gohy
*a
aUnité de Chimie des Matériaux Inorganiques et Organiques (CMAT) and Research Center in Micro- and Nanoscopic Materials and Electronic Devices (CeRMiN), Université catholique de Louvain, Place L. Pasteur 1, 1348 Louvain-la-Neuve, Belgium
bLaboratory of Macromolecular Chemistry and Nanoscience, Eindhoven University of Technology and Dutch Polymer Institute (DPI), Den Dolech 2, 5600 MB, Eindhoven, The Netherlands
cUnité de Chimie et de Physique des Hauts Polymères (POLY) and CeRMiN, Université catholique de Louvain, Place Croix du Sud 1, 1348 Louvain-la-Neuve, Belgium
First published on 10th October 2006
Abstract
The formation of micelles on surfaces by spin-coating dilute solutions of diblock, triblock, and tetrablock copoly(2-oxazoline)s in a non-selective solvent is demonstrated. The micelles are not preexistent in the initial solution but are formed during the evaporation of the solvent by precipitation of the least-soluble block. The morphology and size of the micelles vary according to the fraction of this block but are not dependent on the block order in the copolymer.
Introduction
Spontaneous self-assembly of macromolecules, and in particular of block copolymers, is a major field in current research. Under the proper conditions, block copolymers can indeed give rise to nanostructures in the solid state (thin films or bulk) or in solution (formation of micelles).1 In this context, block copolymers have been considered as starting materials for the preparation of the so-called nanostructured surfaces, that show chemical and/or topographical features at the nanoscale. Such surfaces can be prepared from block copolymers by two main methods. Firstly, thin films prepared by spin-coating a solution of the copolymer in a non-selective solvent on a substrate is a method that could lead to chemically heterogeneous surfaces at the nanoscale due to phase separation. The resulting morphology depends on the degree of incompatibility and of the volume fraction of each block. Secondly, if a selective solvent of one of the blocks is used, the polymer chains are dissolved in their unimeric form under the critical micellar concentration (cmc), micelles being formed at higher concentration. Further deposition or adsorption of the block copolymer micelles finally result in topographically and chemically heterogeneous nanostructured surfaces.In this paper we report on an intermediate case: the formation of micelles during the spin-coating of dilute solutions of block copolymers in a non-selective solvent. Formation of micelle-like structures on surfaces has already been reported in the literature but was either due to the use of a selective solvent, and thus was in fact a deposition of micelles pre-existing in the solution,2–4 or was due to the preferential interaction of one of the blocks with the substrate surface.5–7 A typical example of this last case is the deposition of PS-b-PVP onto mica; the PVP block spreading on the substrate because of strong interactions with the mica and the PS block-forming clusters on the surface of the samples. In the case of micelle deposition from a selective solvent, the micelles can, according to the studied system, either keep their structure and form a dotted surface,2–3 or they can rearrange into a thin film-like morphology.3–4 In our case, the micelles form during the spin-coating process since a non-selective solvent is used, and all the blocks have the same interaction with the substrate. Such laterally patterned surfaces may have potential as functional substrates, or as templates in diverse fields such as for example nanotechnology, biomineralization or cell growth.
In this study, copoly(2-oxazoline)s have been selected as the starting materials for the formation of nanostructured surfaces by evaporation-induced micellization during spin-coating. The poly(2-oxazoline) family is highly interesting because of the large number of differently substituted monomers that can be readily prepared.8–9 These 2-oxazoline monomers can undergo living cationic ring-opening polymerization under the appropriate conditions, resulting in well-defined polymers with narrow molecular weight distributions. Moreover, their living character allows the synthesis of multiblock copolymers. However, the rather long reaction times (up to several days) for the polymerization of 2-oxazolines has been an important problem, limiting potential applications. Recently, an improved polymerization procedure which overcomes this drawback has been reported.10 This procedure uses closed reaction vials and microwave irradiation yielding an acceleration of the cationic ring-opening polymerization of 2-oxazolines by a factor of 400 when compared to conventional reflux polymerizations. Thanks to this improvement, series of diblock,11–14 triblock15 and even tetrablock16 copolymers were synthesized and characterized. Those multiblock copolymers are thus ideal candidates for our study since we can vary systematically the number of blocks (and thus the volume fraction of each constituent) and the position of each block in the copolymer.
Experimental
Microwave-assisted synthesis of diblock, triblock and tetrablock copoly(2-oxazoline)s
All polymerizations were performed under inert conditions in solutions of acetonitrile in a single-mode microwave reactor at 140 °C, using methyl tosylate as an initiator. The first blocks were polymerized at a monomer concentration of 4 M. The block copolymer were prepared by sequential addition of the different monomers to this initial mixture after complete consumption of the previous monomer. The required polymerization times for complete monomer consumption were calculated from a precedent kinetic study. The copolymer composition was determined by a combination of 1H NMR and size exclusion chromatography. For more details on the synthesis and characterization, see ref. 12, 14, 15, 16.Sample preparation
Silicon wafers were cleaned with a piranha solution (H2SO4–H2O2 30%, 70 : 30), followed by copious rinsing with ultra-pure water. The copoly(2-oxazoline)s were dissolved in ethanol at a concentration of 1 g L−1. These solutions were spin-coated onto the silicon wafers at 2000 rpm for 40 s.Scanning force microscopy
Scanning force microscopy (SFM) measurements were performed in the tapping mode with a Veeco Nanoscope IV Multimode microscope operated in air. Cantilevers (NCH type, Nanosensors) with a resonance frequency of approximately 330 kHz and a spring constant of 42 N m−1 were used.Results and discussion
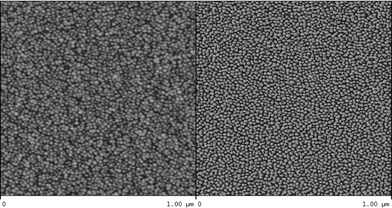
The compositions of the different copolymers used in this study are summarized in Table 1. The copolymers were built from three different monomers, namely 2-methyl- (MeOx), 2-ethyl- (EtOx), and 2-phenyl-2-oxazoline (PhOx). Diblock, triblocks and tetrablocks were synthesized using sequential monomer addition, changing the combination of blocks as well as the block order while maintaining similar degree of polymerization for each block. The selected set of 2-oxazoline monomers that was used for the synthesis of the copolymers yield polymers of different polarity. pMeOx and pEtOx are hydrophilic, while pPhOx is more hydrophobic. Nevertheless, all copolymers were perfectly soluble in ethanol at the concentration used for the spin-coating (1 g L−1). This was verified by performing dynamic light scattering (DLS) on these solutions. In all cases, a single peak was observed on the CONTIN size distribution histograms (not shown) corresponding to a Rh of about 3 nm, and attributed to free copolymer chains. After checking that only free chains were present in the solutions, those solutions were spin-coated onto silicon wafers and the samples were analyzed by SFM. Representative images are shown in Fig. 1, 2 and 3 for the EtOx33–PhOx33, MeOx33–EtOx33–PhOx33, and MeOx25–EtOx25–PhOx25–EtOx25 copolymers, respectively. Nanostructures are clearly seen for all samples, presenting a semi-continuous network of worm-like structures in the case of the diblock (Fig. 1), and spherical features for the triblock and tetrablock (Fig. 2 and 3). We postulate that these nanostructures are formed by the micellization of the copolymers during the spin-coating process as the solvent evaporates and the concentration increases. More precisely, the 2-phenyl-2-oxazoline block is thought to be responsible for this observation because it precipitates during the spin-coating. This behavior is also thought to be closely related to the non-selective solvent used. Indeed, ethanol is a non-selective solvent for this set of poly(2-oxazoline)s. Nevertheless, it is a rather polar solvent and the solubility parameters of the different blocks with ethanol are certainly not equal, and thus the phase diagrams of the investigated blocks in ethanol will be different. Because the PhOx block is more hydrophobic than the other two, the solubility limit of PhOx in ethanol could be crossed as the concentration increases during the evaporation resulting from the spin-coating process. The insoluble hydrophobic PhOx block could then aggregate, resulting in the formation of micellar structures. In order to give credit to this hypothesis, two complementary experiments have been performed. Firstly, when a solution of a MeOx33–EtOx33 diblock in ethanol at the same concentration is spin-coated onto a silicon wafer, no structures are obtained. This confirms that ethanol is a non-selective solvent for the MeOx and EtOx blocks in the whole concentration range. Secondly, whenever the same experiments are carried out in a better, non-selective, solvent for all blocks (N,N-dimethylformamide, DMF), no well-defined micellar structures are observed upon spin-coating the different copolymers. This confirms that DMF is a good solvent of the PhOx block in the whole concentration range, and therefore impedes the formation of micellar structures during spin-coating. In that case, islands of non-structured materials are observed on the surface. | ||
| Fig. 1 SFM image (left: height image, right: phase image) of a spin-coated sample from a 1 g L−1 solution of the EtOx33–PhOx33 copolymer in ethanol. | ||
 | ||
| Fig. 2 SFM image (left: height image, right: phase image) of a spin-coated sample from a 1 g L−1 solution of the MeOx33–EtOx33–PhOx33 copolymer in ethanol. | ||
 | ||
| Fig. 3 SFM image (left: height image, right: phase image) of a spin-coated sample from a 1 g L−1 solution of the MeOx25–EtOx25–PhOx25–EtOx25 copolymer in ethanol. | ||
| Copolymer composition | M n/PDI |
|---|---|
| MeOx33–PhOx33 | 12.3 kDa/1.14 |
| EtOx33–PhOx33 | 13.7 kDa/1.14 |
| MeOx33–EtOx33–PhOx33 | 11.7 kDa/1.24 |
| EtOx33–MeOx33–PhOx33 | 12.4 kDa/1.23 |
| EtOx33–PhOx33–MeOx33 | 16.2 kDa/1.20 |
| MeOx25–EtOx25–PhOx25–MeOx25 | 14.3 kDa/1.20 |
| MeOx25–EtOx25–PhOx25–EtOx25 | 13.6 kDa/1.19 |

|
|
The morphology of the obtained nanostructures is directly related to the fraction of the PhOx block in the copolymer. A semi-continuous worm-like structure is obtained for the diblocks where the weight fraction of the PhOx block is around 60%, and spheres are obtained with the copolymers that have the PhOx as their minor block, i.e., the triblocks and tetrablocks. For those two types of copolymers, the spheres are smaller (diameter around 8 nm) in the case of the tetrablocks compared to the triblocks (diameter around 13 nm). One can therefore conclude that the lower the volume fraction of PhOx block, the lower the size of the micelles formed during spin-coating. Because the average total degree of polymerization is the same for the investigated tri- and tetrablock copolymers, this observation indirectly indicates that micelles with a lower number of aggregation are formed in case of the tetrablock copolymers.
The other diblock, triblock and tetrablock copolymers yielded results (not shown) very similar to those presented in Fig. 1 to 3. This evidences that the block order in the copolymers has no influence on the morphology and size of the nanostructures formed and that only the composition of the copolymer is important. We have also studied the influence of the solution concentration on the features of the nanostructures. Different concentrations were tried, starting from 0.5 g L−1 and increasing to above 5 g L−1. The best results, whatever the copolymer type, are those discussed above for a concentration of 1 g L−1. Above 5 g L−1, homogeneous and featureless films are obtained as previously reported for films of diblock copoly(2-oxazoline)s.13 Between 1 and 0.5 g L−1, micelles of the same shape and size as those shown in Fig. 1 to 3 were obtained, but more widely spread on the substrate. This is another indication that the observed nanostructures are indeed micelles since the same objects are obtained upon dilution of the starting solution, but in smaller numbers.
In the present study, the evaporation-induced micellization is clearly the most probable origin of the observed nanostructures. In addition to the observations described above, we would like to point out that there is no preferential interaction between the surface of the substrate and one of the blocks, since they are all poly(2-oxazoline)s and bear thus the same functional groups. Moreover, it has been reported that pMeOx, pEtOx, and pPhOx have very similar surface tensions,11 and that they are (partially) miscible, probably due to the rather short block length of the investigated copolymers.13 Films of several diblock copoly(2-oxazoline)s spin-coated from more concentrated solutions only showed smooth and featureless surfaces without any sign of a phase separation.13 Lastly, we have annealed the different samples at 140 °C under vacuum for 5 h to check if the nanostructures obtained are at equilibrium. After this annealing the surfaces of the samples were featureless, evidencing that the structures obtained are indeed micelles formed and trapped during the spin-coating process.
Conclusions
The formation of micelles on surfaces by spin-coating dilute solutions of multiblock copoly(2-oxazoline)s in a non-selective has been reported. Those micelles are not preexistent in the initial solution but are formed during the evaporation of the solvent by the precipitation of the least soluble block, i.e., the 2-phenyl-2-oxazoline. The morphology and size of the micelles vary according to the fraction of the PhOx block but are not dependent on the block order in the copolymer. This process could have some application for the creation of nanostructured surfaces. It also shows that care must be taken when spin-coating dilute solutions of non aggregated block copolymers, the observed structures can not always attributed to dewetting or to phase separation in the starting solution.Acknowledgements
CAF is Chargé de Recherches FNRS. HH, AMJ and JFG thank the “Fondation Louvain” for financial support (mécénat Solvay). RH and USS would like to thank the Dutch Polymer Institute (DPI), the Nederlandse Wetenschappelijk Organisatie (NWO), and the Fonds der Chemischen Industrie for financial support as well as Henkel for providing the oxazolines.References
- I. W. Hamley, The Physics of Block Copolymers, Oxford University Press, Oxford, UK, 1998 Search PubMed.
- G. B. Webber, E. J. Wanless, S. P. Armes, Y. Tang, Y. Li and S. Biggs, Adv. Mater., 2004, 16, 1794 CrossRef CAS; J. Hahn and S. E. Webber, Langmuir, 2004, 20, 4211 CrossRef CAS.
- J. P. Spatz, S. Sheiko and M. Möller, Macromolecules, 1996, 29, 3220 CrossRef CAS.
- Z. Li, Y. Liu, M. H. Rafailovich, J. Sokolov, K. Khougaz, A. Eisenberg, R. B. Lennox and G. Krausch, J. Am. Chem. Soc., 1996, 118, 10892 CrossRef CAS.
- J. Zhao, S. Tian, Q. Wang, X. Liu, S. Jianga, X. Ji, L. An and B. Jiang, Eur. Phys. J. E, 2005, 16, 49 CrossRef CAS.
- J. P. Spatz, M. Möller, M. Noeske, R. J. Behm and M. Pietralla, Macromolecules, 1997, 30, 3874 CrossRef CAS.
- I. I. Potemkin, E. Y. Kramarenko, A. R. Khokhlov, R. G. Winkler, P. Reineker, P. Eibeck, J. P. Spatz and M. Möller, Langmuir, 1999, 15, 7290 CrossRef CAS.
- T. G. Bassiri, A. Levy and M. Litt, J. Polym. Sci., Part B, 1967, 5, 871 Search PubMed.
- R. Hoogenboom, M. W. M. Fijten, H. M. L. Thijs, B. M. Van Lankvelt and U. S. Schubert, Des. Monomers Polym., 2005, 8, 659 Search PubMed.
- F. Wiesbrock, R. Hoogenboom, C. H. Abeln and U. S. Schubert, Macromol. Rapid Commun., 2004, 25, 1895 CrossRef CAS; R. Hoogenboom, F. Wiesbrock, M. A. M. Leenen, M. A. R. Meier and U. S. Schubert, J. Comb. Chem., 2005, 7, 10 CrossRef CAS; F. Wiesbrock, R. Hoogenboom, M. A. M. Leenen, M. A. R. Meier and U. S. Schubert, Macromolecules, 2005, 38, 5025 CrossRef CAS.
- S. Wijnans, B. J. de Gans, F. Wiesbrock, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2004, 25, 1958 CrossRef CAS.
- F. Wiesbrock, R. Hoogenboom, M. A. M. Leenen, S. F. G. M. van Nispen, M. van der Loop, C. H. Abeln, A. M. J. van den Berg and U. S. Schubert, Macromolecules, 2005, 38, 7957 CrossRef CAS.
- S. Hoeppener, F. Wiesbrock, R. Hoogenboom, H. M. L. Thijs and U. S. Schubert, Macromol. Rapid Commun., 2006, 27, 405 CrossRef CAS.
- R. Hoogenboom, M. W. M. Fijten, R. M. Paulus, H. M. L. Thijs, S. Hoeppener, G. Kickelbick and U. S. Schubert, Polymer, 2006, 47, 75 CrossRef CAS.
- R. Hoogenboom, F. Wiesbrock, H. Huang, M. A. M. Leenen, H. M. L. Thijs, S. F. G. M. van Nispen, M. van der Loop, C. A. Fustin, A. M. Jonas, J. F. Gohy and U. S. Schubert, Macromolecules, 2006, 39, 4719 CrossRef CAS.
- R. Hoogenboom, F. Wiesbrock, M. A. M. Leenen, H. Huang, C. A. Fustin, P. Guillet, J.-F. Gohy and U. S. Schubert, submitted.
| This journal is © The Royal Society of Chemistry 2007 |
