Long-living channels of well defined radius opened in lipid bilayers by polydisperse, hydrophobically-modified polyacrylic acids
Florent
Vial
a,
Abdel Ghani
Oukhaled
b,
Loic
Auvray
b and
Christophe
Tribet
*a
aLaboratoire de Physico-chimie des Polymères et des Milieux Dispersés, University Pierre et Marie Curie and CNRS UMR 7615, ESPCI, 10 rue Vauquelin, F-75005, Paris, France. E-mail: christophe.tribet@espci.fr; Fax: +33 (0)140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40; Tel: +33 (0)140
40; Tel: +33 (0)140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45
bMatériaux Polymères aux Interfaces, University of EVRY and CNRS UMR 7581, Bd F. Mitterrand, F-91025, Evry, France. E-mail: loic.auvray@physique.univ-evry.fr; Fax: +33 (0)169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27; Tel: +33 (0)169
27; Tel: +33 (0)169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47
47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13
13
First published on 2nd November 2006
Abstract
In the presence of random amphiphilic polymers devoid of secondary structure, lipid membranes become leaky to albumin and dextran (fluorescence of giant unilamellar vesicles) and their conductance (black lipid films) shows the transient opening of channels of typical radius 1–5 nm.
Peptide and protein channels that can cross biological membranes play crucial roles in processes such as the exchange of ions and molecular substrates between cell compartments.1 Their ubiquitous functions are fairly well understood with respect to their ability to form assemblies spanning lipid bilayers. The formation of hollow bundles of amphiphilic peptides or toroidal pores is accordingly recognized as the basis of their important antimicrobial activity.2 Similarly, the design of synthetic permeabilizers and new therapeutic tools has been based on assemblies or molecules of limited lateral extension to mimic peptide channels (e.g., modified cyclodextrins3 or crown ethers4). Free diffusion in membranes of amphiphiles such as biocides induces, in contrast, large and erratic thermal fluctuations of pore size. Surfactants decrease the free energy of the edges of transmembrane pores, with no barrier to the growth of channel radii beyond several tens of nanometers.5
Here we show that well-defined channels of a few nanometers in diameter can be achieved with macro-surfactants devoid of well-defined structure. These polydisperse and random copolymers belong to a class of amphiphilic macromolecules called amphipols (Scheme 1) and are used to stabilize membrane proteins.6
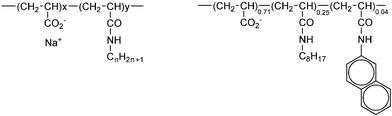 | ||
| Scheme 1 Structure of amphipols 5-yCn and 5-25C8-4Np. The parent poly(acrylic) acids have a molecular weight of 5000 g mol−1.6 According to the amphipol nomenclature,9 “5” stands for the molar mass of the parent chain, and Cn stands for the n-alkyl side groups (octyl C8 or dodecyl C12). The digit adjacent to the letter C refers to the molar percent of alkylacrylamide. Np stands similarly for “naphthylacrylamide”. | ||
Of practical importance, amphiphilic macromolecules are developed for controlled drug delivery and help the release of liposome contents in the cytosol after cellular uptake.7,8 The triggered delivery of drugs is typically associated with surfactant-like behaviour and a disruption of endosomal membranes.7 Our work suggests a stage of milder perturbation sharing some similarities with peptide channels. We investigated the behaviour of several short amphiphilic macromolecules in terms of their permeabilizing properties towards giant unilamellar vesicles. The size and dispersity of the channels were obtained by measurement of ionic current passing through planar bilayers.
To characterize the permeabilization of lipid bilayers, we observed the leakage of giant unilamellar vesicles (GUVs), loaded with fluorescent particles, with radii of a few nanometers. Giant vesicles having a diameter in the range 5–50 µm were prepared by swelling a film of lipids (egg phosphatidylcholine, dipalmitoylphosphatidic acid, and Rhodamine-modified phosphatidylethanolamine 88 : 10 : 2 mol%) in 150 mM NaCl, 10 mM BisTRIS pH 6.8 buffers containing sucrose and a soluble fluorescent probe, FITC–albumin or FITC–dextran (FITC stands here for conjugates with fluorescein isothiocyanate). Dilution of the samples in buffer with no fluorescent probe† enabled us to observe FITC–albumin (or FITC–dextran) trapped in the GUVs' internal compartment.10Fig. 1A,B show representative images of GUVs with membrane fluorescence excited at 515–560 nm, while internal fluorescence is excited at 460–500 nm. The initial internal fluorescence reflects the homogeneity of the population of GUV (Fig. 1E).
 | ||
| Fig. 1 Internal and membrane fluorescence of GUVs in presence of polymer 5-25C8-4Np. (A, B) After times of 5 min and 30 min, respectively, following polymer addition; scale bar 10 µm. (C) Internal fluorescence (marked “fluo” on the y-axis in arbitrary units). The x-axis is the distance along the dotted lines shown in A. (D) Internal fluorescence vs. position on the dotted line of the vesicle shown in B. (E) Histograms of internal fluorescence of ∼50 GUVs at time = 5 min (black) and 30 min (grey), with background fluorescence arbitrarily fixed at zero. | ||
The polymer was prepared in the same buffer as GUV samples and typically a 25 µL polymer solution was added to a 25 µL GUV sample (final concentrations ca. 10 mg L−1 lipids and 0.5 g L−1 polymer). The release of FITC–dextran was reflected by the presence of GUVs having lost their internal fluorescence (Fig. 1B,D), with no visible change of the fluorescence of the membrane (Rhodamine-modified lipid). The preservation of the spherical shape and homogeneity of membrane fluorescence pointed to the absence of membrane breakage at the resolution of photon microscopy.11 At a given incubation time after polymer injection, the histogram of the observed internal fluorescence of GUVs clusters the vesicles into two populations. As shown in the Fig. 1E, which is representative of all polymers and conditions tested, “empty” GUVs coexist with GUVs having essentially the same internal fluorescence as at time zero.
The fraction of empty GUVs increased slowly with incubation time, with no change in either membrane fluorescence or vesicle size. With 5-25C8-4Np at pH 6.8 and 150 mM NaCl, full leakage was reached at time 3 h (Table 1). In reference to samples with either no polymer or with the poly(acrylic) acid parent chains, no leakage was observed during incubation for 48 h. The leakage was accelerated by increasing the density of hydrophobes on the polymer or by increasing the length of the hydrophobic tails (Table 1, C12 vs. C8). Altogether, these results suggest a dominant role of hydrophobicity in the triggering of membrane permeability. Recalling that full leakage was obtained with no variation of the number of GUVs in the samples and homogeneous membrane fluorescence, the release of albumin or dextran clearly points to the formation of small pores through the bilayers with a minimum diameters of a few nanometers and a maximal diameter below the optical resolution of ca. 0.5 µm. Absence of visible membrane disruption (fluorescence excited at 515 nm) may also point to pores with a life-time too short to be observed by microscopy.
| Polymer | Buffer pH/[NaCl] |
t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min min |
t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min min |
t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
t![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) = =![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 h 24 h |
|---|---|---|---|---|---|
| a GUV loaded with FITC–albumin. | |||||
| 5-0 parent | 6.8/150 mM | 0 | 0 | 0 | 5% |
| 5-5C8 | 6.8/150 mM | 5% | 10% | 25% | 65% |
| 5-6C12 | 6.8/150 mM | 5% | 10% | 40% | 75% |
| 5-12C12 | 6.8/150 mM | 5% | 15% | 75% | 100% |
| 5-25C8-4Np | 6.8/150 mM | 15% | 50% | 100% | 100% |
| 5-25C8-4Np | 7.5/150 mM | 0 | 0 | 10% | 25% |
| 5-25C8-4Np | 6.8/10 mM | 0 | 0 | 0 | 5% |
| 5-25C8-4Np | a6.8/150 mM | 20% | 50% | 90% | 100% |
| 5-25C8-40C3 | a6.8/150 mM | 30% | 60% | 100% | — |
We determine whether permeability was associated with channels of defined size or with more erratic transient “rips”. The permeability events were investigated by measurement of conductance of planar egg PC–DPPA (9 : 1) membranes, a routine technique in biophysics.12 Black lipid films were made from a lipid solution in decane that was deposited on a hole of 150 µm diameter separating two chambers filled with an aqueous solution of KCl and 10 mM BisTRIS buffer pH 6.8. Electrical current was recorded using Ag/AgCl electrodes and an Axopatch 200B patch clamp amplifier (Axon Instruments Inc.). In the absence of polymer, no current was detected, though applying a potential of 100 mV resulted in high frequency current fluctuations (0 ± 10 pA) due to thermal motion of ions. Similarly, no current was observed for hours in the presence of parent polyacrylic acid. The addition of 5-25C8-4Np trigerred abrupt jumps between the zero-current state and a conductive state after an incubation time the duration of which fluctuated between a few minutes and up to hours. Fig. 2A–C are representative of the currents measured at fixed polymer concentration in the cathodic compartment. Abrupt current swings took place within less than one millisecond. Between two sharp transitions of conductivity, the membrane sustained for up to several seconds, fairly constant current states within typically 10–20% fluctuations, and no drift. Stepwise transitions clearly point to channel-like behaviours.
 | ||
| Fig. 2 Current traces through a lipid bilayer in presence of polymer 5-25C8-4Np and 1 M KCl. (A) 0.005 g L−1 polymer, (B, C) 0.01 g L−1 polymer, (C) is a zoom of B. Scale bars above the traces give on the y-axis the magnitude of the current and on the x-axis the time scale. (D, E) Point histograms of the number of times the current was measured during a run of 10 min with a sampling time of 0.1 ms; polymer 0.005 and 0.01 g L−1, respectively. | ||
In surfactant-containing membranes,5 including amphiphilic macromolecules,13 discrete conductance states are rare events among predominant erratic and fast flickering current variations. In our case, bursts of high conductances (such as those unzoomed in Fig. 2B) were in contrast not statistically significant. Histograms in Fig. 2D,E show that the plot of residence time of the membrane vs. the currents sampled for ∼10 min clusters the membrane conductivities into only two peaks, corresponding to either zero-conductivity or an “open” permeable state (e.g., 4 pA at 0.005 g L−1 polymer; 250 pA at 0.01 g L−1; and 430 pA at 0.02 g L−1 with typical fluctuations of ±10%). The histogram distribution did not vary during the life-time of the bilayer. Repeated measurements on freshly layered membranes, gave remarkably the same magnitude of the initial current jump within 3% uncertainty. Finally, these results are suggestive of the rapid formation (within a millisecond) of a well-defined channel that can last for several seconds before closing.
To convert measured currents into the apparent sizes of the channels, we assumed that the channels had a cylindrical geometry and performed calibration with α-hemolysin (a transmembrane peptide known to form channels with diameter of 1.5 nm). The conductance of ∼0.04, 2.5 and 4.3 nS observed for polymer concentrations 0.005, 0.01, and 0.02 g L−1, respectively, correspond accordingly to apparent diameters of 0.3, 2.4 ± 0.3 nm and 3.0 ± 0.5 nm (4.8 ± 0.7 nm was obtained at 0.01 g L−1 polymer and 0.15 M KCl). The small diameter of 3 Å obtained with the polymer at 0.005 g L−1 is comparable with the size of a monomer and is, presumably, indicative of a very narrow disorganisation of lipids throughout the membrane. At higher polymer concentrations, however, the apparent diameters are fairly compatible with channels containing several chains (Scheme 2). Channel radii of above 4 nm afford permeability to nanometer-large albumin and dextran, which is consistent with our results on the leakage of GUV. Because the order of magnitude of the surface of a GUV is comparable with the surface of the black lipid films used in this study, we can compare the behavior of the two systems. The rare occurrence of formation of an isolated channel in black lipid films should accordingly correspond to a low probability of formation of a single channel in GUV. The kinetics of leakage when a GUV contains a single channel can be estimated from the diffusivity of the probe and the Fick's law. We estimated that the half-release time of BSA is shorter than ∼10 min, assuming a diffusion coefficient of 7.10−7 cm2 s−1 for BSA, a channel diameter of 10 nm, and ascribing the concentration gradient between the internal compartment (GUV diameter of 10 µm) and the outer buffer to evolve across the membrane thickness of 5 nm. The absence of GUV with intermediate internal concentrations is thus consistent with the size of channels found on black films. The long incubation required to reach 100% release agrees with the rare occurrence of channel formation and incubation times of 10 min to hours observed before a first channel opens on black films.
 | ||
| Scheme 2 Tentative sketch of polymer–membrane interactions with increasing polymer concentration from left to right. | ||
The sizes obtained are similar to those of channel-forming peptides in membranes,1,14 with in addition, a similar sensitivity of channel size to polymer concentration.15 Finally, the behaviour of random & polydisperse acrylic copolymers resembles those of monodisperse & folded peptides. It is not surprising that amphiphilic macromolecules stabilize the saddle-like curvature of a channel and form sufficiently large pathways to let proteins go through the membrane. Because of amphipol's polydispersity and lack of secondary structure, the strikingly well-defined channels obtained in egg PC–DPPA bilayers point, however, to a general size-limiting effect that is not related to the size and folding of a polymer chain. Polymer segments bound on the edge of a pore may display repulsions (e.g., Coulombic) that control the pore.
Achieving subtle control of permeability in lipid membranes is an essential aspect in drug delivery as well as in cellular biology. Work is in progress to characterize the channel structure, by analysing the fluctuations of current through supported lipid films, and using various probe-particles as reporters of leakage kinetics. Polymer concentration and ionic strength were found to be key parameters that deserve investigation.
Notes and references
- J. M. Sanderson and S. Yazdani, Chem. Commun., 2002, 1154–1155 RSC; B. Hille, Ionic Channels of Excitable Membranes, Sinauer Press, Sunderland, 1992 Search PubMed.
- Z. Oren and Y. Shai, Biopolymers, 1998, 47, 451–463 CrossRef CAS.
- L. Bacri, A. Benkhaled, P. Guegan and L. Auvray, Langmuir, 2005, 21, 5842–5846 CrossRef CAS.
- G. W. Gokel, Chem. Commun., 2000, 1, 1–9 RSC.
- C. N. Armah, A. R. Mackie, C. Roy, K. Price, A. E. Osbourn, P. Bowyer and S. Ladha, Biophys. J., 1999, 76, 281–290 CrossRef CAS.
- Y. Gohon, G. Pavlov, P. Timmins, C. Tribet, J.-L. Popot and C. Ebel, Langmuir, 2006, 22, 1281–1290 CrossRef CAS.
- M.-A. Yessine, M. Lafleur, C. Meier, H. U. Petereit and J.-C. Leroux, Biochim. Biophys. Acta, 2003, 1613, 28–38 CAS.
- T. Chen, D. McIntosh, Y. H. He, J. Kim, D. A. Tirrell, P. Scherrer, D. B. Fenske, A. P. Sandhu and P. R. Cullis, Mol. Membr. Biol., 2004, 21, 385–393 CrossRef CAS.
- F. Vial, S. Rabhi and C. Tribet, Langmuir, 2005, 21, 853–862 CrossRef CAS.
- D. Needham and R. S. Nunn, Biophys. J., 1990, 58, 997–1009 CrossRef CAS.
- C. Ladaviere, C. Tribet and S. Cribier, Langmuir, 2002, 18, 7320–7327 CrossRef CAS.
- P. Mueller and D. O. Rudin, Nature, 1968, 217, 713–719 CAS.
- J. C. Chung, D. J. Gross, J. L. Thomas, D. A. Tirrell and L. R. Opsahl-Ong, Macromolecules, 1996, 29, 4636–4641 CrossRef CAS.
- L. Yang, T. A. Harroun, T. M. Weiss, L. Ding and H. W. Huang, Biophys. J., 2001, 81, 1475–1485 CrossRef CAS.
- M.-T. Lee, F. Y. Chen and H. W. Huang, Biochemistry, 2004, 43, 3590–3599 CrossRef CAS.
Footnote |
| † GUVs were diluted 20-fold in the buffer without fluorescent probe and containing glucose instead of sucrose (to balance internal and external osmolarities). GUVs loaded with sucrose internal solution sedimented in the glucose solution of lower density and concentrated at the bottom of the observation cell (the glass plates were pre-incubated with bovine serum albumin and rinsed with water to prevent adhesion of GUVs on bare silica). Excellent contrast was accordingly achieved between the internal fluorescence of GUV and the outer solution. The polymer was typically added at time 0 to a 25 µL GUV solution sample by gentle injection of 25 µL of polymer solution prepared in the same buffer with glucose. In our hands, all polymers except 5-25C8-40C3 did not affect neither the size, shape, nor homogeneity of GUVs during a 24 h incubation (observations of the fluorescent membranes excited at 515 nm). |
| This journal is © The Royal Society of Chemistry 2007 |
