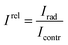Effect of low-level α-radiation on bioluminescent assay systems of various complexity
Tatiana V.
Rozhko
,
Nadezhda S.
Kudryasheva
*,
Alexander M.
Kuznetsov
,
Galina A.
Vydryakova
,
Lydia G.
Bondareva
and
Alexander Ya.
Bolsunovsky
Institute of Biophysics SB RAS, Krasnoyarsk, 660036, Russia. E-mail: gutniktv72@mail.ru; Fax: (3912) 43-34-00; Tel: (3912) 49-42-42
First published on 8th December 2006
Abstract
This study addresses the effects of low-level α-radiation on bioluminescent assay systems of different levels of organization: in vivo and in vitro. Three bioluminescent assay systems are used: intact bacteria, lyophilized bacteria, and bioluminescent system of coupled enzyme reactions. Solutions of 241Am(NO3)3 are used as a source of α-radiation. It has been shown that activation processes predominate in all the three bioluminescent assay systems subjected to short-term exposure (20–55 h) and inhibition processes in the systems subjected to longer-term exposure to radiation. It has been found that these effects are caused by the radiation component of 241Am3+ impact. The intensity of the 241Am3+ effect on the bioluminescent assay systems has been shown to depend on the 241Am3+ concentration, level of organization and integrity of the bioluminescent assay system. The bioluminescent assay systems in vivo have been found to be highly sensitive to 241Am3+ (up to 10−17 M).
Introduction
For several decades, the effect of ionizing radiation on living organisms has been intensively studied. Numerous experimental results show that in the cell, irreparable damage is done to the most important macromolecules—DNA and proteins.1,2,3 There are a great number of hypotheses on the mechanisms of radioactive impact, and none of them has become universally accepted.4,5 A large part of the studies addressed the effect of high-level radiation on biological objects. However, increasing radioactive contamination of the environment makes the question about effects of low and super low radiation on living organisms very important. The problem of low and super low dose radiation is related to the radiobiological paradox—an insignificant amount of absorbed ionizing energy causes an extremely pronounced biological effect.1–3,6–9 For example, a biological effect of α-particles on cell cultures is registered even under the impact of 0.05–0.3 particle per cell.10,11Detailed investigations of the effect of low dose radiation on living organisms are conducted using simple assay systems such as microorganisms, bacteria, plant and mammal cells, tissues of laboratory mice, drosophila flies, freshwater crustaceans, etc.12–16 Assay systems based on luminous bacteria can be good candidates for such investigations. Bacterial bioluminescent assays are widely used to monitor environmental toxicity.18,19 The tested parameter is the luminescence intensity of bacteria, which can be easily measured instrumentally. The advantages of the bioluminescent assays are their rapidity, sensitivity, simplicity, and availability of the devices for toxicity registration.19,20
Bioluminescent bacteria have been used as a bioassay for almost a half of a century. The bioassay was described in its current form in 1969.21 Later, it was modified by different researchers and adapted for their specific purposes; numerous applications of the bacterial bioassay are known.21–30 In 1990 the in vitro bioluminescent system of enzymatic coupled reactions was suggested as a toxicity assay;31 its applications were developed.32–34 Now, the bioluminescent ecological assay is a traditional and important biotechnological application of the bioluminescence phenomenon.20–23,35
General radiotoxicity was already investigated using bioluminescence of recombinant Escherichia coli strains under γ-ray irradiation.36 However, bioluminescent bioassays have not been used yet to study the effect of low-level α-ray irradiation. In their current forms they are not sensitive to a low-level α-ray irradiation; hence, the bioluminescent technique should be modified: bioluminescent reagents should be incubated with radionuclides.
A number of researchers,4,11,12 assumed that there is a relationship between the level of organization of a biological object and its response to low-level radiation exposure, which can be of both theoretical and practical interest. Bioluminescent assay systems can be conveniently used to verify this assumption as they can be based on biological objects of different levels of organization, bacteria-based and enzyme-based bioassays (in vivo and in vitro), providing comparison of the effect of low-level α-ray irradiation on enzymes and bacterial cells.
Thus, the purpose of this study was to investigate the effect of low-level α-radiation on bioluminescent assay systems of various complexity (in vivo and in vitro).
Materials and methods
Objects
Three bioluminescent assay systems (AS) were applied: AS(1)—cell suspension of 16 h P. phosphoreum 1883 IBSO culture from the Collection of Luminous Bacteria CCIBSO-836, AS(2)—a microbiotest 677F preparation i.e. lyophilized luminous bacterial cells of P. phosphoreum 1883 IBSO, and AS(3)—a kit of reagents for analytical bioluminescence, which includes lyophilized preparations of luciferase (0.5 mg ml−1) and NADH:FMN-oxidoreductase (0.15 units of activity). All the ASs were produced in the Institute of Biophysics SB RAS, Krasnoyarsk, Russia.As the source of ionizing radiation we used solutions of 241Am(NO3)3 of concentrations 10−15, 5 × 10−16, and 10−17 M in 1.1 M NaNO3 (pH 7.0). Activities of these solutions were 12000, 6000, and 1000 Bq l−1, respectively.
To investigate the effect of low-level α-radiation, the radioactive and control samples were prepared as follows.
Radioactive samples: solutions of 241Am(NO3)3 in 1.1 M NaNO3 were added to AS(1), AS(2), or AS(3).
Control samples: a 1.1 M NaNO3 solution was added to AS(1), AS(2), or AS(3).
Radioactive and control samples were maintained at constant temperature: +4 °C for AS(1), and −5 °C for AS(2) and AS(3). In some time-periods (0.5 h at the beginning of exposure to radiation and up to 10 h at the end of the exposure) the test samples (radioactive and control) were prepared and the bioluminescent intensity was measured at 20 °C. Measuring of bioluminescent intensity was carried out using standard procedure;17–20 it went on until the bioluminescent intensity in the control samples decreased to 20% of the maximal one. Time-courses of the bioluminescent intensity in the control and radioactive samples are presented in Fig. 1 taking AS(1) as an example.
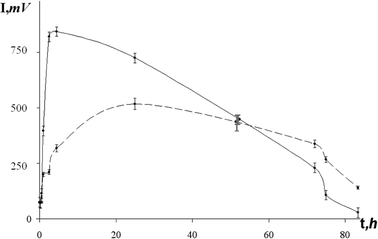 | ||
| Fig. 1 Bioluminescent intensity (I) versus time (t). | ||
The composition of the test samples and the procedure of radiotoxicity evaluation are described below.
Compositions of the test samples
(a) Radioactive (or control) test samples based on AS(1) or AS(2): 50 µl of radioactive (or control) samples based on AS(1) or AS(2), 500 µl of the 3% NaCl solution.(b) Radioactive (or control) test samples based on AS(3): 10 µl of radioactive (or control) samples based on AS(3), 50 µl of the 0.0025% tetradecanal solution, 200 µl of the 0.05 M potassium phosphate buffer, pH 6.8, 50 µl of 5 × 10−4 M FMN solution, and 200 µl of 4 × 10−4 M NADH solution. The reaction was initiated by addition of NADH solution.
Evaluation of radiotoxicity of the test samples
Kinetics of bioluminescence signal was measured using a CL3606 Biochemiluminometer (SEDD “Nauka”, Russia). The relative bioluminescent intensity Irel was calculated according to:where: Irad is the bioluminescence intensity in the radioactive test sample and Icontr is the bioluminescence intensity in the control test sample.
Values of Irel were plotted vs. time of exposure to 241Am3+. To evaluate the radiotoxicity of the samples, the maximal and the minimal relative bioluminescent intensities (Irelmax and Irelmin) were determined. Values of Irelmax correspond to the bioluminescence activation in the early stage of exposure; Irelmin values characterize the bioluminescence inhibition in the final stage of exposure.
Results and discussion
The following characteristics of the three ASs were under consideration: AS(1) contained whole bacterial cells; in AS(2) some of the cell walls were considerably damaged by lyophilization (freezing and rehydration)17 and penetration of low-molecular substances into the cells was facilitated;18 AS(3) included NADH:FMN-oxidoreductase and luciferase-enzymes catalyzing bioluminescent system of coupled reactions (1) and (2): | (1) |
 | (2) |
Using AS(1) and AS(3), one can compare radionuclide effects on the whole cells and enzymes. Hence, it is a way to evaluate the significance of the system's level of organization in its response to radiation exposure. A comparison of responses of AS(1) and AS(2) allows one to determine the role of cell integrity in this process.
It was found that the bioluminescence intensity in AS(1), AS(2), or AS(3) depended upon the time of their exposure to 241Am3+. Fig. 2 shows the relationship of Irel to exposure time for AS(1) at the highest 241Am3+ concentration used (C = 10−15 M). The Irelmax and Irelmin are indicated in the figure.
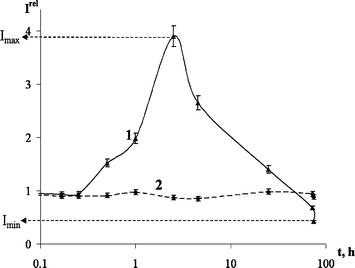 | ||
| Fig. 2 Relative bioluminescent intensity Irelvs. time of exposure (t/h) to: 1. Am3+: CAm = 10−15 M, AS(1) and 2. Eu3+: CEu = 10−15 M, AS(1). | ||
It can be seen that, first, irradiation activates the bioluminescence (Irel > 1) during 55 h of exposure, and, second, during the final stage of exposure, 55–85 h, the bioluminescence is inhibited (Irel < 1).
Similar relationships between bioluminescence intensity and exposure time were obtained for AS(2) and AS(3). Activation predominated for 35 and 20 h of the exposure in AS(2), and AS(3), respectively. Values of Irelmax were found at 6, 4, and 2 h, and values of Irelmin at approximately 85, 50 and 35 h of exposure in AS(1), AS(2), and AS(3), respectively.
Fig. 3 shows Irelmax and Irelmin for all the three systems: AS(1), AS(2), and AS(3) at three concentrations of 241Am3+.
 | ||
| Fig. 3 Effect of different concentrations of 241Am3+ on AS(1), AS(2) and AS(3); (a) bioluminescence activation, (b) bioluminescence inhibition. | ||
It can be seen in this graph that, the Irelmax value decreases and the Irelmin value increases as 241Am3+ concentration gets lower in every system. The most pronounced effects (Irelmax = 4; Irelmin = 0.4) were registered in AS(1) at the highest 241Am3+ concentration (C = 10−15 M). Irelmax values decrease from AS(1) to AS(3) for all the three 241Am3+ concentrations (Fig. 3(a)), while the Irelmin values change insignificantly from AS(1) to AS(3) (Fig. 3(b))
Thus, a comparison of the 241Am3+ effect on the bioluminescent ASs suggests that there are two kinds of effects: activation and inhibition. The more complex and the less damaged is the system, the more strongly pronounced is the activation.
Differences in the response of AS(1) and AS(2) can be accounted for by differences in the state of the cells in them (whole cells and damaged ones). It should be noted that bioluminescent activation was observed even in the enzymatic reactions—AS(3).
The impact of 241Am3+ on the bioluminescent assay systems can include a radiation component and a chemical one. To isolate the radiation component, we compared effects of 241Am3+ with that of trivalent nonradioactive metals Eu3+ and Fe3+. According to the periodic table, europium is the closest analog of americium in its electronic structure and, hence, chemical properties.
Model solutions of europium(III) nitrate and iron(III) chloride were used to estimate the chemical component. Within the concentration range from 10−7 to 10−17 M, neither europium nitrate nor iron chloride affected AS(1), AS(2) or AS(3). The absence of the effect of europium nitrate on AS(1) is demonstrated in Fig. 2 as an example. Hence, all the effects described above can be ascribed to the radiation component of the 241Am3+ impact.
Activation of the vital function of various organisms is a well-known phenomenon. As suggested by some researchers,37–43 the activation can be accounted for by unfavorable influence triggering cell defence responses. As soon as recovery processes in the system become active, radioactivity impact decreases.44,45 In our study we showed experimentally that ionizing radiation, within a certain range of low intensities, stimulates the bioluminescence intensity. (The bioluminescence intensity is supposed to be the main vital function of bacteria.21–25) These data are consistent with the results obtained for other organisms.15,16,44–51
The molecular mechanism of a change in bioluminescent intensity can be explained as follows: activation of bioluminescence must be caused by formation of radicals under the impact of ionizing radiation, and inhibition by their excess amount. A similar explanation for activation and inhibition of bacterial bioluminescence under the impact of UV irradiation was given by Czyz and colleagues.52
It was also reported in ref. 52 that the death rate of UV-irradiated bacterial cells with a luminescent system was lower than that of their dark variants. It is quite possible that the luminescent system takes part in neutralizing free radicals. Additionally, the authors speculated that one of the possible roles of bacterial luminescence is efficient DNA repair in a photoreactivation-like reaction. The same assumption can be made in the case of ionizing radiation. However, the noticeable response of the enzyme system to the ionizing radiation (AS(3) in Fig. 3(a),(b)), when there cannot be any DNA repair, is indicative of the complex mechanism of the cell defence responses.
The results obtained for the simplest assays can be taken into consideration for predicting effects of low-level α-radiation on complex organisms, including humans.
Conclusions
(1) There were two effects in bioluminescence dynamics (activation and inhibition), which corresponded to different times of exposure to 241Am3+. Irradiation activates bioluminescence during 20–55 h of exposure, and inhibits it during the final stage of exposure. (2) The intensity of the 241Am3+ effect on a bioluminescent assay system depended on: (i) 241Am3+ concentration (higher values of Irelmax and lower values of Irelmin were observed under higher concentrations of 241Am3+.); and (ii) level of organization of the bioluminescent systems and cell integrity (the most pronounced effect was observed in the intact bacteria, and the least in the enzyme reaction). (3) Bioluminescent assay systems in vivo are highly sensitive to 241Am3+ (up to 10−17 M). (4) The radiation component of the 241Am3+ effect contributed for the most part to activation and inhibition of bioluminescence.Acknowledgements
The work was supported by a joint grant of the Krasnoyarsk Regional Scientific Foundation and Russian Foundation for Basic Research N05-03-97701-R_Yenisei; Award No. RUX0-002-KR-06 of the U.S. Civilian Research & Development Foundation (CRDF) and RF Ministry of Education and Science, BRHE program; grant of the “Molecular and Cellular Biology” program of the Russian Academy of Science.References
- J. Pohl-Ruling, P. Fischer and O. Haas, Effect of low dose acute X-irradiation on the frequencies of chromosomal aberrations in peripheral lymphocytes in vitro, Mutat. Res., 1983, 100, 71 CrossRef.
- T. Lackey, Physiological benefits from low levels of ionizing radiation, Health Phys., 1982, 43, 771.
- E. Ron, B. Modan and D. Preston, Thyroid neoplasia following low-dose radiation in childhood, Radiat. Res., 1989, 120, 516–531 CrossRef CAS.
- A. M. Kuzin, Possible mechanisms of contribution of natural radiation background (NRB) to cell division stimulation, Radiats. Biol., Radioekol., 1994, 34, 398 Search PubMed.
- V. G. Zainullin, Genetic effects of chronic low-level radiation exposure, Radiats. Biol., Radioekol., 1997, 37, 555 Search PubMed.
- M. Pollycove, Nonlinearity of radiation health effects, Environ. Health Perspect., 1998, 10, 363.
- V. G. Zainullin and A. A. Moskalev, Effect of chronic low-dose radiation exposure and etoposide on lifetime of mei-41 Drosophila melanogaster flies, Genetika, 2000, 36, 578 Search PubMed.
- P. H. Abelson, Risk assessment of low level exposure, Science, 1994, 265, 1507 CrossRef CAS.
- J. Booz and L. E. Feinendegen, A microdosimetric understanding of low-dose radiation effects, Int. J. Radiat. Biol., 1988, 53, 13 CrossRef CAS.
- V. G. Zainullin, M. V. Shaposhnikov and I. N. Yuraneva, Genetic effects in Drosophila melanogaster induced by chronic low-dose radiation exposure, Radiats. Biol., Radioekol., 2000, 40, 567 Search PubMed.
- H. Nagasawa and J. B. Little, Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect, Radiat. Res., 1999, 152, 552 CrossRef CAS.
- A. V. Nikolsky and A. N. Koterov, Radioadaptive response of mammalian cells, Med. Radiol. Radiats. Bezop., 1999, 44, 5 Search PubMed.
- V. P. Lyutykh and A. P. Dolgikh, Clinical aspects of the effect of low-dose ionizing radiation on the human, Med. Radiol. Radiats. Bezop, 1998, 43, 28 Search PubMed.
- A. M. Kuzin, Ideas of radiation hormesis in the atomic century, Nauka, Moscow, 1995 Search PubMed.
- D. M. Spitkovsky, On some new biophysical and biological aspects of mechanisms under the impact of “low” and near-low doses of ionizing radiation on eukaryotic cells, Radiats. Biol., Radioekol., 1999, 39, 145 Search PubMed.
- A. N. Koterov and A. V. Nikolsky, Adaptation to irradiation in vivo, Radiats. Biol., Radioekol., 1999, 39, 648 Search PubMed.
- A. M. Kuznetsov, E. K. Rodicheva and E. V. Shilova, Bioassay based on lyophilized bacteria, Biotekhnologiya, 1996, 9, 57 Search PubMed.
- A. M. Kuznetsov, N. A. Tyulkova, V. A. Kratasyuk, V. V. Abakumova and E. K. Rodicheva, A study of properties of reagents for bioluminescent bioassays, Sibirskii Ekologicheskii Zhurnal, 1997, 75, 459 Search PubMed.
- N. Kudryasheva, V. Kratasyuk, E. Esimbekova, E. Vetrova and I. Kudinova, Development of the bioluminescent bioindicators for analyses of pollutions, Field Anal. Chem. Technol., 1998, 2, 277 Search PubMed.
- J. I. Gitelson and V. A. Kratasyuk, Photobiophysics of ecosystems, Ecol. Biophys., 2002, 1 Search PubMed.
- E. Grabert and F. Kossler, About the effects of nutrients on the luminescent bacteria test, in Bioluminescence and Chemiluminescence, ed. J. W. Hastings, L. J. Kricka and P. E. Stanley, John Wiley & Sons, Chichester, 1997, 291 Search PubMed.
- A. Roda, P. Pasini, M. Mirasoni, E. Michchelini and M. Guardigli, Biotechnological application of bioluminescence and chemiluminescence, Trends Biotechnol., 2004, 22, 295 CrossRef CAS.
- V. A. Kratasyuk and I. I. Gitelson, Use of bacterial bioluminescence and bioluminescent assay, Usp. Mikrobiol., 1987, 21, 3 Search PubMed.
- N. S. Kudryasheva, Bioluminescence and exogenous compounds. Physico-chemical basis for bioluminescent assay (Review), J. Photochem. Photobiol., B, 2006, 1, 77 CrossRef.
- N. S. Kudryasheva, Nonspecific effects of exogenous compounds on bacterial bioluminescent enzymes: Fluorescence study (Review), Curr. Enzyme Inhibition, 2006, 4, 363 Search PubMed.
- D. I. Stom, T. A. Geel, A. E. Balayan, A. M. Kuznetsov and S. E. Medvedeva, Bioluminescent method in studying the complex effect of sewage components, Arch. Environ. Contam. Toxicol., 1992, 22, 203 CrossRef CAS.
- K. V. Wood and M. G. Gruber, Transduction in microbial biosensors using multiplexed bioluminescence, Biosens. Bioelectron., 1996, 11, 207 CrossRef CAS.
- L. Guzzella, C. Bartone, P. Ross, G. Tartari and H. Mutau, Toxicity evaluation of lake Orta (Northen Italy) sediments using the Microtox system, Ecotoxicol. Environ. Saf., 1995, 35, 231.
- A. A. Bulish and D. L. Isenberg, Use of the luminescent bacterial system for rapid assessment of aquatic toxicity, ISA Trans., 1981, 20, 29.
- G. Natecz-Jawecki, B. Rudz and J. Sawicki, Evaluation of toxicity of medical devices using Spirotox and Microtox tests: I. Toxicity, of selected toxicants in various diluents, Biomed. Mater. Res., 1997, 35, 101 Search PubMed.
- V. A. Kratasyuk, Principles of luciferase biotesting in B. Jezowska-Trzebiatowska, ed. B. Kochel, J. Stawinski and I. Strek, Biological Luminescence, World Scientific, Singapore, 1990, 550 Search PubMed.
- E. V. Vetrova, V. A. Kratasyuk and N. S. Kudryasheva, Bioluminescent characteristics map of Shira lake water, Aquatic Ecol., 2002, 36, 309 Search PubMed.
- N. S. Kudryasheva, E. N. Esimbekova, N. N. Remmel, V. A. Kratasyuk, A. J. W. G. Visser and A. van Hoek, Effect of quinones and phenols on the triple enzymic bioluminescent system with protease, Luminescence, 2003, 18, 224 CrossRef CAS.
- N. Kudryasheva, E. Vetrova, A. Kuznetsov, V. Kratasyuk and D. Stom, Bioluminescent assays: effects of quinones and phenols, Ecotoxicol. Environ. Saf., 2002, 53, 221 CrossRef CAS.
- V. A. Kratasyuk, E. N. Esimbekova, M .I. Gladyshev, E. B. Khromichek, A. M. Kuznetsov and E. A. Ivanova, The use of bioluminescent biotests for study of natural and laboratory aquatic ecosystems, Chemosphere, 2001, 42, 909 CrossRef CAS.
- J. Min, C. W. Lee and M. B. Gu, Gamma-radiation dose-rate effects on DNA damage and toxicity in bacterial cells, Radiat. Environ. Biophys., 2003, 42, 189 Search PubMed.
- R. M. Macklin and B. Beresford, Radiation hormesis, J. Nucl. Med., 1991, 32, 350.
- P. A. Parsons, Radiation hormesis: an evolutionary expectation and the evidence, Appl. Radiat. Isot., 1990, 41, 857 CrossRef CAS.
- E. B. Burlakova, Effect of super-low doses, Vestnik RAN, 1994, 4, 80 Search PubMed.
- E. B. Burlakova, F. K. Goloshchapov and N. V. Gorbunova, Specific features of biological effect of low-dose radiation exposure, Radiats. Biol., Radioekol., 1996, 36, 610 Search PubMed.
- A. M. Kuzin, Strukturno-metabolicheskaya teoriya v radiobiologii, Nauka, Moscow, 1986, 282 Search PubMed.
- D. E. Li, Deistviye ioniziruyushchikh izluchenii na zhivyye kletki, Atomizdat, Moscow, 1962, 58 Search PubMed.
- I. I. Pelevina, G. G. Afanasyev and A. V. Aleshchenko, Radiation-induced adaptive response in children and effect of outer and inner factors, Radiats. Biol., Radioekol., 1999, 39, 106 Search PubMed.
- S. P. Yarmonenko, Problems of radiation biology at the end of the 20th century, Radiats. Biol., Radioekol., 1997, 36, 488 Search PubMed.
- V. G. Zainullin, M. V. Shaposhnikov, A. A. Moskalev and A. I. Taskaev, Sovremennyye aspekty radiobiologii Drosophila melanogaster, Nauka, Yekaterinburg, 2001, 102 Search PubMed.
- B. E. Lehnert and R. Iyer, Exposure to low-level chemicals and ionizing radiation: reactive oxygen species and cellular pathways, Hum. Exp. Toxicol., 2002, 21, 65 CrossRef CAS.
- A. M. Kuzin, On differences in leading molecular mechanisms under the impact of high- and low-dose γ-radiation on the organism, Izvestiya AN SSSR, Seriya Biol., 1980, 6, 883 Search PubMed.
- A. B. Schneider, E. Ron and J. Lubin, Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid, J. Clin. Endocrinol. Metab., 1993, 77/2, 362 CrossRef CAS.
- J. Edward, L. Calabrese and A. Baldwin, Radiation Hormesis and Cancer, Human Ecol. Risk Assess., 2002, 8, 327 Search PubMed.
- T. Lackey, Ionizing radiation promotes protozoan reproduction, Radiat. Res., 1986, 108, 215 CrossRef.
- T. M. Zukhbaya and O. A. Smirnova, An experimental and mathematical analysis of lymphopoiesis dynamics under continuous irradiation, Health Phys., 1991, 61, 87 CAS.
- A. Czyz, B. Wróbel and G. Wegrzyn, Vibrio harveyi bioluminescence plays a role in stimulation of DNA repair, Microbiology, 2000, 146, 283 CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |