The solar UV radiation level needed for cutaneous production of vitamin D3 in the face. A study conducted among subjects living at a high latitude (68° N)
Kåre
Edvardsen
*ab,
Magritt
Brustad
a,
Ola
Engelsen
b and
Lage
Aksnes
c
aInstitute for Community Medicine, University of Tromsø, Tromsø, Norway. E-mail: kare.edvardsen@nilu.no; Fax: +47 77 64 48 31; Tel: +47 77 64 48 16
bNorwegian Institute for Air Research, The Polar Environmental Centre, Tromsø, Norway
cDepartment of Clinical Medicine, Section of Paediatrics, University of Bergen, Bergen, Norway
First published on 10th November 2006
Abstract
Populations at high latitudes experience several winter months with insufficient UV solar radiation to induce a significant cutaneous production of vitamin D. This unique study was designed to pursue an in vivo threshold of UV radiation needed for cutaneous production of vitamin D to take place if only the face was exposed to UV radiation. The vitamin D status were measured by analyzing blood samples weekly from a study group of 15 subjects over a period of 2 months during late winter, when UV radiation can be expected to increase substantially from rising solar elevations. Statistical analysis showed no significant positive association between the mean UV radiation dose and the mean 25(OH)D (25-hydroxy vitamin D) for the group. On an individual basis, however, we found indications that subjects with very low initial concentration of 25(OH)D (<30 nmol l−1) seemed to respond to UV radiation as early as in the beginning of March. For other individuals diet seemed to be the dominant controlling factor for 25(OH)D levels.
Introduction
By far the most important source of vitamin D for people living at mid- and low-latitudes is the induced synthesis in the human skin from solar UV radiation.†1 For people living at higher latitudes where the solar UV radiation is too weak during winter for the synthesis to take place,1 it is of great importance to obtain adequate vitamin D through diet (food or supplement) to avoid vitamin D insufficiency.2 Not only is vitamin D essential for normal calcium metabolism and maintenance of bone density but some autoimmune diseases3–8 and forms of cancer (breast, prostate and colon cancer) have been associated to vitamin D insufficiency through epidemiological studies.8–11 In addition it has been suggested that diagnoses during summer and fall, the time of year with the highest level of vitamin D, reveal the lowest risk of cancer death.12–15 Very few natural sources of food contain the vitamin and the most important ones are fatty fish, fish liver and fortified butter and margarine. For people living in the coastal areas of Norway, cod liver and cod-liver oil have played an important role as a vitamin D source in the traditional diet16 and most importantly as a substitute source during the winter when the UV radiation is absent, and thus no cutaneous production of vitamin D occur.Still, one unsolved question has been: “When is the UV radiation strong enough for a sufficient synthesis to take place”? The answer to this question is not only a function of solar elevation. The total amount of atmospheric ozone and cloudiness are also important controlling factors of UV radiation.1 In addition, the area of exposed skin17 and skin type18 matter.
In northern Norway freezing temperature often prevail until end of April. For comfort, most of its population expose no more than the face (about 3% of the body surface) to sunlight during this cold period. This reduces the cutaneous vitamin D production substantially.
The study was primarily designed to find the time of year when UV radiation is strong enough to result in sufficient cutaneous vitamin D production from facial exposure. We discuss here whether the subjects in our test group did respond in vitamin D status by staying outdoors at daytime more than 20 min every day during the test period of February 8–April 12, 2005. To our knowledge no other study has examined vitamin D status along with accurate and extensive measurements of UV exposure and vitamin D weighted UV-doses.
Materials and methods
Statistics
A group of 15 people, 4 females and 11 males, at the age of 34 to 58 years, from Andenes (68.2° N), Norway, participated in the study. The main reason for choosing this group was that they all worked at the Andøya Airport Station of the Royal Norwegian Air Force which is at sea level about 5.5 km from the UV instrument location (see below). Due to the scarce UV radiation level at this time of the year, the study participants were instructed to stay outdoors as much as possible and at least 20 min at noon every day if possible, with only the face exposed to daylight (20 min was chosen after negotiation with the subjects, as they had to take time off from work). They also had access to the same military nurse who collected a blood sample every week during the test period. The subjects were asked not to travel out of Andenes, not to use sun bed and not to start taking cod-liver oil if they had not already done so, or other dietary supplements. Violating any of these criteria would lead to the exclusion from the study.Questionnaire
Prior to the blood sampling, all participants answered a slightly altered version of the Norwegian Women and Cancer Study (NOWAC) food frequency questionnaire, which was used to estimate the usual daily vitamin D intake. Both the NOWAC questionnaire and the altered version have been described in detail elsewhere.19–21 When the baseline blood samples were drawn, the participants answered questions on hours spent in daylight the previous week, as well as sun-seeking holidays and use of solarium during the last month prior to the study. These questions have been validated.22 The questionnaire also contained questions on gender, age, height, and weight of the respondents. At the last blood sampling, all participants answered an additional questionnaire on fish-liver consumption related to the last fish-liver season (fish liver is consumed in the coastal areas of Norway from January to March/April).To record the subject's time spent outside in daylight, all subjects marked in a timetable with 1 min resolution when they were outside after 8:00 AM and before 16:00 PM. This was done for each person every day through the whole period, including weekends.
Software tools
For the statistical analysis we mainly used R, version 2.1.1 (2005-06-20), which is a computer language and environment for statistical analyses and graphics (http://www.r-project.org/). The data were also cross-checked with both the SAS and SBSS software for statistical analyses and graphics.25-Hydroxyvitamin D
Blood plasma was collected weekly from February 8 to April 12, 2005 and kept at −80 °C until analysis for 25(OH)D (25-hydroxy vitamin D) according to a modified version of the method described by Aksnes.23 Briefly, 0.25 ml plasma samples were spiked with 3H-25(OH)3 for calculation of recovery, and 25(OH)D was extracted with methanol and n-hexane. The n-hexane phase was collected, evaporated to dryness and ejected into a reverse-phase high performance liquid chromatography system. Elution of 25(OH)D was performed with methanol–water (85 : 15, v/v) and the elute was monitored at 265 nm by a diode-array detector (UV6000; ThermoFinnigan, San Jose, CA, USA) equipped with a 5 cm detector cuvette. The mean recovery of 25(OH)D was 77.2% (SD 3.9%) and the interassay variation was 6%, with a detection limit of 6.0 nmol l−1. The method determined the sum of 25(OH)D2 and 25(OH)D3.Solar UV radiation measurements
To ensure high accuracy measurements, the instrument used for the UV radiation measurements was a Brewer MK-III spectrophotometer manufactured by Kipp & Zonen, Delft, Netherlands (http://www.kippzonen.com), especially designed to measure spectral UV irradiance and total atmospheric ozone. It has a spectral response function with full width of the half maximum (FWHM) of ∼0.6 nm and is calibrated on a yearly basis by International Ozone Services, Toronto, CA. To compensate for eventual change in accuracy the instrument is run through a relative stability check several times a day. No significant change of accuracy of the instrument was detected throughout the study period. The instrument is placed at the Arctic Lidar observatory for Middle Atmosphere Research (ALOMAR, http://alomar.rocketrange.no) 380 m above sea level, near the hill ‘Røyken’, and about 5.5 km from Andøya Airport Station. The horizon at both ALOMAR and Andenes is very open, ensuring the same UV radiation conditions at both places.Biologically effective UV dose rate on vitamin D (BED-rate)
From experiments on human skin24 (in vitro) a minimum level of about 0.024 and 1.0 mW m−2 at 300 nm and 306 nm respectively, was found necessary for the vitamin D synthesis to take place. Based on this result Brustad et al.22 established a biological effective UV dose rate (BED-rate) for photo-conversion of 7-dehydrocholesterol (7-DHC) to previtamin D in skin with a threshold level of 0.472 mW m−2.Verified through continuous solar UV radiation measurements nearby Andenes, this value is normally reached daytime during the test period of February 6–April 12, 2005, (Fig. 1, panel (a)). MacLaughlin et al.25 obtained an action spectrum for the production of previtamin D3 from 7-DHC in human epidermis and this action spectrum has been standardized by the Commission Internationale de l'Eclariage (CIE).26 By applying this action spectrum on the measured high resolution UV radiation spectra near Andenes, it was possible to calculate the ambient BED-rate for vitamin D for the whole wavelength area of interest. Webb et al.24 used only three wavelengths (296, 300 and 306 nm) to describe the measured UV radiation. A BED-rate computed from only these few wavelengths is different from the true vitamin D effective BED-rate, accurately integrated over the full range of relevant wavelengths (eqn (1), Fig. 1).
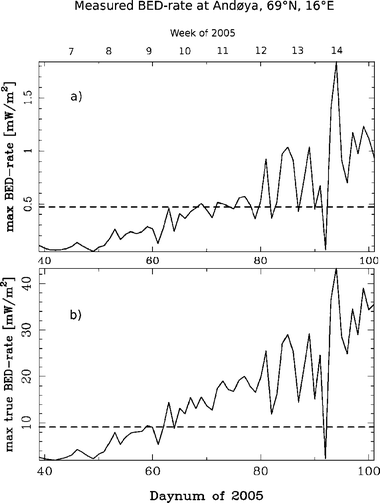 | ||
| Fig. 1 (a) The daily maximum BED-rate through the test period of February 6–April 12, using the method outlined in Brustad et al.22 The dashed line indicates the threshold level for photo-conversion of 7-dehydrocholesterol (7-DHC) to previtamin D in skin found in ref. 24. (b) The daily maximum true BED-rate for the same period using the method of weighting the measured spectrum by the normalized vitamin D action spectrum and integrated over all wavelengths of interest. | ||
Based on the work by Webb et al.,24 we established a true vitamin D effective UV dose rate for the photo-conversion of 7-dehydrocholesterol (7-DHC) to previtamin D in skin. From the spectral measurements of 0.024, 1 and 10 mW m−2 nm−1 at 300, 306 and 316 nm respectively, we have estimated the rest of the spectrum by matching the above measurements with simulations from the well established libRadtran model.27 Using estimated input parameters for solar zenith angle, ozone, cloud condition and aerosols, we were able to reproduce a spectrum where the calculated values at 300, 306 and 316 nm are within 0.5%, 3% and 19% of the measured values, respectively. Under the assumption that the our calculated spectrum pertains to the conditions during the experiment of Webb et al.,24 it was weighted by the normalized vitamin D action spectrum and integrated over all wavelengths provided by the Brewer instrument (295–330 nm):
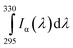 | (1) |
The preferred model spectrum to represent the conditions during the experiment of Webb et al.24 was calculated with input parameters of solar zenith angle of 57°, close to local noon in Boston (42.2° N), for the 15th of February, 1986, TOMS ozone (TOMS: total ozone mapping spectrometer, http://toms.gsfc.nasa.gov) was 404 DU, strong urban aerosol (aerosol optical depth AOD (0.55 µm) = 0.79), and scattered clouds (total water column of 460 g m−2) with a 45% cloud coverage located at an altitude between 2 and 4 km. However, it is not in the scope of this work to reconstruct the overall conditions during the experiment of Webb et al.24 The important and difficult issue is to find a spectrum most likely to represent the UV radiation conditions during their experiment in order to establish a realistic BED-rate threshold.
As the subjects kept a record on time spent outdoors exposing only their face to daylight, we were able to calculate the weekly vitamin D effective UV dose they where exposed to. In calculation of the weekly vitamin D effective UV dose, we assumed all subjects to receive the same UV dose if they were outdoors at the same time.
Results
Blood sample and diet results
We found a relatively large variation in mean 25(OH)D over the period between the subjects, ranging from 23.9 to 74.8 nmol l−1 (results not shown here). Unfortunately, we have no information on specific vitamin D intake throughout the test period other than the daily mean value calculated from the diet information in the questionnaires. This number provides information on each subject's intake through diet in general. We found the subjects with very low initial 25(OH)D value to have the lowest intake. Seven subjects had lower intake than the 7.5 µg per day recommended by the Norwegian health authorities. Five of these subjects had concentration of <37.5 nmol l−1, which has been used as an indicator for moderate hypovitaminosis D.28We found no significant relations between age, body mass index, sex and 25(OH)D levels for our participants.
Vitamin D and UV radiation
BED-rate measurements relative to the threshold value are higher when we integrate over the full range of relevant wavelengths (eqn (1), Fig. 1).Statistical analysis showed there were no significant positive association between mean BED and mean 25(OH)D for the group (Fig. 2). The minimum mean BED was measured after the first week of the test period (February 15, 2005, 701 J m−2) and the maximum mean BED was measured right after week 12 (March 29, 2005, 9890 J m−2). For BED lower than ∼7000 J m−2 there is a negative gradient in the mean 25(OH)D, corresponding to the time before week 12 (due to Easter holidays we have no 25(OH)D measurements for week 12). For BED above ∼7000 J m−2 there is a slight positive gradient in mean 25(OH)D, corresponding to the time after week 12.
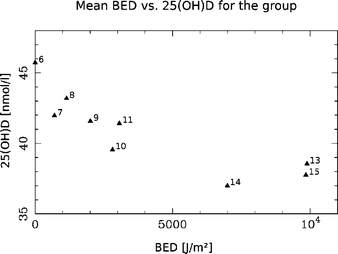 | ||
| Fig. 2 The figure shows how mean vitamin D for the group decreases for BED under ∼7000 J m−2 and slightly increase for BED above ∼7000 J m−2. Each point is marked with the corresponding week of 2005. The maximum value of week 13 corresponds to the Easter. | ||
Discussion
Exposure to sunlight and its vitamin D effective part of the spectrum is the simplest, cheapest and most effective source of vitamin D. The stronger the radiation is, the more effective the photochemical transformation of provitamin D3 to previtamin D3.We have extended the method for calculation of BED22 to take into account all wavelengths covered by the vitamin D action spectrum. Based on the results from facial UV exposure we cannot find a well defined and statistically significant, BED-threshold needed for the cutaneous vitamin D synthesis to take place. However, for our group the results suggests a BED above ∼7000 J m−2 in order to incite an apparent cutaneous vitamin D production (Fig. 2).
The measurements in Fig. 1, panel (b) show slightly higher values relative to the threshold value than in panel (a), suggesting better conditions for cutaneous production of vitamin D than first anticipated, especially during the days 62–92. Introducing this new “true BED-rate” shortens the vitamin D winter by at least ten days.
We find indications on the production in the subjects with low initial 25(OH)D levels as early as in late February (min. SZA = 76°) with only their faces exposed (Fig. 3), and we find no association between 25(OH)D and BED for the subjects with higher initial levels of 25(OH)D for the same period (Fig. 4).
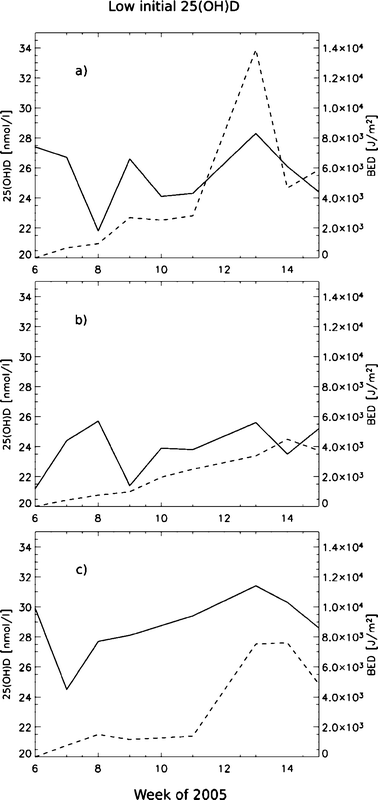 | ||
| Fig. 3 :The graphs (a), (b),and (c) show the individual 25(OH)D (solid) and BED (dashed) vs. time for the three subjects in the group with a low initial 25(OH)D status (<30 nmol l−1). | ||
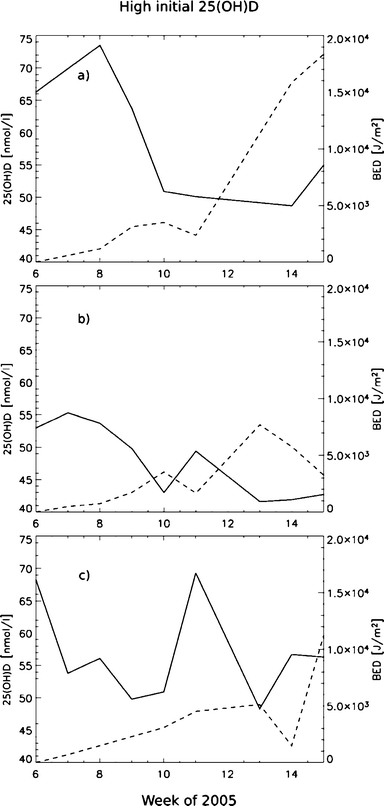 | ||
| Fig. 4 The graphs (a), (b), and (c) show the individual 25(OH)D (solid) and BED (dashed) vs. time for the three subjects in the group with higher initial 25(OH)D status (>30 nmol l−1). | ||
Webb et al.24 reported no detectable cutaneous production of previtamin D3 until mid-March. An interesting point is that our UV radiation measurements show that the irradiance Webb et al.24 measured in mid-February on a clear day in Boston (42° N) may be obtained at Andøya (68.2° N) only 16 days later. However, the difference in solar zenith angle at local noon between these two dates is ∼15° (Boston having the sun higher in the sky). With Andøya having this high irradiance with the sun 15° lower in the sky than Boston, is a direct effect of less pollution (only background aerosols), low total atmospheric ozone (290 DU vs. Boston’s 406 DU) and a quite high albedo (a measure of the reflection of radiation from the ground) at Andenes due to the snow covered ground.
Our results indicate that the vitamin D winter is shorter than anticipated24 and end as early as in week 8 or 9 at a high latitude of 69° N, at which the minimum SZA is around 76°. This is consistent with the results of Engelsen et al.1 However, the solar UV irradiance conditions are highly dependent on the cloud cover conditions, total atmospheric ozone content, aerosols and albedo. This means that on a local geographical basis, local atmospheric conditions may play a significant role for cutaneous production of vitamin D along with the latitudinal effect on the solar zenith angle,1 thus the UV radiation intensity. Therefore a latitudinal effect may only be investigated for equal atmospheric conditions of clouds, ozone, aerosols and albedo.
From the radiative transfer modeling we found a relatively large discrepancy in the model result compared with the measured result from Webb et al.24 in the UVA region (+19% at 316 nm). This could be a result of several conditions, like calibration errors of the spectroradiometer and change in light conditions during the measurements, or wrong input parameters to the model. Most likely it is a combination of all. The model simulations are by no means perfect, and the quality assurance procedures where clearly not as good back in the 80s as they are now. Uncertainty issues regarding measurements and modeling are further discussed in Engelsen et al.1 However, relatively large errors (measured or modeled) in the UVA region will only introduce minor errors in the calculation of the BED as the total UVA contribution to the BED is less than 10% under these conditions.
When designing this study, we expected a reduction in the subject 25(OH)D levels up to about week 9 (around March 6, SZA = 76°) based on the work by Engelsen et al.,1 since the UV radiation intensity before this date is probably too low to initiate any cutaneous production of vitamin D. Another question rising is whether it is enough to only have the face exposed to ambient UV light during daytime after week 9 (Fig. 3) In a study like this it is not possible to get an exact measure on how much vitamin D effective UV radiation dose each subject receive through their facial skin. We can only measure the ambient UV radiation and assume all subjects to behave equally over time with respect to the orientation of their face. Furthermore, a larger area of skin exposure than just the face may have resulted in more obvious and statistically significant associations within this study.
On an individual basis, the variation between subjects is quite large, but subjects with initial 25(OH)D values less than ∼30 nmol l−1 seem to respond easier to UV radiation than subjects with higher initial 25(OH)D values than that (Fig. 3 and 4). Both the studies of Mawer et al.29 and Snell et al.30 showed that absolute rise in 25(OH)D concentration was inversely related to the basal 25(OH)D concentration. Our results seem to be consistent with these studies.
For our group, the minimum mean 25(OH)D was measured after week 14 of 2005 (Fig. 2), while the subjects with lower initial 25(OH)D values than 30 nmol l−1 had reached their maximum 25(OH)D values by this time (Fig. 3). This means that the BED-threshold is not an absolute and independent value, but varies with the subjects basal 25(OH)D concentration. Skin type is also believed to be important, but in this experiment all had very similar skin complexion (skin type II).31
In order to determine a more precise connection between cutaneous production of vitamin D and UV radiation exposure for our group, the duration of the experiment should have been expanded until we had a significant increase in the subject 25(OH)D concentration. Unfortunately we were not able to monitor the 25(OH)D concentration during the experiment, as the analyses of the blood samples had to be sent away to a laboratory after the experimental period was over. Bad weather during the last three weeks of the period also prevented the UV radiation to become as high as we had hoped.
Conclusions
We have carried out a unique quantitative analysis of vitamin D status, diet and UV radiation exposure of a study group of 15 subjects living at a high latitude, over a time period of 9 weeks during springtime when the solar UV radiation seems to reach adequate levels to have a sufficient facial cutaneous vitamin D production.The vitamin D winter does not seem as long for pristine atmospheric conditions at high latitudes for snow covered ground as first anticipated. However, UV radiation forcing parameters (ozone, aerosols, clouds and albedo) may vary substantially from the normal, forcing a change in the UV radiation level in either directions causing a longer or shorter vitamin D winter than normal.
We found that the initial 25(OH)D concentration is important for the vitamin D synthesis. Only the subjects with very low initial (<30 nmol l−1) 25(OH)D concentrations seem to respond to UV radiation during the test period. For the subjects with higher initial 25(OH)D concentrations diet seems to be the dominant factor.
Acknowledgements
We thank all the participants from Andenes Airport for taking part in this study. Our thanks also goes to the military nurse, Øyvind Aas, for handling the blood sampling, and The Norwegian Pollution Control Authority for providing the Brewer spectroradiometer data. We appreciate the financial support from The Norwegian Cancer Society and The Research Council of Norway. Finally we thank Professor Eiliv Lund and fellow Tonje Braathen for comments on the manuscript.References
- O. Engelsen, M. Brustad, L. Aksnes and E. Lund, Daily duration of vitamin D synthesis in human skin with relation to latitude total ozone, altitude, ground cover, aerosols and cloud thickness, Photochem. Photobiol., 2005, 81, 1287–1290 CrossRef CAS.
- A. R. Webb and M. F. Holick, The role of sunlight in the cutaneous production of vitamin D, Annu. Rev. Nutr., 1988, 8, 375–399 CrossRef CAS.
- E. Hypponen, E. Laara, A. Reunanen, M. R. Jarvelin and S. M. Virtanen, Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study, Lancet, 2001, 358, 1500–1503 CrossRef CAS.
- K. V. Pinette, Y. K. Yee, B. Y. Amegadzie and S. Nagpal, Vitamin D receptor as a drug discovery target, Mini Rev. Med. Chem., 2003, 3, 193–204 Search PubMed.
- M. T. Cantorna, C. E. Hayes and H. F. DeLuca, 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis, J. Nutr., 1998, 128, 68–72 CAS.
- M. T. Cantorna, Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease, Prog. Biophys. Mol. Biol., 2006, 92, 60–64 CrossRef CAS.
- M. T. Cantorna and B. D. Mahon, Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence, Exp. Biol. Med. (Maywood), 2004, 229, 1136–1142 Search PubMed.
- W. B. Grant, Epidemiology of disease risk in relation to vitamin D insufficiency, Prog. Biophys. Mol. Biol., 2006, 92, 65–79 CrossRef CAS.
- E. Giovannucci, The epidemiology of vitamin D and cancer incidence and mortality: A review (United States), Cancer Causes Control, 2005, 16, 83–95 CrossRef.
- E. Giovannucci, Y. Liu, E. B. Rimm, B. W. Hollis, C. S. Fuchs, M. J. Stampfer and W. H. Willett, Prospective study of predictors of vitamin D status and cancer incidence and mortality in men, J. Natl. Cancer Inst., 2006, 98, 451–459 CrossRef CAS.
- Y. Cui and T. E. Rohan, Vitamin D, calcium, and breast cancer risk: a review, Cancer Epidemiol. Biomarkers Prev., 2006, 15, 1427–1437 Search PubMed.
- H. S. Lim, R. Roychoudhuri, J. Peto, G. Schwartz, P. Baade and H. Moller, Cancer survival is dependent on season of diagnosis and sunlight exposure, Int. J. Cancer, 2006, 119, 1530–1536 CrossRef CAS.
- J. Moan, A. C. Porojnicu, T. E. Robsahm, A. Dahlback, A. Juzeniene, S. Tretli and W. Grant, Solar radiation, vitamin D and survival rate of colon cancer in Norway, J. Photochem. Photobiol., B, 2005, 78, 189–193 CrossRef CAS.
- A. C. Porojnicu, T. E. Robsahm, A. H. Ree and J. Moan, Season of diagnosis is a prognostic factor in Hodgkin's lymphoma: a possible role of sun-induced vitamin D, Br. J. Cancer, 2005, 93, 571–574 CrossRef CAS.
- T. E. Robsahm, S. Tretli, A. Dahlback and J. Moan, Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway), Cancer Causes Control, 2004, 15, 149–158 CrossRef.
- M. Brustad, T. Sandanger, T. Wilsgaard, L. Aksnes and E. Lund, Change in plasma levels of vitamin D after consumption of cod liver and fresh cod liver oil as part of the traditional north Norwegian fish dish ‘mølje’, Int. J. Circumpolar Health, 2003, 62, 40–53 Search PubMed.
- L. Y. Matsuoka, J. Wortsman, M. J. Dannenberg, B. W. Hollis, Z. Lu and M. F. Holick, Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3, J. Clin. Endocr. Metab., 1992, 75, 1099–1103 Search PubMed.
- L. Y. Matsuoka, J. Wortsman, J. G. Haddad, P. Kolm and B. W. Hollis, Racial pigmentation and the cutaneous synthesis of vitamin D, Arch. Dermatol., 1991, 127, 536–538 CAS.
- A. Hjartake, E. Lund and K. S. Bjerve, Serum phospholipid fatty acid composition habitual intake of marine foods registered by a semi-quantitative food frequency questionnaire, Eur. J. Clin. Nutr., 1997, 51, 736–742 CrossRef CAS.
- M. Brustad, T. Braaten and E. Lund, Predictors for cod-liver oil supplements use-the Norwegian Women and Cancer Study, Eur. J. Clin. Nutr., 2003, 51, 128–136.
- M. Brustad, T. Sandanger, L. Aksnes and E. Lund, Vitamin D status in a rural population of northern Norway with high fish liver consumption, Publ. Health Nutr., 2004, 7, 783–789 Search PubMed.
- M. Brustad, E. Alsaker, O. Engelsen, L. Aksnes and E. Lund, Vitamin D status of middle-aged women at 65–71° N in relation to dietary intake and exposure to ultraviolet radiation, Publ. Health Nutr., 2004, 7, 327–335 Search PubMed.
- L. Aksnes, Simultaneous determination of retinol, alphatocopherol, and 25-hydroxyvitamin D in human serum by high-performance liquid chromatography, J. Pediatr. Gastr. Nutr., 1994, 18, 339–343 Search PubMed.
- A. R. Webb, M. F. Holick and L. Kline, Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin, J. Clin. Endocr. Metab., 1988, 67, 373–378 Search PubMed.
- J. A. MacLaughlin, R. R. Anderson and M. F. Holick, Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin, Science, 1982, 216, 1001–1003 CAS.
- M. F. Holick, R. Bouillon, J. Eisman, M. Garabedian, J. Kleinschmidt, T. Suda, I. Terentskaya and A. R. Webb, Commission Internationale de l'Eclariage (CIE), Technical Committe 6-54, Technical report: Action spectrum for the production of previtamin D3 in human skin, CIE, 2006, vol. 174 Search PubMed.
- B. Mayer and A. Kylling, Technical note: The libRadtran software package for radiative transfer calculations-description and examples of use, Atmos. Chem. Phys., 2005, 5, 1855–1877 CAS.
- M. K. Thomas, J. D. Lloyd, R. I. Thadhani, A. C. Shaw, D. J. Deraska, B. T. Kitch, E. C. Vamvakas, I. M. Dick, R. L. Prince and J. S. Finkelstein, Hypovitaminosis in medical inpatients, N. Engl. J. Med., 1998, 338, 777–783 CrossRef CAS.
- E. B. Mawer, J. L. Berry, E. Sommer-Tsilenis, W. Beykirch, A. Kuhlwein and B. T. Rohde, Ultraviolet irradiation increases serum 1,25-dihydroxyvitamin D in vitamin-D-replete adults, Miner. Electrol. Metab., 1984, 10, 117–121 Search PubMed.
- A. P. Snell, W. J. MacLennan and J. C. Hamilton, Ultra-Violet irradiation and 25-hydroxy-vitamin D levels in sick old people, Age Ageing, 1978, 7, 225–228 Search PubMed.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I trough VI, Arch. Dermatol., 1988, 124, 869–871 CAS.
Footnote |
| † UV radiation (or irradiance) is here defined as the amount of solar UV radiation reaching a horizontal ground surface in W m−2. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |
