A dye-sensitized solar cell driven electrochromic device
Henriette
Santa-Nokki
*,
Jani
Kallioinen
and
Jouko
Korppi-Tommola
Department of Chemistry/Nanoscience Center, University of Jyväskylä, P.O. Box 35, FIN-40014, Finland. E-mail: hebusi@cc.jyu.fi; Fax: +358 14 2604756
First published on 10th November 2006
Abstract
A new dye-sensitized solar cell driven electrochromic device has been fabricated. The device consists of an electrochromic display and a solar cell in a single nanocrystalline film. The optimization of the electrochromic and the solar cell functions was carried out. An applied potential of 1.0 V was required for coloring and the best solar energy conversion efficiency 1.1% was achieved. The efficiency may be compared to an efficiency of 4.6% obtained in a similar dye-sensitized solar cell without the display property. Coloring and bleaching times of the device were less than one second and a transmittance change from 38.7% (bleached) to 15.9% (colored) at best was achieved. The optimization of the electrochromic property of the device lead to decreasing efficiency of the solar cell and vice versa.
1 Introduction
Dye-sensitized solar cells (DSSCs) have attracted much attention over the past decade since they have been reported to have a high light into electricity conversion efficiency (up to 11%)1–3 and potential for low-cost production.4–7 A typical DSSC consists of two transparent conductive glasses or plastic substrates, a ruthenium dye-sensitized TiO2 film, a Pt catalyst layer and a liquid electrolyte containing the I−/I3− redox couple. During illumination, a dye molecule attached to the surface of a nanoparticle absorbs a photon. Following the excitation, an electron is injected into the conduction band of the nanoparticulate TiO2. The injected electrons are transported through the network of TiO2 into an outer electrical circuit. The oxidized dye is regenerated by the I−/I3− redox couple in an electrolyte solution. The I3− ions formed in regeneration are thought to diffuse to the cathode, where they are reduced by the returning electrons from the electrical circuit.In an electrochromic (EC) device a reversible optical change in transmittance and/or reflectance occurs as a consequence of an electrochemically induced oxidation–reduction reaction.8–10 Several materials have electrochromic properties with different colored states, e.g. transition metal oxides,11–13 viologens14–16 and conducting polymers.17 The structure of a viologen based electrochromic device is similar to the dye-sensitized solar cell, but the electron transfer direction is opposite to that of the electron transfer in solar cells. These devices need an applied external voltage to be operational.
Fitzmaurice et al. have reported a heterosupramolecular assembly,18–22 which consists of a covalently linked ruthenium complex and a viologen ligand. In such a device, it is possible to potentiostatically modulate the direction of the light-induced electron transfer from the ruthenium complex to TiO2 and to the viologen and thus the darkness of the system. Bonhote et al.23 have introduced a ruthenium complex that contains an attachment group and an electrochromic group. They have also described an anode consisting of a distinct electrochromic molecule with an attachment group co-absorbing with the dye. However, these articles discuss only the electrochromic properties of the devices.
In the present paper we report on the performance of a combined DSSC and EC device that includes two dyes, an electrochromic dye (a modified viologen) for coloring and a ruthenium dye for light-harvesting. This device has a dual function, it works both as a solar cell and as an electrochromic display in such way that two opposite electron transfer processes occur in the same film (electrode), i.e. the solar cell and the EC device are totally integrated. The space requirement is high, especially if the solar cell generates a voltage that is needed to the EC-reaction and both devices are arranged side-by-side. However, the space of solar cells and EC devices is usually very limited (for example in portable devices) and therefore it is advantageous that specific areas have multiple functions. The optimization of the electrochromic and the solar cell parts of the device is discussed.
2 Experimental
Photoactive electrodes were made on conductive glass substrate (F-doped SnO2, 8 Ω square−1). The films were spread using anatase TiO2 paste (Solaronix SA) by the doctor-blade technique using an adhesive tape as a frame. The film thicknesses were ∼3 µm and ∼6 µm. After spreading, the film was left to dry in air for few minutes and then fired at ∼450 °C for 30 min. The fired films were cooled down to ∼80 °C and immersed in a 4 mM bis(2-phosphonoethyl)-4,4′-pyridinium dichloride in formamide or in methanol and water mixture (1 : 1) to anchor the viologen on the surface of the titanium dioxide nanoparticles. The film was kept in solution from 2 min to 20 min. After attachment, the TiO2–viologen layer was rinsed with ethanol and sensitized with 0.3 mM Ru(2,2′-bipyridyl-4,4′-dicarboxylate)2(NCS)2(TBA)2H2·4H2O in ethanol overnight. The DSSC was sensitized direct with 0.3 mM Ru(2,2′-bipyridyl-4,4′-dicarboxylate)2(NCS)2(TBA)2H2·4H2O in ethanol and the EC-film direct with 4 mM bis(2-phosphonoethyl)-4,4′-pyridinium dichloride in methanol and water mixture (1 : 1). Sensitized films were rinsed with ethanol prior to cell assembly.The counter electrode was made by spraying 5 mM H2PtCl6 (Aldrich) in 2-propanol onto heated (80 °C) conducting glass substrate (F-doped SnO2, 8 Ω square−1) followed by heating in an oven for 15 min while the temperature was increased from room temperature to 385 °C and then held at 385 °C for 10 min.24
A hotmelt polymer foil (Surlyn 1702, DuPont) was used as a spacer frame between the electrodes. The empty volume between electrodes was filled with an electrolyte consisting of 0.5 M LiI (Aldrich, 99.9%) and 0.05 M I2 (Aldrich, 99.8%) in propylene carbonate (Aldrich, 99%)12 in the EC device and combined DSSC–EC device. The electrolyte in the DSSC consisting of 0.1 M LiI (Aldrich, 99.9%), 0.05 M I2 (Aldrich, 99.8%), 0.3 M tert-butylpyridine (Aldrich, 99%), 0.5 M 1-hexyl-3-methylimidazolium iodide (HMII, was synthesized according to ref. 25) in 3-methoxypropionitrile (Fluka, 99%). The electrodes were pressed together with clamps. Finally, a silver paste was added on clean areas of the conducting glass to enhance conductivity and to ensure good contact during measurements. A schematic representation of the device is presented in Fig. 1. Fabricated cells had a surface area of about 1.0 cm2.
 | ||
| Fig. 1 Description of the dual function cell. (A) F-doped SnO2 coated glass, (B) TiO2 layer containing ruthenium and viologen dye, (C) F-doped SnO2 coated glass containing Pt, and (D) electrolyte. | ||
Transmittance and kinetic measurements were carried out with a Varian Cary 100 UV/VIS spectrophotometer. For characterization, solar cells were illuminated with a halogen lamp (Solux) having an intensity of 0.1 Sun (10 mW cm−2). A calibrated solar cell was used as reference. A KG3 filter was used to adjust the illumination level of the halogen lamp. Current–voltage curves were collected by using a Keithley 2400 source measurement unit and LabView 6.1 software for both electrochromic and solar cell functions.
3 Results and discussion
We have studied the performance of a device having both a dye-sensitized solar cell and an electrochromic display functions. Both characterization of the solar cell conversion efficiency and electrochromic darkening behavior of the system were studied. Several nanocrystalline TiO2 films sensitized with both viologen and ruthenium were made. The color of the films changed from transparent to red due to the dye under zero applied voltage. An applied potential (≥0.8 V) caused darkening of the device (see Fig. 2). Coloring and bleaching responses were relatively fast (<1 s). The color did not change under illumination. | ||
| Fig. 2 Demonstration of a dual function device having a TiO2 film thickness of 6 µm. The applied potential is 0 V (left) or 1.0 V (right). | ||
Transmittance spectra of colored and bleached states for 3 µm and 6 µm TiO2 layers (both 20 min in viologen solution) are shown in Fig. 3. A typical change in transmittance for the 3 µm thick TiO2 layer at wavelength about 630 nm was from 39% to 20% and the response time about 0.6 s. The current consumption of the cell was 10.0 mA cm−2 at an applied voltage of 1.0 V. The kinetics of coloration and bleaching was tested by measuring the change in transmittance as a function of time at 630 nm (Fig. 4). The device was colored with an applied potential of 1.0 V and bleached at 0.0 V. The voltage was changed every 3 s. A typical change in transmission for the 6 µm thick TiO2 layer at 675 nm was 23%.
 | ||
| Fig. 3 Transmittance spectra of colored and bleached states of the dual function device. TiO2 film thickness of 3 µm (solid line) and 6 µm (dashed line). An applied voltage of 1.0 V was used for coloring. | ||
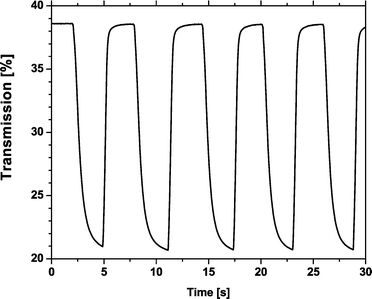 | ||
| Fig. 4 Periodic transmission response at wavelength of 630 nm (3 µm thick TiO2 film). | ||
The darkest color under applied voltage was achieved in the 6 µm TiO2 layer (see Fig. 3 and Table 1). However, the conversion efficiency of the solar cell was slightly better (0.1–0.2%) for the 3 µm TiO2 layer. The solar cell function improved, when viologen was dissolved in formamide instead of a methanol–water mixture (1 : 1). The best solar cell efficiency of 1.1% was achieved with the 3 µm thick TiO2 film, which was kept for 2 min in the viologen formamide solution. The following operational parameters were obtained: ISC = 0.67 mA cm−2, VOC = 0.27 V, IMPP = 0.57 mA cm−2, VMPP = 0.20 V, filling factor (FF) = 0.62 and Pmax = 0.11 mW cm−2 under 0.1 Sun illumination. When the 3 µm thick TiO2 layer was kept for 20 min in the viologen formamide solution, an efficiency of 0.9% was obtained. By using the methanol–water mixture instead of formamide for viologen sensitization, the efficiency dropped to 0.3% for the 6 µm thick TiO2 film kept for 20 min in the viologen methanol–water solution. The presence of water in the electrolyte has been shown to decrease also the performance of conventional DSSCs.26
| No | TiO2 film thickness/µm | Time in viologen solution/min | Conversion efficiency (%) | Transmittance change ΔTa (%) | Power consumption/mW cm−2 |
|---|---|---|---|---|---|
| a Zerobias, high transmittance (Th); 1V bias, low transmittance (Tl); ΔT = Th − Tl. b Transmission change at 630 nm (maximum). c Transmission change at 675 nm (maximum). | |||||
| 1 | 3 | 2 | 1.1 | 13.3b | 10 |
| 2 | 3 | 20 | 0.9 | 18.8b | 10 |
| 3 | 6 | 2 | 1.0 | 16.5c | 11 |
| 4 | 6 | 20 | 0.7 | 22.8c | 13 |
Tert-butylpyridine (TBP) has been widely used as an additive in conventional organic liquid electrolytes to improve the open-circuit voltage (VOC) in DSSCs.27,28 In our case however the presence of 0.5 M TBP in the electrolyte decreased dramatically the efficiency of the combined device. For a cell consisting of the sensitizer dyes on a 3 µm thick TiO2 layer and containing TBP in the electrolyte the best efficiency obtained was only 0.06%.
I–V curves of the devices for different preparation parameters are shown in Fig. 5. The color change was best for the cells for which viologen was introduced by keeping the TiO2 film in a viologen formamide solution for 20 min (Table 1). The optimization of the electrochromic part of the device decreased the efficiency of the solar cell and vice versa.
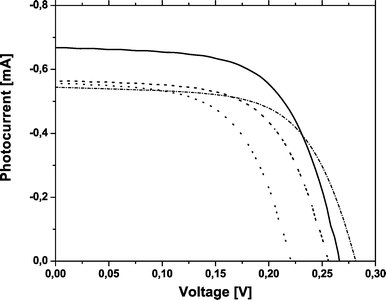 | ||
| Fig. 5 I–V curves of dual function devices with different preparation parameters. Solid line—3 µm TiO2 kept for 2 min in viologen, dashed line—3 µm TiO2 kept for 20 min in viologen, dashed–dotted line—6 µm TiO2 kept for 2 min in viologen and dotted line—6 µm TiO2 kept for 20 min in viologen. | ||
A typical DSSC consists of a ∼10 µm thick ruthenium dye-sensitized TiO2 film and 3-methoxypropionitrile (MPN) based electrolyte. Our reference DSSC consisted of a 6 µm thick TiO2 film sensitized with the ruthenium dye and the electrolyte was 0.1 M LiI, 0.05 M I2, 0.3 M TBP, 0.5 M HMII in 3-methoxypropionitrile. An efficiency of 4.6% was obtained under 0.1 Sun. The combined DSSC and EC device yielded a light to electricity conversion efficiency of 1.0%. A reference cell with the same electrolyte as in the combined DSSC–EC device yielded an efficiency of 3.7%. Fig. 6 shows the current–voltage curves of three different cells made from the same type of TiO2 films. The reference cell containing TBP in the electrolyte has the highest VOC and ISC. The light to electricity conversion efficiency of the combined DSSC–EC device is more than three times lower than that of a DSSC with the same electrolyte and film thickness. One of the reasons for the lower efficiency is related to the competing electron transfer reaction from the ruthenium dye to viologen and back to the electrolyte. However, an applied voltage of 2.4 V was needed for coloration in the bare EC device containing a 3 µm thick TiO2 layer and the same electrolyte that was used in the combined device. The power consumption (25.0 mA cm−2) of the EC device was more than two times greater compared to the power consumption (10.0 mA cm−2) of the combined device. This may be due to the presence of the DSSC. The bare EC-device needs an applied voltage of 2.4 V for coloration and the combined device needs only 1.0 V. The applied voltage in the combined device is smaller because DSSC generates a voltage (partially) to the EC-reaction.
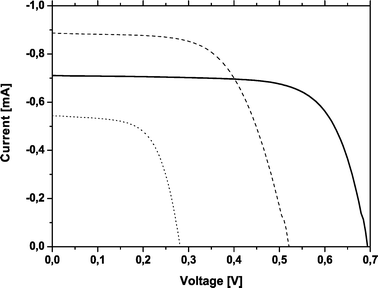 | ||
| Fig. 6 I–V curves of different types of cells made with a 6 µm thick TiO2 film. DSSC reference cell (solid line), DSSC containing an electrolyte consisting of 0.5 M LiI and 0.05 M I2 in propylene carbonate (dashed line) and combined DSSC and EC device containing an electrolyte consisting of 0.5 M LiI and 0.05 M I2 in propylene carbonate (dotted line). | ||
4 Conclusions
A combined dye-sensitized solar cell (DSSC) and electrochromic (EC) device has been built and characterized. In this device an electrochromic dye (viologen) co-absorbs with the sensitizer dye and the device can be used as a solar cell as well as an electrochromic device in such way that two opposite electron transfer processes occur in the same electrode. The space requirement is high if the solar cell generates a voltage to the EC-reaction and the devices are arranged side-by-side. However, the space of solar cells and electrochromic devices is usually very limited, especially in portable devices. Therefore it is advantageous that they are totally integrated into the same area. For the studied combined device an applied potential of 1.0 V was required for coloring and a power of 10 mW cm−2. In the best bifunctional device the solar energy conversion efficiency was 1.1%. Coloring and bleaching times were less than one second and a transmittance change from 39% (bleached) to 16% (colored) was obtained in the best case. The solar cell part of the combined device reduced the applied voltage that was needed for coloration in the bare EC-device. The optimization of the electrochromic function reduced the performance of the solar cell and vice versa.Acknowledgements
This work has been funded by the Technology Agency of Finland (TEKES). H. S.-N. is grateful to the Finnish Academy of Science and Letters, Vilho, Yrjö and Kalle Väisälä Foundation for support.References
- M. Grätzel, Mesoscopic Solar Cells for Electricity and Hydrogen Production from Sunlight, Chem. Lett., 2005, 34, 8–13 CrossRef.
- J. M. Kroon, N. J. Bakker, H. J. P. Smit, P. Liska, K. R. Thampi, M. Grätzel, A. Hinsch, S. Hore, J. R. Durrant, E. Palomares, H. Pettersson, T. Gruszecki, J. Walter, K. Skupien and G. Tulloch, New Concepts and Materials for World-Class Dye Sensitized Solar Cells, Energy Research Centre of The Netherlands (ECN), The Netherlands, Report ECN-RX-04-057, 2004, see: http://www.ecn.nl/library/reports/2004e/rx04057.html Search PubMed.
- M. Grätzel, Conversion of Sunlight to Electric Power by Nanocrystalline Dye-Sensitized Solar Cells, J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef CAS.
- B. O'Reagan and M. Grätzel, A Low Cost, High Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films, Nature, 1991, 353, 737–740 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Light-Induced Redox Reactions in Nanocrystalline Systems, Chem. Rev., 1995, 95, 49–68 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Molecular Photovoltaics, Acc. Chem. Res., 2000, 33, 269–277 CrossRef CAS.
- M. Grätzel, Photoelectrochemical Cells, Nature, 2001, 414, 338–344 CrossRef CAS.
- R. D. Rauh, Electrochromic Windows: An Overview, Electrochim. Acta, 1999, 44, 3165–3176 CrossRef CAS.
- N. M. Rowley and R. J. Mortimer, New Electrochromic Material, Science Progress, 2002, 85, 243–262 Search PubMed.
- R. J. Mortimer, Organic Electrochromic Materials, Electrochim. Acta, 1999, 44, 2971–2981 CrossRef CAS.
- B. A. Gregg, Photoelectrochromic Cells and Their Applications, Endeavour, 1997, 21, 52–55 CrossRef CAS.
- A. Hauch, A. Georg, S. Baumgärtner, U. O. Krasovec and B. Orel, New Photoelectrochromic Device, Electrochim. Acta, 2001, 46, 2131–2136 CrossRef CAS.
- S. K. Deb, Opportunities and Challenges of Electrochromic Phenomena in Transition Metal Oxides, Sol. Energy Mater. Sol. Cells, 1992, 25, 327–338 CrossRef CAS.
- R. Cinnsealach, G. Boschloo, S. N. Rao and D. Fitzmaurice, Coloured Electrochromic Windows Based on Nanostructured TiO2 Films Modified by Adsorbed Redox Chromophores, Sol. Energy Mater. Sol. Cells, 1999, 57, 107–125 CrossRef.
- P. Bonhote, E. Gogniat, F. Campus, L. Walder and M. Grätzel, Nanocrystalline Electrochromic Displays, Displays, 1999, 20, 137–144 CrossRef CAS.
- R. Cinnsealach, G. Boschloo, S. N. Rao and D. Fitzmaurice, Electrochromic Windows Based on Viologen-Modified Nanostructured TiO2 Films, Sol. Energy Mater. Sol. Cells, 1998, 55, 215–223 CrossRef CAS.
- A. A. Argun, P.-H. Aubert, B. C. Thompson, I. Schwendeman, C. L. Gaupp, J. Hwang, N. J. Pinto, D. B. Tanner, A. G. MacDiarmid and J. R. Reynolds, Multicolored Electrochromism in Polymers: Structures and Devices, Chem. Mater., 2004, 16, 4401–4412 CrossRef CAS.
- G. Will, G. Boschloo, S. N. Rao and D. Fitzmaurice, Potentiostatic Modulation of the lifetime of Light-Induced Charge Separation in a Heterosupermolecule, J. Phys. Chem. B, 1999, 103, 8067–8079 CrossRef CAS.
- G. Will, G. Boschloo, R. Hoyle, S. N. Rao and D. Fitzmaurice, Potentiostatic Modulation of the Direction of Light-Induced Electron Transfer in a Heterosupermolecule, J. Phys. Chem. B, 1998, 102, 10272–10278 CrossRef CAS.
- A. Merrins, C. Kleverlaan, G. Will, S. N. Rao, F. Scandola and D. Fitzmaurice, Time-Resolved Optical Spectroscopy of Heterosupramolecular Assemblies Based on Nanostructured TiO2 Films Modified by Chemisorption of Covalently Linked Ruthenium and Viologen Components, J. Phys. Chem. B, 2001, 105, 2998–3004 CrossRef CAS.
- G. Will, J. Sotomayor, S. N. Rao and D. Fitzmaurice, Heterosupramolecular Optical Write-Read-Erase Device, J. Mater. Chem., 1999, 9, 2297–2299 RSC.
- J. Sotomayor, G. Will and D. Fitzmaurice, Photoelectrochromic Heterosupramolecular Assemblies, J. Mater. Chem., 2000, 10, 685–692 RSC.
- P. Bonhote, L. Walder and M. Grätzel, Electrochromic or Photoelectrochromic Device, US Pat. 6734305, 2004 Search PubMed.
- N. Papageorgiou, W. F. Maier and M. Grätzel, An Iodide/Triiodide Reduction Electrocatalyst for Aqueous and Organic Media, J. Electrochem. Soc., 1997, 144, 876–884 CAS.
- P. Bonhote, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS.
- S. M. Zakeeruddin, Md. K. Nazeeruddin, R. Humphry-Baker, P. Pechy, P. Quagliotto, C. Barolo, G. Ciscardi and M. Grätzel, Design, Synthesis, and Application of Amphiphilic Ruthenium Polypyridyl Photosensitizers in Solar Cells based on Nanocrystalline TiO2 Films, Langmuir, 2002, 18, 952–954 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Light-Induced Redox Reactions in Nanocrystalline Systems, Chem. Rev., 1995, 95, 49–68 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulos and M. Grätzel, Conversion of Light to Electricity by cis-X2Bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium-(II) Charge-Transfer Sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on Nanocrystalline TiO2 Electrodes, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |
