Differential interactions of phytochrome A (Pr vs. Pfr) with monoclonal antibodies probed by a surface plasmon resonance technique
Chihoko
Natori†
ab,
Jeong-Il
Kim†
cd,
Seong Hee
Bhoo†
e,
Yun-Jeong
Han
cd,
Hiroko
Hanzawa
a,
Masaki
Furuya
*a and
Pill-Soon
Song
*df
aHitachi Advanced Research Laboratory, Hatoyama, Saitama 350-0395, Japan. E-mail: mfuruya@cd6.so-net.ne.jp
bHitachi Instruments Service Co., Yotsuya, Shinjuku-ku, Tokyo 160-0004, Japan
cDepartment of Biotechnology (BK21 program) and Kumho Life Science Laboratory, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, 500-757, South Korea
dEnvironmental Biotechnology National Core Research Center, Gyeongsang National University, Jinju, 660-701, South Korea. E-mail: pssong@cheju.ac.kr; Fax: +82-64-726-3395; Tel: +82-64-754-3395
eGraduate School of Biotechnology and Plant Metabolism Research Center, Kyung Hee University, Suwon, 449-701, South Korea
fFaculty of Biotechnology, College of Applied Life Sciences, Cheju University, Jeju, 690-756, South Korea
First published on 8th December 2006
Abstract
Phytochromes are red- and far-red light-reversible photoreceptors for photomorphogenesis in plants. Phytochrome A is a dimeric chromopeptide that mediates very low fluence and high irradiance responses. To analyze the surface properties of phytochrome A (phyA), the epitopes of 21 anti-phyA monoclonal antibodies were determined by variously engineered recombinant phyA proteins and the dissociation constants of seven anti-phyA monoclonal antibodies with phyA were measured using a surface plasmon resonance (SPR)-based resonant mirror biosensor (IAsys). Purified oat phyA was immobilized on the sensor surface using a carboxymethyl dextran cuvette in advance, and the interactions of each chosen monoclonal antibody against phyA in either red light absorbing form (Pr) or far-red light absorbing form (Pfr) at different concentrations were monitored. The binding profiles were analyzed using the FAST Fit program of IAsys. The resultant values of dissociation constants clearly demonstrated the differential affinities between the phyA epitopes and the monoclonal antibodies dependent upon Pr vs. Pfr conformations. Monoclonal antibody mAP20 preferentially recognized the epitope at amino acids 653–731 in the Pr form, whereas mAA02, mAP21 and mAR07/mAR08 displayed preferential affinities for the Pfr's surfaces at epitopes 494–601 (the hinge region between the N- and C-terminal domains), 601–653 (hinge in PASI domain), and 772–1128 (C-terminal domain), respectively. The N-terminal extension (1–74) was not recognized by mAP09 and mAP15, suggesting that the N-terminal extreme is not exposed in the native conformation of phyA. On the other hand, the C-terminal domain becomes apparently exposed on Pr-to-Pfr phototransformation, suggesting an inter-domain cross-talk. The use of surface plasmon resonance spectroscopy offers a new approach to study the surface properties of phytochromes associated with the photoreversible structural changes, as well as for the study of protein–protein interactions of phytochromes with their interacting proteins involved in light signaling events in plants.
Introduction
Light is essential for the growth and development of plants as energy for photosynthesis and as environmental signals for photomorphogenesis, circadian rhythm and phototropism. Plants have developed sophisticated light sensing systems in response to light wavelengths, intensity, duration and direction.1 There are three well-known major classes of plant light-sensing photoreceptors, namely the red/far-red light-sensing phytochromes,2,3 and the blue light-sensing cryptochromes4 and phototropins.5 Among them, phytochromes are widely distributed in plants, algae and also in certain fungi and bacteria.6 Phytochromes are dimeric chromopeptides (monomer sizes of 120–130 kDa) with a covalently linked open-tetrapyrrole chromophore called phytochromobilin. They display two spectrally distinct forms, the red light (660 nm) absorbing Pr and the far-red light (730 nm) absorbing Pfr. The functional activities of the phytochromes are modulated by the photochromic transformation between these two forms. The activated Pfr form elicits the light-signaling cascade through interactions with upstream and downstream signaling components, eventually regulating the gene expressions involved in photomorphogenesis.7–9Protein–protein interactions are essential in many processes of living cells. Phytochrome signaling is also believed to be transmitted through the protein–protein interactions with downstream phytochrome interacting proteins (PIPs) such as PIF3 (phytochrome interacting factor 3),10–12 NDPK2 (nucleoside di-phosphate kinase 2),13,14 PKS1 (phytochrome kinase substrate 1),15 PAPP5 (phytochrome-associated protein phosphatase 5)16 and others.17 Since the photochromism of phytochromes results in differential exposures of the surface properties of Pr vs. Pfr forms of the proteins,8 the interactions between the photoreceptor and the PIPs would depend on the attendant conformational changes in phytochromes. However, it has been difficult to measure quantitatively such interactions in cells because of the complexity of the intracellular reactions. Therefore, we aimed to establish a quantitative assay system for in vitro binding between purified phytochrome A (phyA) and anti-phyA monoclonal antibodies as a model system for the protein–protein interactions.
Recently, surface plasmon resonance-based optical techniques have been widely used for quantitative studies of the protein–protein interactions.18 The surface plasmon resonance spectroscopy (SPR) is a sensitive real-time measurement method for the characterization of macromolecular interactions. It uses the evanescent wave phenomenon to measure changes in refractive index very close to the sensor surface. The binding between an analyte in solution and its ligand immobilized on the sensor surface results in a change in the refractive index . The interaction is monitored in real time and the amount of bound ligand and the rates of association and dissociation can be measured with high precision. Thus, these optical biosensors are a powerful tool for probing the surface topography and the protein–protein interactions of phytochromes on the basis of both kinetic and equilibrium parameters.19,20 In the present study, we have established a quantitative assay method for the phytochrome binding with monoclonal antibodies using a commercially available SPR biosensor instrument, IAsys.
Experimental
Preparation of truncated phytochrome A constructs
A full-length cDNA of pea (Pisum sativa L.) phytochrome A (phyA) was cloned in pBluescript (Stratagene, CA) at BamHI/SalI sites and named as pPP800.21 This construct was used as a starting material for the construction of all deletion mutants of pea phyA. Two N-terminal [Δ(N-74), Δ(N-248)], and five C-terminal deletion mutants [Δ(494-C), Δ(601-C), Δ(653-C), Δ(731-C), Δ(772-C)], were generated by using polymerase chain reaction (PCR ) and replaced the full-length pea phyA gene in pPP800. Full-length and truncated phyA genes were cleaved out from pPP800 by using BamHI/SalI and ligated to the BglII/SalI digested yeast expression vector pAA7,22,23 because BamHI and BglII generate complementary cohesive ends to ligate each other. All constructs were confirmed by restriction enzyme mapping and by DNA sequencing.Several recombinant oat phyA, including full-length (1–1129 aa), Δ65 (66–1129 aa; NTE-deleted), A875 (1–875 aa; HKRD-deleted), A610 (1–610 aa; PRD/HKRD-deleted), A407 (1–407 aa; PHY/PRD/HKRD-deleted) were prepared as previously described.14,24
Expression of truncated phytochromes
A protease-deficient Saccharomyces cerevisiae strain BJ5465 (ura3-52, trp1, leu2Δ1, his3Δ200, pep4:HIS3, prb1Δ1.6R, can1, GAL) (Yeast Genetic Stock Center, CA) was used for the expression of phytochrome mutants that are cloned in the pAA7 yeast expression vector. About 1 µg of each mutant DNA construct was transformed into BJ5465 strain using the Alkali-Cation yeast transformation kit (Bio 101, CA). For protein expression, several selected colonies were inoculated into 10 ml liquid minimal medium and incubated at 30 °C for 20–24 h with shaking at 250 rev min–1. These cultures were used for the inoculation of 400 ml complete medium. The YPG [1% (w/v) yeast extract, 2% (w/v) bactopeptone, 2% (v/v) glycerol] liquid medium was used as a complete medium to avoid the medium change for induction. The inoculated YPG medium was incubated at 30 °C with shaking at 250 rev min–1 until OD600 (optical density at 600 nm) reached around 1.5. To induce the expression of recombinant protein, 20% galactose was added to the cultures to obtain a final concentration of 0.2%. The cultures were then incubated for further 7–8 h at the same conditions. Cells were harvested by centrifugation (3000 g, 10 min at 4 °C) and washed with 20 ml of buffer A (100 mM Tris–HCl, 2 mM EDTA pH 8.0). The washed cell pellets were resuspended with an equal volume of buffer A containing 1 mM phenylmethyl-sulfonyl fluoride (PMSF). Resuspended yeast cells were constantly dropped into liquid nitrogen in which homogenization was carried out with a Ultra-Turrax T25 Homogenizer (Janke & Kunkel, Germany) for 20 min at 13000 rev min–1. The liquid nitrogen was evaporated after cell disruption. The disrupted yeast powder samples were stored at –70 °C for western blot analysis.Recombinant proteins of oat phyA constructs were expressed in the Pichia pastoris protein expression system and purified by streptavidin affinity chromatography as previously described.14,24
Preparation of native oat phyA
Native phyA (∼98% pure) was prepared from etiolated oat (Avena sativa L.) seedlings with a specific absorbance ratio (SAR for Pr, A666![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A280 = 1.03). The protein was obtained essentially by using an ammonium sulfate back-extraction procedure and gel filtration in a 20 mM Tris–HCl buffer, pH 7.8, containing 1 mM dithiothreitol (DTT) on a ToyoPearl HW-65 column.25
A280 = 1.03). The protein was obtained essentially by using an ammonium sulfate back-extraction procedure and gel filtration in a 20 mM Tris–HCl buffer, pH 7.8, containing 1 mM dithiothreitol (DTT) on a ToyoPearl HW-65 column.25
Production of monoclonal anti-phytochrome A antibodies
Monoclonal antibodies against purified pea phyA apoprotein , mAP05, mAP09, mAP10, mAP13, mAP14, mAP15, mAP16, mAP18, mAP19, mAP20, mAP21, mAP22, mAP23, mAP25, mAP28, mAP29, and mAP30,26,27 and those against rye phyA, mAR07 and mAR08,28 were produced and have been stocked in our laboratory. Monoclonal antibodies against recombinant Arabidopsis phyA apoprotein , mAA01 and mAA02, were produced by Dr H. Hanzawa and Dr A. Nagatani.29Epitope mapping and cross-reactivity analysis
To check the binding region of monoclonal antibodies, 600 ml of clarified protein samples from the disrupted yeast powders were resolved by SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad, CA), and the membrane was incubated with each monoclonal antibody for 2 h and developed using 5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium (BCIP–NBT) chromogenic substrates or an ECL™ western blotting analysis system (Amersham Pharmacia Biotech).The cross-reactivity analyses with monoclonal antibodies derived from pea (mAP), rye (mAR) and Arabidopsis (mAA) were performed with native oat phyA proteins by western blotting. With selected monoclonal antibodies that showed cross-reactivity to native oat phyA, we performed more western blot analyses with different domain-deleted recombinant oat phyA proteins to confirm the mapped epitopes . The constructs, full-length (FL, 1–1129 aa), Δ65 (66–1129 aa; NTE-deleted), A875 (1–875 aa; HKRD-deleted), A610 (1–610 aa; PRD/HKRD-deleted), and A407 (1–407 aa; PHY/PRD/HKRD-deleted) were included in these analyses.
Binding assay with IAsys
We analyzed the interactions between the oat phyA and the monoclonal anti-phytochrome antibodies using IAsys (Manual type, Affinity Sensors, Cambridge, UK). Prior to each experiment, phyA in either Pr or Pfr form (30 µg ml–1 in acetate buffer, pH 5.50) was immobilized onto the carboxylate cuvette in phosphate-buffered saline, pH 5.0, as running buffer. After a 15 min immobilization process at room temperature, the immobilized phyA in the cuvette was rinsed several times with a phosphate buffer at pH 7.4, and kept in the phosphate buffer at pH 7.4 before and during the binding assay. To prevent the phototransformation of immobilized phyA by the measuring laser beam of 670 nm, the laser beam was blocked with a light shield at all other times (see Fig. 1). As a measure of the binding affinity, the dissociation equilibrium constants (KD) were evaluated by using the FAST Fit software, as described elsewhere.19,20 The binding data with various ligate concentrations yield both the association rate constants (kass) and the dissociation rate constants (kdiss). The term (kass[ligate] + kdiss) is the apparent pseudo first-order rate constant (kon) with unit of s–1. From the slope of the plot for the kon as a function of antibody (i.e. ligate) concentrations, KD was determined as the ratio of slope![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intercept. The kinetic expression for the dissociation constant is given by KD = kdiss/kass.
intercept. The kinetic expression for the dissociation constant is given by KD = kdiss/kass.
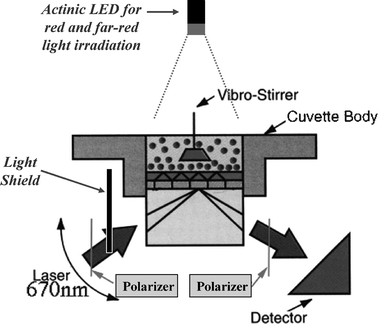 | ||
| Fig. 1 Schematic drawing of the SPR-based biosensor in IAsys. Actinic LED of red and far-red light were used to photoconvert phytochromes in solution prior to immobilization. To prevent the phototransformation of immobilized phytochromes by the measuring laser beam, the laser beam was blocked with a light shield so that the phyA samples were only exposed to the measuring beam during the measurement for a short time. Without the light shield, the immobilized phyA reached its photostationary state during the assay. During the IAsys assay all operations were conducted in a dark room. | ||
Preparation of phytochrome interacting factor 3 (PIF3)
The phytochrome signal transducer, PIF3, was subcloned into pGEX 4T (Amersham Pharmacia Biotech) for the protein expression in E. coli. The primers used were 5′-CTCGGATCCATGCCTCTGTTTGAGCTTTTCAG-3′ (BamH1) and 5′-CGGAATTCTCACGACGATCCACAAAACTG-3′ (EcoR1) for full-length PIF3. The construct was transformed into E. coli strain BL21 (Invitrogen). Protein purification was performed using glutathioneaffinity chromatography according to the manufacture's instruction (Amersham Pharmacia Biotech). Binding of PIF3 to phytochrome A was assayed according to the IAsys procedure described above. The binding was conducted at three different concentrations of PIF3, namely 10, 100, and 200 µg PIF3 with phyA (Pr/Pfr).Results and discussion
Epitope mapping of diverse anti-phyA monoclonal antibodies
Prior to IAsys analysis, the epitopes of 21 anti-phyA monoclonal antibodies in our stock were determined by using variously engineered recombinant phyA proteins. We produced many monoclonal antibodies against phytochromes of several plants, including 17 monoclonal antibodies against pea phyA (mAP05, mAP09, mAP10, mAP13, mAP14, mAP15, mAP16, mAP18, mAP19, mAP20, mAP21, mAP22, mAP23, mAP25, mAP28, mAP29 and mAP30), two monoclonal antibodies against Arabidopsis phyA (mAA01 and mAA02), and two monoclonal antibodies against rye phyA (mAR07 and mAR08). They were tested with various deletion apo-pea phyA proteins by western blot analysis. As shown in Fig. 2, the epitopes of pea phyA were mapped by using 21 different phyA-specific monoclonal antibodies (Fig. 2B). We also checked the cross-reactivities of antibodies with native oat phyA (Fig. 2C). Monoclonal antibodies against rye (mAR7, mAR8) and Arabidopsis (mAA01, mAA02) reacted with native oat phyA, whereas a few monoclonal antibodies against pea (mAP05, mAP15, mAP20, mAP21) reacted with oat phyA.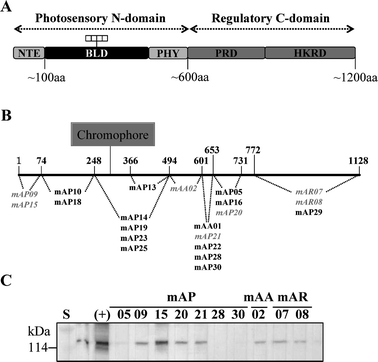 | ||
| Fig. 2 Epitope mapping of phytochromes with anti-phytochrome monoclonal antibodies. (A) Schematic diagram of a phytochrome monomer domain structure. Phytochromes consist of a N-terminal photosensory domain and a C-terminal regulatory domain. NTE, N-terminal extension; BLD, bilin lyase domain; PHY, phytochrome-specific domain; PRD, PAS-related domain; HKRD, histidine kinase-related domain. (B) Mapped epitopes of 21 monoclonal antibodies; the 7 antibodies used for IAsys analyses are shown in gray and italics. (C) The cross-reactivities of monoclonal antibodies with native oat phyA. | ||
Phytochrome proteins consist of two structural domains, a ∼65 kDa chromophore bearing globular N-terminal domain and a ∼55 kDa conformationally extended C-terminal domain connected by a flexible hinge region (Fig. 2A).30 The N-terminal domain is a light-sensing domain that is necessary for light perception by the chromophore attached to the apophytochrome. The N-terminal domain could be further divided into three subdomains by their specific roles: N-terminal extension (NTE), bilin lyase domain (BLD) and phytochrome domain (PHY).1,30 The NTE is not important for the chromophore attachment but is necessary for biological activity. The serine rich NTE regulates light responses and nuclear localization of phytochrome A, and the amphipathic α-helix motif of NTE31 plays a critical role in phytochrome signaling.8,32 The BLD contains the catalytic site for the chromophore ligation.6 The PHY is considered to interact with the D ring of the chromophore to stabilize the Pfr form of phytochrome.33
The C-terminal domain of phytochrome is functionally important for its interactions with several signaling proteins. The C-terminal domain consists of a pair of Per-Arnt-Sim (PAS) repeats (PAS-related domain, PRD) and histidine kinase-related domain (HKRD). The PAS repeats are important for nuclear localization and possibly involved in dimerization and protein–protein interactions,6,34,35 with proteins such as PIF3, NDPK2, PKS1, and FyPP.17,30 The HKRD is homologous to histidine kinases, and might be involved in a regulatory function in phytochrome signaling as well as in dimerization .
Based on epitope mapping, we selected antibodies with epitopes in the different domains of phytochromes, such as mAP09 and mAP15 bound to the NTE domain, mAR07 and mAR08 bound to HKRD, mAP20 and mAP21 bound to PRD, and mAA02 bound to the PHY domain. We then confirmed their binding domains using purified recombinant oat phyA fragments, including full-length (FL, 1–1129 aa), NTE-deleted (Δ65, 66–1129 aa), HKRD-deleted (A875, 1–875 aa), both PRD and HKRD-deleted (A610, 1–610 aa), and PHY/PRD/HKRD-deleted (A407, 1–407 aa). As shown in Fig. 3, mAP09 binds to the NTE domain, because it binds to FL, A875, A610 and A410 but not to NTE-deleted Δ65 (same result with mAP15). mAA02 binds to FL, Δ65, A610 and A410 but not to HKRD-deleted A875, which indicates the binding region of mAA02 as HKRD. Similarly, mAP20 binds to PRD (same result with mAP21), and mAR07 binds to PHY domain (same result with mAR08).
 | ||
| Fig. 3 Epitope confirmation for selected monoclonal antibodies using purified recombinant oat phyA proteins with different domain deletions. (A) Schematic representation of the constructs used for the epitope confirmation. FL, full-length (1–1129 aa); Δ65 (66–1129 aa; NTE-deleted); A875 (1–875 aa; HKRD-deleted); A610 (1–610 aa; PRD/HKRD-deleted); A407 (1–407 aa; PHY/PRD/HKRD-deleted). (B) SDS-PAGE (left) and Zn2+-blot analysis (right) of purified oat phyA fragments. Protein standards, S, are shown in lane 1. (C)–(G) Representative western blot results for selected monoclonal antibodies with oat phyA fragments. Polyclonal antibodies against oat phyA were used as a control (C); mAP9 (D), mAA2 (E), mAP20 (F) and mAR7 (F) are shown as representatives of antibodies bound to the NTE, HKRD, PRD, and PHY domain, respectively. | ||
Our results of epitope mapping showed that the monoclonal antibodies specifically bound to various regions of phytochrome A (Fig. 2B and Fig. 3). For example, both mAP09 and mAP15 specifically interacted with NTE, and mAR07 and mAR08 interacted with the region of HKRD. These results suggest that the monoclonal antibodies would be useful for domain specific studies of phytochromes. However, the epitope mapping was done in denaturing gels, so it is uncertain if these antibodies interact with native phytochromes in solution. Thus, we performed an IAsys analysis of the antibodies with immobilized native phyA. For these analyses, we chose the following antibodies: NTE-specific antibodies (mAP09 and mAP15), PHY-specific antibody (mAA02), PRD-specific antibodies (mAP20 and mAP21) and HKRD-specific antibodies (mAR07 and mAR08).
Interactions between oat phyA and monoclonal anti-phyA antibodies
We first established a method for the immobilization of oat phyA dimer molecules in Pr or Pfr onto a commercially available IAsys carboxymethyl dextran cuvette using actinic irradiation with red and far-red light (Fig. 1) and determined the amounts of Pr and Pfr. The results showed that the Pfr form of oat phyA bound significantly more to the cuvette surface than the Pr form of phyA (Table 1). The binding profiles between mAA02 antibody and oat phyA were then analyzed as a model system by using FAST Fit program. The results showed some differences in the interactions of mAA02 antibodies with phyA, depending on Pr and Pfr forms (Fig. 4). An example of the dissociation equilibrium constant (KD value) for measuring binding affinity is shown in Fig. 5. The interaction between the oat phyA and the monoclonal antibody varied depending upon the Pr or Pfr form of phytochrome A (see below).| Immobilized amount | ||
|---|---|---|
| Arcsecond | ng mm–2 | |
| Pr form of phyA | 530 | 0.89 |
| Pfr form of phyA | 803 | 1.3 |
 | ||
| Fig. 4 Binding curves of a monoclonal antibody, mAA02, to Pr (left) and Pfr (right) forms of phyA at different concentrations. The IAsys signals in arcseconds are traced along the time course for Pr (left panel) and Pfr (right panel) at different concentrations in µg ml–1. | ||
![Determination of the dissociation constant KD. As a measure of the binding affinity, the dissociation equilibrium constants (KD) were evaluated by using FAST Fit software. (A) Binding curve of Pr and mAA02 (0.83 µM). (B) The apparent pseudo first-order rate constants (kon) were calculated and plotted from the binding curves of Pr and various mAA02 concentrations. Since the graph represents y = kass[mAA02] + kdiss, the association rate constant (kass, the slope) was determined as 15089 s–1 M–1, and the dissociation rate constant (kdiss, y-intercept) was determined as 0.004368 s–1. Thus, the dissociation constant (KD = kdiss/kass) was calculated as 2.9 × 10–7 M.](/image/article/2007/PP/b611077k/b611077k-f5.gif) | ||
| Fig. 5 Determination of the dissociation constant KD. As a measure of the binding affinity, the dissociation equilibrium constants (KD) were evaluated by using FAST Fit software. (A) Binding curve of Pr and mAA02 (0.83 µM). (B) The apparent pseudo first-order rate constants (kon) were calculated and plotted from the binding curves of Pr and various mAA02 concentrations. Since the graph represents y = kass[mAA02] + kdiss, the association rate constant (kass, the slope) was determined as 15089 s–1 M–1, and the dissociation rate constant (kdiss, y-intercept) was determined as 0.004368 s–1. Thus, the dissociation constant (KD = kdiss/kass) was calculated as 2.9 × 10–7 M. | ||
Differential surface topography of phyA
The surface topography of phyA is important in determining the protein–protein interactions in the phytochrome-mediated signaling, especially in the photoactivation of phytochrome from its Pr to Pfr form. Previously, we used small molecules to probe the surface properties of phyA, gaining a limited insight into the phototransformation-induced differential exposures of Trp and Cys residues. However, monoclonal antibodiess are better suited for probing the domain surfaces, especially in the absence of the three-dimensional structure of a plant phytochrome (the structure of Deinococcus bacteriophytochrome has been elucidated recently).36We determined the dissociation constants (KD) of 7 anti-phyA monoclonal antibodies that have different epitopes against phyA dimers (see Fig. 2). Table 2 shows the KD values (average of triplicates) for each monoclonal antibody. The monoclonal antibodies can be classified into three different types: (1) monoclonal antibodies (mAP09 and mAP15) without specific affinity toward native phyA; (2) monoclonal antibodies (mAP21, mAA02, mAR07, and mAR08) showing stronger affinity to Pfr than to Pr; and (3) monoclonal antibody (mAP20) with stronger affinity to Pr than to Pfr.
| Monoclonal antibodies | K D a /nM | Affinity | |
|---|---|---|---|
| Pfr | Pr | ||
| a The values are average of triplicate measurements; “—” means no binding. | |||
| mAP09 | — | — | — |
| mAP15 | — | — | — |
| mAP20 | 1200 | 650 | Pr > Pfr |
| mAP21 | 1400 | 3100 | Pfr > Pr |
| mAA02 | 45 | 310 | Pfr > Pr |
| mAR07 | 130 | 650 | Pfr > Pr |
| mAR08 | 81 | 130 | Pfr > Pr |
Both mAP09 and mAP15 interacted with NTE of phyA by western blot analysis (see Fig. 2B). Since the western blot analysis was performed under denaturing conditions, these monoclonal antibodies can be considered specific to the NTE polypeptide sequence when fully accessible. Thus, these antibodies are different from Oat23 and Oat25 antibodies that bind to the native phyA and affect the kinetics of primary photoprocess.37
The Pr-to-Pfr phototransformation of phytochromes is accompanied by changes in the protein conformation. On Pr-to-Pfr phototransformation of phyA, the N-terminal extension undergoes a conformational change from random coil to amphiphilic α-helix which interacts with the chromophore in the Pfr form.8,31 Also, the hinge region is preferentially exposed in the Pfr form.24 The surface topography of Pr and Pfr phytochromes includes differential exposure of tryptophan and cysteine residues.38,39 Trp-773 and Trp-777 in PRD are preferentially exposed in the Pfr form,38 and Cys-311 near the chromophore showed a significant dependence of its surface exposure on the Pr-to-Pfr phototransformation.39 The preferentially exposed domains of the Pfr form of phyA induced by the phototransformation can be defined through the interactions of monoclonal antibodies mAP21, mAA02, mAR07 and mAR08 with the phyA dimer. Only mAP20 showed stronger interactions with the Pr than with Pfr form. Since this antibody interacts with the PAS domain, the preferential binding with Pr reflects a conformational change in the PRD domain that retracts the mAP20 epitope .
Further comments on other phytochrome-interacting proteins (PIPs)
Since we had successfully established the conditions for the interactions between phytochrome and its monoclonal antibodies based on the IAsys-sensor, we have applied the method to other phytochrome-interacting proteins (unpublished results). We performed an IAsys analysis of immobilized phyA in the presence of PIF3 (Fig. 6). The Pfr form of phytochrome A interacted with PIF3 more strongly than with the Pr form, consistent with a previous report.11 In another preliminary IAsys experiment, NDPK2 known to interact with the C-terminal domain of phyA as bait in the yeast-two hybrid screening13 did not competitively inhibit the binding of mAR08 (data not shown). This observation suggests that NDPK2 and the antibody bind at different sites/domains. | ||
| Fig. 6 Application of the IAsys analysis to the protein–protein interaction between phytochrome and its interacting protein, PIF3. Binding between the PIF3 and phytochrome A was demonstrated in triplicate at a PIF3 concentration of 100 µg. The Pfr form of phyA interacted more efficiently with PIF3 than with the Pr form. | ||
In conclusion, the present approach of SPR-based IAsys analysis for the quantitative measurement of the binding affinities of monoclonal antibodies to the phytochromes provides additional insights into the conformation and mechanism of phytochrome-mediated light signaling in plants.
Acknowledgements
This work was supported by grants from HARL (B2023) and the Program for Promotion of Basic Research Activity for Innovative Biosciences (1995–2001, to M. F.), the KOSEF/MOST to the Environmental Biotechnology National Core Research Center (NCRC) (to J.-I. K. and P.-S. S.; grant # R15-2003-012-01003-0), BioGreen 21 Program (Code # 20050401034689) from Rural Development Administration of Republic of Korea, and in part by KOSEF/MOST through the Plant Metabolism Research Center, Kyung Hee University (to S. H. B.; grant # R11-2000-081). We thank Dr Thummala Chandrasekhar for his careful reading of the manuscript.References
- M. Chen, J. Chory and C. Fankhauser, Light signal transduction in higher plants, Annu. Rev. Genet., 2004, 38, 87–117 CrossRef CAS.
- H. Smith, Phytochromes and light signal perception by plants - an emerging synthesis, Nature, 2000, 407, 585–591 CrossRef CAS.
- C. Fankhauser, The phytochromes, a family of red/far-red absorbing photoreceptors, J. Biol. Chem., 2001, 276, 11453–11456 CrossRef CAS.
- C. Lin and D. Shalitin, Cryptochrome structure and signal transduction, Annu. Rev. Plant Biol., 2003, 54, 469–496 Search PubMed.
- W. R. Briggs and J. M. Christie, Phototropins 1 and 2: versatile plant blue-light receptors, Trends Plant Sci., 2002, 7, 204–210 CrossRef CAS.
- N. C. Rockwell, Y. S. Su and J. C. Lagarias, Phytochrome structure and signaling mechanisms, Annu. Rev. Plant Biol., 2006, 57, 837–858 Search PubMed.
- P. H. Quail, Phytochrome photosensory signaling networks, Nat. Rev. Mol. Cell Biol., 2002, 3, 85–93 CrossRef CAS.
- C. M. Park, S. H. Bhoo and P.-S. Song, Inter-domain crosstalk in the phytochrome molecules, Semin. Cell Dev. Biol., 2000, 11, 449–456 CrossRef CAS.
- H. Wang and X.-W. Deng, Dissecting the phytochrome A-dependent signaling network in higher plants, Trends Plant Sci., 2003, 8, 172–178 CrossRef CAS.
- M. Ni, J. M. Tepperman and P. H. Quail, PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein, Cell, 1998, 95, 657–667 CAS.
- Y. Zhu, J. M. Tepperman, C. D. Fairchild and P. H. Quail, Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 13419–13424 CrossRef CAS.
- J. Kim, H. Yi, G. Choi, B. Shin, P.-S. Song and G. Choi, Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction, Plant Cell, 2003, 15, 2399–2407 CrossRef CAS.
- G. Choi, H. Yi, Y.-K. Kwon, M.-S. Soh, B. Shin, Z. Luka, T.-R. Hahn and P.-S. Song, Phytochrome signaling is mediated through nucleoside diphosphate kinase 2, Nature, 1999, 401, 610–613 CrossRef CAS.
- Y. Shen, J.-I. Kim and P.-S. Song, NDPK2 as a signal transducer in the phytochrome-mediated light signaling, J. Biol. Chem., 2005, 280, 5740–5749 CAS.
- C. Fankhauser, K. C. Yeh, J. C. Lagarias, H. Zhang, T. D. Elich and J. Chory, PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis, Science, 1999, 284, 1539–1541 CrossRef CAS.
- J. S. Ryu, J.-I. Kim, T. Kunkel, B. C. Kim, D. S. Cho, S. H. Hong, S.-H. Kim, A. P. Fernndez, Y. Kim, J. M. Alonso, J. R. Ecker, F. Nagy, P. O. Lim, P.-S. Song, E. Schäfer and H. G. Nam, Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer, Cell, 2005, 120, 395–406 CrossRef CAS.
- E. Schäfer and C. Bowler, Phytochrome-mediated photoperception and signal transduction in higher plants, EMBO Rep., 2002, 3, 1042–1048 Search PubMed.
- J. M. McDonnell, Surface plasmon resonance: towards an understanding of the mechanisms of biological molecular recognition, Curr. Opin. Chem. Biol., 2001, 5, 572–577 CrossRef CAS.
- W. M. Mullet, E. P. C. Lai and J. M. Yeung, Surface plasmon resonance-based immunoassays, Methods, 2000, 22, 77–91 Search PubMed.
- P. R. Edwards, C. H. Maule, R. J. Leatherbarrow and D. J. Winzor, Second-order kinetic analysis of IAsys biosensor data: its use and applicability, Anal. Biochem., 1998, 263, 1–12 CrossRef CAS.
- K. Tomizawa, N. Ito, Y. Komeda, T. Q. P. Uyeda, K. Takio and M. Furuya, Characterization and intracellular distribution of pea phytochrome I polypeptides expressed in E. coli, Plant Cell Physiol., 1999, 32, 95–102.
- H. Abe, H. Handa, Y. Hogi and T. Fukazawa, Efficient usage of a galactose-inducible expression vector for the production of heterologous protein in yeast, Agric. Biol. Chem., 1991, 52, 2035–2041.
- L. Deforce, K. Tomizawa, N. Ito, D. Farrens, P.-S. Song and M. Furuya, In vitro assembly of apophytochrome and apophytochrome deletion mutants expressed in yeast with phycocyanobilin, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10392–10396 CrossRef CAS.
- J.-I. Kim, Y. Shen, Y.-J. Han, D. Kirchenbauer, J.-E. Park, M.-S. Soh, F. Nagy, E. Schäfer and P.-S. Song, Phytochrome phosphorylation modulates light signaling by influencing the protein–protein interaction, Plant Cell, 2004, 16, 2629–2640 CrossRef CAS.
- V. N. Lapko and P.-S. Song, A simple and improved method of isolation and purification for native oat phytochrome, Photochem. Photobiol., 1995, 62, 194–198 CrossRef CAS.
- A. Nagatani, K. T. Yamamoto, M. Furuya, T. Fukumoto and A. Yamashita, Production and characterization of monoclonal antibodies which distinguish different surface structures of pea (Pisum sativum cv. Alaska) phytochrome, Plant Cell Physiol., 1984, 25, 1059–1068 CAS.
- A. Nagatani, P. J. Lumsden, A. Konomi and H. Abe, Application of monoclonal antibodies to phytochrome studies, in Phytochrome and photoregulation in plants, ed. M. Furuya, Academic Press, 1987, pp. 95–114 Search PubMed.
- P. J. Lumsden, K. T. Yamamoto, A. Nagatani and M. Furuya, Effect of monoclonal antibodies on the in vitro Pfr dark reversion of pea phytochrome, Plant Cell Physiol., 1985, 26, 1313–1322 CAS.
- T. Shinomura, A. Nagatani, H. Hanzawa, M. Kubota, M. Watanabe and M. Furuya, Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana., Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 8129–8133 CrossRef CAS.
- J.-I. Kim, J.-E. Park, X. Zarate and P.-S. Song, Phytochrome phosphorylation in plant light signaling, Photochem. Photobiol. Sci., 2005, 4, 681–687 RSC.
- W. Parker and P.-S. Song, Location of helical regions in tetrapyrrole containing proteins by a helical hydrophobic moment analysis. Application to phytochrome, J. Biol. Chem., 1990, 265, 17568–17575 CAS.
- J. J. Casal, S. J. Davis, D. Kirchenbauer, A. Viczian, M. J. Yanovsky, R. C. Clough, S. Kircher, E. T. Jordan-Beebe, E. Schäfer, F. Nagy and R. D. Vierstra, The serine-rich N-terminal domain of oat phytochrome A helps regulate light responses and subnuclear localization of the photoreceptor, Plant Physiol., 2002, 129, 1127–1137 CrossRef CAS.
- B. L. Montgomery and J. C. Lagarias, Phytochrome ancestry: Sensors of bilins and light, Trends Plant Sci., 2002, 7, 357–366 CrossRef CAS.
- J.-I. Kim and P.-S. Song, A Structure-function model based on inter-domain crosstalks in phytochromes, in Light Sensing in Plants, ed. M. Wada, K. Shimazaki and M. Iino, Springer-Verlag, Tokyo, 2005, pp. 53–63 Search PubMed.
- J.-I. Kim, S. H. Bhoo, Y.-J. Han, X. Zarate and P.-S. Song, The PAS-2 domain is required for dimerization of phytochrome A, J. Photochem. Photobiol., A, 2006, 178, 115–121 CrossRef CAS.
- J. R. Wagner, J. S. Brunzelle, K. T. Forest and R. D. Vierstra, A light sensing knot revealed by the structure of the chromophore binding domain of phytochrome, Nature, 2005, 438, 325–331 CrossRef CAS.
- P. Lindemann, S. E. Braslavsky, M. M. Cordonnier, L. H. Pratt and K. Schaffner, Photochem. Photobiol., 1993, 58, 417–424 CrossRef CAS.
- T. A. Wells, M. Nakazawa, K. Manabe and P.-S. Song, A conformational change associated with the phototransformation of Pisum phytochrome A as probed by fluorescent quenching, Biochemistry, 1994, 33, 708–712 CrossRef CAS.
- V. N. Lapko, X. Y. Jiang, D. L. Smith and P.-S. Song, Surface topography of phytochrome A deduced from specific chemical modification with iodoacetamide, Biochemistry, 1998, 37, 12526–12535 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |
