Fluorescent dyes undergoing intramolecular proton transfer with improved sensitivity to surface charge in lipid bilayers
Gora
M'Baye
a,
Andrey S.
Klymchenko
*a,
Dmytro A.
Yushchenko
a,
Volodymyr V.
Shvadchak
a,
Turan
Ozturk
b,
Yves
Mély
a and
Guy
Duportail
a
aDépartement de Pharmacologie et Physicochimie, Photophysique des Interactions Biomoléculaires, UMR 7175, Institut Gilbert Laustriat, 74 Route du Rhin, Université Louis Pasteur (Strasbourg I), BP 60024, 67401, Illkirch, France. E-mail: aklymchenko@pharma.u-strasbg.fr
bIstanbul Technical University, Science Faculty, Chemistry Department, Organic Chemistry, Maslak 34469, Istanbul, Turkey
First published on 23rd November 2006
Abstract
4′-(Dialkylamino)-3-hydroxyflavones are characterized by an excited-state proton transfer reaction between two tautomeric excited states, which results in two emission bands well separated on the wavelength scale. Due to the high sensitivity of the relative intensities of the two emission bands to solvent polarity, hydrogen bonding and local electric fields, these dyes found numerous applications in biomembrane studies. In order to further improve their fluorescence characteristics, we have synthesized new dyes where the 2-phenyl group is substituted with a 2-thienyl group. In organic solvents, the new dyes exhibit red shifted absorption and dual fluorescence. Although they show lower sensitivity to solvent polarity and H-bond donor ability (acidicity) than their parent 3-hydroxyflavone dyes, they exhibit a much higher sensitivity to solvent H-bond acceptor ability (basicity). Moreover, when tested in lipid vesicles of different surface charge, the new dyes show much better resolved dual emission and higher sensitivity to the surface charge of lipid bilayers than the parent dyes. The response of the new dyes to surface charge is probably connected with the H-bond basicity of the membrane surface, which is the highest for negatively charged surfaces. As a consequence, the new dyes appear as prospective fluorophores for the development of new fluorescent probes for biomembranes.
Introduction
3-Hydroxychromone dyes are dual emission fluorophores due to an excited state intramolecular proton transfer (ESIPT) that results in two excited state forms: a normal (N*) and a tautomer (ESIPT product, T*) forms.1 Both N* and T* forms are highly emissive exhibiting well separated bands in the emission spectra. Importantly, these dyes can report the physicochemical properties of their microenvironment both by the positions and the relative intensities of their two emission bands.2 Due to these unique features, numerous 3-hydroxychromone dyes have been synthesized and studied during the recent years, resulting in the development of new fluorescence probes to study solvent polarity,2–4 ion binding5 and electric fields,6,7 with applications in the field of polymers,8 reverse micelles,9,10 lipid vesicles,7,11–14 cellular membranes15 and proteins.16–18 The most prospective biochemical targets for these dyes are biomembranes, which are characterized by relatively low fluidity and polarity. In this respect, the most suitable dyes are 4′-(dialkylamino)-3-hydroxyflavone derivatives (see the typical representative dye 1 in Fig. 1), since the polarity range of their dual emission corresponds well to that of the lipid membranes. However, the main disadvantage of these dyes is their absorption close to 400 nm, which is inconvenient for cellular studies due to the high phototoxicity of short wavelength excitation. In order to improve their absorption and fluorescence properties, several modifications of the basic fluorophore were considered. For instance, an increase in the electron donor ability at the 2-aryl group and an extension of the fluorophore shifted the absorption and emission spectra of the dyes to the red and increased their fluorescence quantum yield.19 Additional red shifts were achieved by the introduction of an electron acceptor group from the opposite side of the fluorophore.20 However, in all cases the shifts to the red were accompanied by strong variations in the intensity ratios. As a consequence, the polarity range where the dual emission can be observed was shifted to lower polarities. For instance, recently developed 3-hydroxychromone dyes absorbing around 440–450 nm show a dual emission only in highly apolar media (dielectric constant, ε = 2–6),19 which are uncommon in biological systems. The most apolar sites in living cells are in biomembranes, where the probes are exposed to an environment that corresponds to ε = 3–20. Therefore, an improved fluorophore for biomembranes should exhibit a dual emission in a more extended polarity range.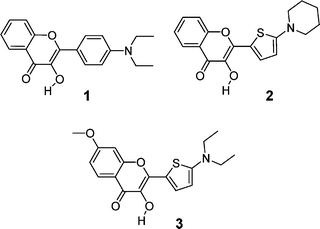 | ||
| Fig. 1 Chemical structures of the 4′-(dialkylamino)-3-hydroxyflavone reference dye 1 and the new 2-(2-thienyl)-3-hydroxychromone dyes 2 and 3. | ||
The other important issue of 3-hydroxychromone (3HC) dyes, which determines their applicability to biological systems, is their sensitivity to specific interactions. Previously, it was shown that H-bonds with water and alcohols strongly modify the ESIPT reaction in a non-substituted 3-hydroxyflavone.21 Moreover, we showed that protic solvents form a H-bond with the 4-carbonyl group of dialkylamino-substituted flavones, inhibiting the ESIPT reaction and favoring the emission of the N* excited state.2,22 However, in these studies the dyes did not show any sensitivity to the H-bond basicity, probably because the complexes of the dyes with H-bond acceptor solvents are not emissive.23
In the present work, we show that substitution of the phenyl ring by thiophene (3-hydroxy-2-(5-(1-dialkylamino)-2-thienyl)chromones, compounds 2 and 3, Fig. 1) strongly red-shifts the absorption and emission spectra. Moreover, the polarity range where the dual emission of this new dye is observed is broader than that of the parent molecule 1. In addition, we found that the new dyes, unlike other studied 3-hydroxychromones, are sensitive to solvent basicity. Furthermore, being bound to lipid vesicles, the new dyes demonstrate improved fluorescence properties as well as higher sensitivity to the vesicle surface charge as compared to the parent 4′-(dialkylamino)-3-hydroxyflavones. This high sensitivity of the new dyes is probably connected to their sensitivity to solvent basicity, suggesting that the dye responds to the basicity differences of lipid bilayers with different surface charge. The improved fluorescence properties as well as the new solvent sensitivity profile make these new fluorophores highly prospective for further applications in biological research, particularly for the development of new biomembrane probes.
Materials and methods
All reagents were purchased from Sigma-Aldrich. Solvents for synthesis were of reagent quality and were appropriately dried if necessary. For absorption and fluorescence studies, solvents were of spectroscopic grade.Absorption and fluorescence spectra were recorded on a Cary 400 spectrophotometer (Varian) and FluoroMax 3.0 spectrofluorimeter (Jobin Yvon, Horiba), respectively. For fluorescence studies, the dyes were used at an absorbance of 0.1 at the 420 nm excitation wavelength. Quantum yields of the dyes were determined with respect to a solution of dye 1 in ethanol as a reference (Φ = 0.52).24 Deconvolution of fluorescence spectra with two overlapping bands was performed with the program Siano, kindly provided by the author (Dr A. O. Doroshenko from the Karazin University, Kharkov, Ukraine).25 The program uses an iterative non-linear least-square method based on the Fletcher–Powell algorithm.25 The shapes of the individual emission bands were approximated by a log-normal function, which accounts for the asymmetry of the spectral bands.
Proton NMR spectra were recorded on a 300 MHz Bruker spectrometer and mass spectra were recorded on a Mariner System 5155 mass spectrometer using the electro-spray ionization (ESI) method. All column chromatography experiments were performed on silica gel (Merck, Kieselgel 60H, Art 7736).
Large unilamellar vesicles (0.11–0.12 µm in diameter) were obtained by extrusion as previously described.26 They were made either of egg yolk phosphatidylcholine (EYPC) and/or phosphatidylglycerol (EYPG), of bovine brain phosphatidylserine (BBPS) and of the synthetic cationic lipid N-[1-(2,3-dimyristoyloxy)-propyl]-N,N,N-trimethylammonium tosylate salt (DMTAP). Natural phospholipids were from Sigma and DMTAP was a gift from Dr Heissler (Institut de Chimie, Strasbourg). Experiments with vesicles were performed in phosphate–citrate buffer at 15 mM ionic strength, pH 7. Vesicles, at a concentration of 200 µM of lipids, were labeled at a lipid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dye ratio of 100 by adding small aliquots (∼2 µl) of DMSO stock solution of dye 2 in 1 ml solutions of vesicles, under vigorous vortexing.
dye ratio of 100 by adding small aliquots (∼2 µl) of DMSO stock solution of dye 2 in 1 ml solutions of vesicles, under vigorous vortexing.
Synthesis of 3-hydroxy-2-(5-(1-piperidinyl)-2-thienyl)chromone (2)
5-(1-Piperidinyl)-2-thiophenecarbaldehyde27 (1 mol) and 2′-hydroxyacetophenone (1 mol) were dissolved in a minimum volume of DMF followed by 3 mol of NaOMe. After the mixture was stirred overnight, it was diluted with ethanol and then, subsequently, 15 mol of sodium methoxide and 12 mol of 30% hydrogen peroxide were added. The mixture was refluxed for 3 min, cooled to room temperature and poured into water. After neutralization with diluted HCl, the resultant precipitate was filtered and the product was purified by column chromatography using as eluent a ethyl acetate–heptane mixture (EtOAc–Hept = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80). Yield 32%. 1H NMR (300 MHz, CDCl3) δ 8.20 (d, J = 8 Hz, 1H), 7.79 (d, J = 4 Hz, 1H), 7.60 (t, J = 7 Hz, 1H), 7.45 (d, J = 7 Hz, 1H), 7.35 (t, J = 8 Hz, 1H), 6.15 (d, J = 4 Hz, 1H), 3.30 (m, 4H), 1.70 (m, 6H). MS (EI): m/z 328.2 (M+). Elemental analysis: C18H17NO3S; Calcd. C 66.03, H 5.23, N 4.28; Found C 66.2, H 5.1, N 4.2%.
80). Yield 32%. 1H NMR (300 MHz, CDCl3) δ 8.20 (d, J = 8 Hz, 1H), 7.79 (d, J = 4 Hz, 1H), 7.60 (t, J = 7 Hz, 1H), 7.45 (d, J = 7 Hz, 1H), 7.35 (t, J = 8 Hz, 1H), 6.15 (d, J = 4 Hz, 1H), 3.30 (m, 4H), 1.70 (m, 6H). MS (EI): m/z 328.2 (M+). Elemental analysis: C18H17NO3S; Calcd. C 66.03, H 5.23, N 4.28; Found C 66.2, H 5.1, N 4.2%.
3-Hydroxy-7-methoxy-2-(5-(1-(N,N-dimethylamino))-2-thienyl)chromone (3)
This was synthesized using the same procedure as dye 2starting from 5-(1-(N,N-diethylamino))-2-thiophenecarbaldehyde and 2′-hydroxy-4′-methoxyacetophenone. It was purified by column chromatography on silica gel (eluent was ethyl acetate–heptane = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60). Yield 37%. 1H NMR (300 MHz, CDCl3) δ 8.08 (d, J = 8 Hz, 1H), 7.72 (d, J = 4 Hz, 1H), 6.9 (d, J = 8 Hz, 1H), 6.86 (s, 1H), 5.95 (d, J = 4 Hz, 1H), 3.89 (s, 1H), 3.40 (q, J = 7 Hz, 4H), 1.25 (t, J = 7 Hz, 6H). MS (EI): m/z 346.2 (M+). Elemental analysis: C18H17NO3S; Calcd. C 62.59, H 5.54, N 4.05; Found C 62.7, H 5.6, N 4.1%.
60). Yield 37%. 1H NMR (300 MHz, CDCl3) δ 8.08 (d, J = 8 Hz, 1H), 7.72 (d, J = 4 Hz, 1H), 6.9 (d, J = 8 Hz, 1H), 6.86 (s, 1H), 5.95 (d, J = 4 Hz, 1H), 3.89 (s, 1H), 3.40 (q, J = 7 Hz, 4H), 1.25 (t, J = 7 Hz, 6H). MS (EI): m/z 346.2 (M+). Elemental analysis: C18H17NO3S; Calcd. C 62.59, H 5.54, N 4.05; Found C 62.7, H 5.6, N 4.1%.
Results and discussion
Absorption and fluorescence properties of dyes 2 and 3 were studied in different organic solvents and compared with those of dye 1. The solvents were chosen to provide maximal variation of the physicochemical properties and were classified into three groups: protic (high H-bonding donor ability, α, Table 1), H-bonding acceptor (large β value) and neutral (low α and β values).28,29| Solvents | f(ε) | β | λ abs/nm | λ N*/nm | λ T*/nm | I N*/IT* | φ (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| a f(ε)—Polarity of a solvent as a function of the dielectric constant f(ε) = (ε + 1)/(2ε − 1); β—Abraham's H-bond basicity introduced in ref. 28 and 29; λabs—position of absorption maxima; λN* and λT*—positions of fluorescence maxima of the N* and T* states; φ is the fluorescence quantum yield; IN*/IT* is the intensity ratio of the two emission bands measured at the peak maxima; HMPA is hexamethylphosphoramide. Data on dye 1 are from ref. 2. | |||||||||||||||||
| Heptane | 0.1889 | 0.00 | 396 | 425 | 432 | 423 | 465 | 453 | 554 | 595 | 600 | 0.011 | 0.092 | 0.069 | 14 | 14 | 14 |
| Toluene | 0.2390 | 0.14 | 409 | 435 | 442 | 456 | 495 | 482 | 566 | 605 | 609 | 0.044 | 0.18 | 0.132 | 14 | 15 | 14 |
| Trichloroethylene | 0.3087 | 0.00 | 412 | 438 | 446 | 462 | 499 | 489 | 563 | 596 | 603 | 0.119 | 0.42 | 0.293 | 19 | 34 | 28 |
| Fluorobenzene | 0.3733 | 0.10 | — | 437 | 444 | — | 509 | 495 | — | 603 | 608 | — | 0.50 | 0.24 | — | 24 | 45 |
| Ethyl acetate | 0.3843 | 0.45 | 401 | 429 | 437 | 475 | 509 | 493 | 570 | 606 | 610 | 0.253 | 0.78 | 0.350 | 5.0 | 14 | 14 |
| Tributylphosphate | 0.414 | 1.21 | 404 | 430 | 438 | 482 | 509 | 492 | 576 | 610 | 612 | 0.594 | 1.79 | 0.90 | 13 | 22 | 23 |
| Dichloromethane | 0.4204 | 0.05 | 411 | 442 | 450 | 492 | 519 | 508 | 568 | 600 | 605 | 0.621 | 0.81 | 0.471 | 17 | 31 | 30 |
| HMPA | 0.475 | 1.0 | 403 | 429 | 435 | 506 | 521 | 501 | 583 | 618 | 618 | 1.45 | 3.20 | 1.57 | 14 | 21 | 18 |
| Acetonitrile | 0.4792 | 0.32 | 404 | 435 | 441 | 509 | 530 | 514 | 574 | 600 | 608 | 1.72 | 1.20 | 0.67 | 9.0 | 18 | 18 |
| Dimethylformamide | 0.4801 | 0.74 | 407 | 440 | 443 | 509 | 530 | 512 | 583 | 600 | 618 | 1.78 | 2.67 | 1.44 | 7.7 | 26 | 18 |
| Dimethyl sulfoxide | 0.4841 | 0.88 | 411 | 442 | 448 | 514 | 534 | 518 | 584 | 610 | 620 | 2.83 | 4.97 | 3.76 | 13 | 39 | 15 |
| 2-Methyl-2-Butanol | 0.3805 | — | 410 | 440 | 448 | 487 | 512 | 507 | 558 | 588 | 592 | 2.38 | 1.55 | 0.90 | 22 | 28 | 28 |
| 1-Butanol | 0.4579 | 0.48 | 415 | 444 | 454 | 517 | 535 | 525 | 567 | 586 | 588 | 6.57 | 2.44 | 1.38 | 58 | 34 | 46 |
| Ethanol | 0.4704 | 0.48 | 413 | 444 | 453 | 521 | 539 | 528 | 570 | 588 | 587 | 10.0 | 3.21 | 1.49 | 52 | 20 | 41 |
The absorption spectra of 2 and 3 exhibit significant red shifts with respect to the parent compound 1 in all studied solvents (Fig. 2, Table 1). With a few exceptions, similar red shifts are also observed in the fluorescence spectra (Fig. 2, Table 1). These red shifts can be related to the substitution of the phenyl ring by the highly polarisable and stronger electron donating thienyl ring, which increases the charge transfer character of the excited state of the dye. Moreover, the thiophene group, being a five-member ring, is probably smaller than the phenyl group and thus, by analogy with furyl-substituted 3HC, may be more planar.18,30 Similarly to 1, the new dyes show dual emission (Fig. 2, Table 1), which could be unambiguously assigned to the emission of the normal (N*) and the tautomer (T*) states in the short-wavelength and long-wavelength spectral regions, respectively. Excitation spectra recorded at the emission maxima are nearly identical (not shown), confirming that both states originate from the same ground state species. The red shifts in the fluorescence spectra of 2 with respect to 1 are generally accompanied by an increase in the ratio of the two emission bands, IN*/IT* (Fig. 2, Table 1). According to our previous studies, this effect is an additional indication of an increased charge transfer of the N* excited state of dye 2, which decreases the energy of this state with respect to that of the T* state and thus increases its relative intensity (i.e. increases the IN*/IT* ratio).18 Meantime, the electron donor methoxy group at the 7-position of dye 3 decreases the IN*/IT* ratio, in line with the previously observed effect of this group in 3HF.19 The red-shifted absorption and emission spectra of the new dyes make them more suitable for biological applications. Noticeably, the absorption of the new dyes around 440 nm makes them suitable for excitation with an He–Cd laser (442 nm). Another important property of the new dyes is their higher fluorescence quantum yield in most of studied solvents as compared to 1. The most notable exceptions are in highly polar protic solvents for which the quantum yields are lower. This last effect was previously observed with other dyes showing higher charge transfer character of their excited state19 and was attributed to solvent quenching effects.
 | ||
| Fig. 2 Normalised absorption (A) and fluorescence (at the N* band maximum) (B) spectra of dyes 1 (solid lines), 2 (dashed lines) and 3 (dotted lines) in dichloromethane. | ||
Similarly to the parent dye 1,2,31 the fluorescence spectra of 2 and 3 show a strong sensitivity to the solvent properties. Indeed, an increase of solvent polarity (a function of the dielectric constant ε)32,33 shifts the N* band to the red and increases the IN*/IT* ratio (Table 1). The logarithm of the IN*/IT* ratio of 2 and 3 increases linearly with the solvent polarity function of neutral solvents (Fig. 3). However, the slope of the linear fit for both dyes is lower than that observed for dye 1, indicating a lower sensitivity to solvent polarity. This implies that the polarity-dependent dual emission of the new dyes can be observed in a broader polarity range.
 | ||
| Fig. 3 Logarithm of IN*/IT* of dyes 2 (A) and 3 (B) vs. the solvent polarity function f(ε) for neutral (○), H-bond donor (protic, (△) and H-bond acceptor (□) solvents. Solid lines correspond to the linear fits for neutral solvents. Dotted lines correspond to the linear fit for dye 1 in neutral and basic solvents based on data from ref. 2. | ||
In H-bond donor (protic) solvents, the log(IN*/IT*) of dye 2 and 3 deviates upwards from the linear function in neutral solvents (Fig. 3), indicating that as in the case of 1, protic solvents inhibit the ESIPT reaction in these dyes. However, when protic and aprotic solvents of close polarity are compared (ethyl acetate and 2-methyl-2-butanol, see Table 1), we observe that the IN*/IT* ratio (8.3-fold) of dye 1 exhibits a dramatic increase in protic solvent, while for dyes 2 and 3 this increase is less pronounced (1.8 and 2.4-fold respectively). This indicates that the dual emission of dyes 2 and 3 is less sensitive to solvent acidicity. For dyes 2 and 3 in basic solvents, an upward deviation of log(IN*/IT*) from the linear function in neutral solvents is also observed (Fig. 3A). Remarkably, this deviation was not detected for dye 1.2 This differential sensitivity of the dyes to solvent basicity can be illustrated from the comparison of the dyes in a pair of solvents of similar polarity but different basicity (acetonitrile and DMF). While the ratio of the two emission bands of dye 1 is similar in these two solvents, we observe a strong increase in the IN*/IT* ratio for dyes 2 and 3 in the more basic DMF (Fig. 4). Therefore, we conclude that unlike 1, the dual emission of dyes 2 and 3 is sensitive to solvent basicity. Basic solvents probably inhibit the ESIPT reaction in 2 and 3, resulting in an increase of the intensity ratio of the N* state with respect to the T* state.
 | ||
| Fig. 4 Effect of solvent basicity on the dual emission of the dyes. Fluorescence spectra of 1 (A), 2 (B) and 3 (C) in acetonitrile (solid line) and dimethylformamide (dashed line). | ||
The inhibition of the ESIPT reaction with basic solvents can be explained by the formation of an intermolecular H-bond between the dye and a molecule of solvent (Fig. 5). This H-bond likely disrupts the intramolecular H-bond in the dye and thus uncouples the ESIPT reaction. In this case, the complexes of dyes 2 and 3 with basic solvents are emissive and contribute to the observed increase in the relative intensity of the N* band (i.e. the IN*/IT* ratio). In contrast, the solvent basicity does not affect the IN*/IT* ratio of the parent compound 1,2 probably because the corresponding H-bonded complex is not emissive.23 In summary, the new dyes 2 and 3 exhibit lower sensitivity to solvent polarity and acidicity than 1, but show a strong sensitivity to solvent basicity, which constitutes a new feature of these dyes.
Having improved the spectroscopic properties and different sensitivity to the environment, the new dyes are attractive for studying lipid bilayers. Previously, we reported that analogs of dye 1 exhibit a high sensitivity to the surface charge in lipid vesicles.12,34 However, the mechanism of this response is still unclear. Initially, it was proposed that the increased IN*/IT* ratio in negatively charged lipid bilayers is due to their higher hydration. In later studies, when we succeeded to separate the “hydrated” and “non-hydrated” states of the dye, we found that the negative surface charge of the bilayers does not affect the hydration of the dye, but influences the IN*/IT* ratio of its non-hydrated form.13 In this respect, examination of dyes 2 and 3 in lipid vesicles of different surface charge may contribute to understand the observed phenomenon.
Binding of dyes 2 and 3 to large unilamellar phospholipid vesicles results in a more than 5000-fold increase of the fluorescence intensity, as it can be seen from the comparison of the quantum yields in lipid vesicles and buffer (Table 2). In vesicles composed of neutral lipid EYPC, the dyes show dual emission (Fig. 6), which could be assigned to N* (short-wavelength) and T* (long-wavelength) bands. Excitation spectra recorded at the two emission bands are the same, confirming the presence of only one ground state species. The resolution between the N* and T* emission bands of dye 2 and especially dye 3 is much better than that observed previously for analogs of dye 1 (Fig. 6),12,34 showing a significant improvement for their further applications as two-band ratiometric probes.
| Lipid vesicles | Surface charge | λ abs/nm | λ N*/nm | λ T*/nm | I N*/IT* | ϕ (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 3 | ||
| a Symbols are as in Table 1. | |||||||||||
| DMTAP + EYPC | +0.5 | 446 | 455 | 525 | 529 | 600 | 605 | 0.957 | 0.56 | 17 | 16 |
| EYPC | 0 | 441 | 450 | 526 | 525 | 598 | 603 | 1.29 | 0.75 | 35 | 52 |
| EYPG + EYPC | −0.5 | 444 | 450 | 526 | 527 | 596 | 601 | 1.58 | 0.94 | 45 | 35 |
| EYPG | −1 | 445 | 455 | 525 | 522 | 591 | 598 | 2.20 | 1.61 | 36 | 34 |
| BBPS | −1 | 445 | 454 | 529 | 524 | 592 | 599 | 2.28 | 1.80 | 34 | 40 |
| Buffer | — | 444 | 465 | 576 | 552 | — | — | — | — | 0.008 | 0.006 |
 | ||
| Fig. 6 Fluorescence spectra of an analog of dye 1, 4′-(dimethylamino)-3-hydroxyflavone (A), 2 (B) and 3 (C) in lipid vesicles of neutral, EYPC (solid curve) and anionic, EYPG (dashed curve) lipids. | ||
To test the sensitivity of the dyes to the surface charge, we performed a series of experiments with vesicles composed of lipids (either pure lipids or equimolar binary mixtures) with differently charged polar heads. As shown in Fig. 6, the decrease of the surface charge (or surface potential) from neutral (EYPC vesicles) to negative value (−1, EYPG or BBPS vesicles) results in a pronounced decrease of the relative intensity of the T* emission (i.e. increase in the IN*/IT* ratio, Table 2). Meantime, the positions of the absorption and emission bands do not show significant changes (Table 2). These effects are in line with those previously observed for analogs of dye 1.12,34 However, the changes in the ratio of the two bands are larger in the case of the new dyes, indicating their higher sensitivity to the surface charge of lipid bilayers. For instance, in the case of dye 3, the intensity ratio at the peak maxima changes 2.4-fold from PC to PS, while this ratio changes only 1.35-fold for the analog of dye 1.12 Several factors could be responsible for the higher sensitivity of the new dyes to the surface charge. First, the improved resolution of the two emission bands evidently decreases the effects of band overlap and can thus increase to some extent the variation in the IN*/IT* ratio as a function of the surface charge. Second, as it was shown above, the new dyes exhibit higher sensitivity to the environment basicity but lower sensitivity to polarity and H-bond donor ability. Therefore, the observed increase in the sensitivity to the surface charge may be related to the H-bond basicity differences between negatively charged bilayers (high basicity) and neutral bilayers (low basicity). This conclusion is in line with previous reports showing that lipid bilayers of higher negative charge deprotonate a 7-hydroxycoumarin derivative.35 Thus, the present results suggest that the changes in the environment basicity could be a general mechanism for the response to the surface charge of the 3-hydroxychromone dyes studied so far.12,34 However, as we already showed, analogs of dye 1 are nearly insensitive to solvent basicity. In lipid vesicles, the situation can be different. Indeed, in a highly rigid environment containing H-bond acceptor groups, complexes with broken intramolecular H-bond can become emissive2,10,21,36 and thus increase the relative intensity of the N* emission. In the case of 2 and 3, the form with broken intramolecular H-bond (Fig. 5) is emissive already in solvents and contributes to the high sensitivity of these dyes to the surface charge of the vesicles.
Conclusions
The aim of the present work was to improve the spectroscopic properties of 3-hydroxychromone dyes by increasing their fluorescence quantum yields and shifting their absorption to longer wavelengths. This was realized by substitution of the phenyl ring of 3-hydroxychromones with a thiophene moiety. In comparison with their 3-hydroxyflavone analogs, the dual emission of the new dyes is highly sensitive to solvent basicity, while their sensitivity to solvent polarity and H-bond donor ability is significantly lower. The new dyes show strong response to the surface charge in lipid vesicles, probably in response to the differences in the H-bond basicity of the membrane surface. These new properties make these dyes prospective candidates for the study of lipid vesicles. In comparison to commonly used membrane dyes such as Prodan, Laurdan and Nile red,35,37–39 the new probes exhibit ESIPT-generated dual emission, which provides an additional information channel2 to study the membrane environment. Finally, substituting dyes 2 and 3 with amphiphilic groups, as it was done with dye 1,12,15 will allow the development of a new generation of membrane probes for surface potential in cellular membranes.Acknowledgements
This work was supported by CNRS and Université Louis Pasteur. The laboratory of André Mann and Jean Suffert is acknowledged for providing support for the organic syntheses. GM is a fellow from the Agence Universitaire de la Francophonie and DAY from the Ministère des Affaires Etrangères (Bourse d'excellence Eiffel). AK and VVS were fellows from the European Project TriOH.References
- P. K. Sengupta and M. Kasha, Excited state proton-transfer spectroscopy of 3-hydroxyflavone and quercetin, Chem. Phys. Lett., 1979, 68, 382–391 CrossRef CAS.
- A. S. Klymchenko and A. P. Demchenko, Multiparametric probing of intermolecular interactions with fluorescent dye exhibiting excited state intramolecular proton transfer, Phys. Chem. Chem. Phys., 2003, 5, 461–468 RSC.
- S. Ercelen, A. S. Klymchenko and A. P. Demchenko, Ultrasensitive fluorescent probe for the hydrophobic range of solvent polarities, Anal. Chim. Acta, 2002, 464, 273–287 CrossRef CAS.
- W. Liu, Y. Wang, W. Jin, G. Shen and R. Yu, Solvatochromogenic flavone dyes for the detection of water in acetone, Anal. Chim. Acta, 1999, 383, 299–307 CrossRef CAS.
- A. D. Roshal, A. V. Grigorovich, A. O. Doroshenko, V. G. Pivovarenko and A. P. Demchenko, Flavonols and crown-flavonols as metal cation chelators. The different nature of Ba2+ and Mg2+ complexes, J. Phys. Chem. A, 1998, 102, 5907–5914 CrossRef CAS.
- A. S. Klymchenko and A. P. Demchenko, Electrochromic modulation of excited-state intramolecular proton transfer: The new principle in design of fluorescence sensors, J. Am. Chem. Soc., 2002, 124, 12372–12379 CrossRef CAS.
- A. S. Klymchenko, G. Duportail, Y. Mély and A. P. Demchenko, Ultrasensitive two-color fluorescence probes for dipole potential in phospholipid membranes, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 11219–11224 CrossRef CAS.
- J. R. Dharia, K. F. Johnson and J. B. Schlenoff, Synthesis and characterization of wavelength-shifting monomers and polymers based on 3-hydroxyflavone, Macromolecules, 1994, 27, 5167–5172 CrossRef CAS.
- S. M. Dennison, J. Guharay and P. K. Sengupta, Excited-state intramolecular proton transfer (ESIPT) and charge transfer (CT) fluorescence probe for model membranes, Spectrochim. Acta, Part A, 1999, 55, 1127–1132 CrossRef.
- A. S. Klymchenko and A. P. Demchenko, Probing AOT reverse micelles with two-color fluorescence dyes based on 3-hydroxychromone, Langmuir, 2002, 18, 5637–5639 CrossRef CAS.
- O. P. Bondar, V. G. Pivovarenko and E. S. Rowe, Flavonols-new fluorescent membrane probes for studying the interdigitation of lipid bilayers, Biochim. Biophys. Acta, 1998, 1369, 119–130 CAS.
- A. S. Klymchenko, G. Duportail, T. Oztürk, V. G. Pivovarenko, Y. Mély and A. P. Demchenko, Novel two-band ratiometric fluorescence probes with different location and orientation in phospholipid membranes, Chem. Biol., 2002, 9, 1199–1208 CrossRef CAS.
- A. S. Klymchenko, G. Duportail, A. P. Demchenko and Y. Mély, Bimodal distribution and fluorescence response of environment-sensitive probes in lipid bilayers, Biophys. J., 2004, 86, 2929–2941 CrossRef CAS.
- A. S. Klymchenko, Y. Mély, A. P. Demchenko and G. Duportail, Simultaneous probing of hydration and polarity of lipid bilayers with 3-hydroxyflavone fluorescent dyes, Biochim. Biophys. Acta, 2004, 1665, 6–19 CAS.
- V. V. Shynkar, A. S. Klymchenko, G. Duportail, A. P. Demchenko and Y. Mély, Two-color fluorescent probes for imaging the dipole potential of cell plasma membranes, Biochim. Biophys. Acta, 2005, 1712, 128–136 CAS.
- S. Ercelen, A. S. Klymchenko and A. P. Demchenko, Novel two-color fluorescence probe with extreme specificity to bovine serum albumin, FEBS Lett., 2003, 538, 25–28 CrossRef CAS.
- A. S. Klymchenko, S. V. Avilov and A. P. Demchenko, Resolution of Cys and Lys labeling of alpha-crystallin with site-sensitive fluorescent 3-hydroxyflavone dye, Anal. Biochem., 2004, 329, 43–57 CrossRef CAS.
- S. V. Avilov, C. Bode, F. G. Tolgyesi, A. S. Klymchenko, J. Fidy and A. P. Demchenko, Temperature effects on alpha-crystallin structure probed by 6-bromomethyl-2-(2-furanyl)-3-hydroxychromone, an environmentally sensitive two-wavelength fluorescent dye covalently attached to the single Cys residue, Int. J. Biol. Macromol., 2005, 36, 290–298 CrossRef CAS.
- A. S. Klymchenko, V. G. Pivovarenko, T. Oztürk and A. P. Demchenko, Modulation of the solvent-dependent dual emission in 3-hydroxychromones by substituents, New J. Chem., 2003, 27, 1336–1343 RSC.
- A. S. Klymchenko and Y. Mély, 7-(2-Methoxycarbonylvinyl)-3-hydroxychromones: new dyes with red shifted dual emission, Tetrahedron Lett., 2004, 45, 8391–8394 CrossRef CAS.
- D. McMorrow and M. Kasha, Intramolecular excited-state proton transfer in 3-hydroxyflavone. Hydrogen-bonding solvent perturbations, J. Phys. Chem., 1984, 88, 2235–2243 CrossRef CAS.
- A. S. Klymchenko, V. G. Pivovarenko and A. P. Demchenko, Elimination of the hydrogen bonding effect on the solvatochromism of 3-hydroxyflavones, J. Phys. Chem. A, 2003, 107, 4211–4216 CrossRef CAS.
- A. S. Klymchenko, V. G. Pivovarenko and A. P. Demchenko, Perturbation of planarity as the possible mechanism of solvent-dependent variations of fluorescence quantum yield in 2-aryl-3-hydroxychromones, Spectrochim. Acta, Part A, 2003, 59, 787–792 CrossRef.
- P.-T. Chou, M. L. Martinez and J. H. Clements, Reversal of excitation behavior of proton-transfer vs. charge-transfer by dielectric perturbation of electronic manifolds, J. Phys. Chem., 1993, 97, 2618–2622 CrossRef CAS.
- D. B. Siano and D. E. Metzler, Band shapes of the electronic spectra of complex molecules, J. Chem. Phys., 1969, 51, 1856–1861 CrossRef CAS.
- M. J. Hope, M. B. Bally, G. Webb and P. R. Cullis, Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential, Biochim. Biophys. Acta, 1985, 812, 55–65 CAS.
- D. Prim, G. Kirsch and J. F. Nicoud, Efficient synthesis of N,N-disubstituted 5-aminothiophene-2-carboxaldehydes by nucleophilic aromatic substitution in water, Synlett., 1998, 383–384 CAS.
- M. H. Abraham, Hydrogen bonding. 31. Construction of a scale of solute effective or summation hydrogen-bond basicity, J. Phys. Org. Chem., 1993, 6, 660–684 CrossRef CAS.
- M. H. Abraham, H. S. Chadha, G. S. Whiting and R. C. Mitchell, Hydrogen bonding. 32. An analysis of water–octanol and water–alkane partitioning and the delta log P parameter of Seiler, J. Pharm. Sci., 1994, 83, 1085–1100 CAS.
- S. O. Yesylevskyy, A. S. Klymchenko and A. P. Demchenko, Semi-empirical study of two-color fluorescent dyes based on 3-hydroxychromone, J. Mol. Struct. (THEOCHEM), 2005, 755, 229–239 CrossRef.
- T. C. Swiney and F. D. Kelley, Proton transfer dynamics in substituted 3-hydroxyflavones: Solvent polarization effects, J. Chem. Phys., 1993, 99, 211–221 CrossRef.
- E. L. Lippert, in Organic Molecular Photophysics, ed. J. B. Birks, Wiley, New York, 1975, vol. 2, p. 1 Search PubMed.
- N. Mataga and T. Kubota, in Molecular interactions and electronic spectra, M. Dekker, New York, 1970 Search PubMed.
- G. Duportail, A. S. Klymchenko, Y. Mély and A. P. Demchenko, Neutral fluorescence probe with strong ratiometric response to surface charge of phospholipid membranes, FEBS Lett., 2001, 508, 196–200 CrossRef CAS.
- J. B. Massey, Effect of cholesteryl hemisuccinate on the interfacial properties of phosphatidylcholine bilayers, Biochim. Biophys. Acta, 1998, 1415, 193–204 CAS.
- A. Sytnik, D. Gormin and M. Kasha, Interplay between excited-state intramolecular proton transfer and charge transfer in flavonols and their use as protein-binding-site fluorescence probes, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 11968–11972 CAS.
- T. M. Parasassi, M. Di Stefano, M. Loiero, G. Ravagnan and E. Gratton, Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe, Biophys. J., 1994, 66, 763–768 CrossRef CAS.
- P. Jurkiewicz, J. Sýkora, A. Olżyńska, J. Humpolickova and M. Hof, Solvent relaxation in phospholipid bilayers: Principles and recent applications, J. Fluoresc., 2005, 15, 883–894 CAS.
- Ira and G. Krishnamoorthy, Probing the dynamics of planar supported membranes by Nile red fluorescence lifetime distribution, Biochim. Biophys. Acta, 1998, 1414, 255–259.
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |

