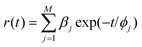Redox compounds influence on the NAD(P)H:FMN–oxidoreductase–luciferase bioluminescent system
E. V.
Vetrova
*a,
N. S.
Kudryasheva
a and
V. A.
Kratasyuk
b
aInstitute of Biophysics SB RAS, Krasnoyarsk, 660036, Russia. E-mail: vetrova@ibp.ru; Fax: +7(3912)433400
bKrasnoyarsk State University, Krasnoyarsk, 660041, Russia
First published on 11th December 2006
Abstract
A review of the mechanisms of the exogenous redox compounds influence on the bacterial coupled enzyme system: NAD(P)H:FMN–oxidoreductase–luciferase has been done. A series of quinones has been used as model organic oxidants. The three mechanisms of the quinones’ effects on bioluminescence were suggested: (1) inhibition of the NADH-dependent redox reactions; (2) interactions between the compounds and the enzymes of the coupled enzyme system; and (3) intermolecular energy migration. The correlation between the kinetic parameters of bioluminescence and the standard redox potential of the quinones proved that the inhibition of redox reactions was the key mechanism by which the quinones decrease the light emission intensity. The changes in the fluorescence anisotropy decay of the endogenous flavin of the enzyme preparations showed the direct interaction between quinones and enzymes. It has been demonstrated that the intermolecular energy migration mechanism played a minor role in the effect of quinones on the bioluminescence. A comparative analysis of the effect of quinones, phenols and inorganic redox compounds on bioluminescent coupled enzyme systems has been carried out.
 E. V. Vetrova E. V. Vetrova | Elena V. Vetrova, Dr. Sc. (Biophysics), Institute of Biophysics, Siberian Branch of Russian Academy of Sciences. Dr Vetrova is a specialist in the physicochemical basis of bioluminescent assays and molecular spectroscopy. Her research focuses on bioluminescent analysis and ecological biophysics with a particular interest in the development of bioluminescent assays to monitor the aquatic ecosystems of marked redox properties. She is the author of eight papers on bioluminescence. |
 N. S. Kudryasheva N. S. Kudryasheva | Nadezhda S. Kudryasheva, Dr. Sc. (Biophysics), Institute of Biophysics, Siberian Branch of Russian Academy of Sciences. Dr Kudryasheva is a specialist in physical chemistry, molecular spectroscopy, the structure of molecules, primary physicochemical processes in biological systems, the physical mechanism of bacterial bioluminescence and bioluminescence applications in ecology and education. She develops hypotheses on the activity of upper electron excited states in bioluminescence and studies trends in the influence of chemical compounds on bioluminescent systems, forecasting a basis for bioluminescent assays. She is the author of more than fifty papers on bioluminescence. |
 V. A. Kratasyuk V. A. Kratasyuk | Professor Valentina A. Kratasyuk, DSc (Biophysics), Head of the Biophysics Department at Krasnoyarsk State University. Prof. Kratasyuk pioneered enzymatic bioluminescent biotests and co-immobilization techniques for luminescent enzyme systems. Her research focuses on bioluminescent analysis and ecological biophysics with a particular interest in design of bioluminescent enzymatic assays for monitoring the wide range of biological and ecological systems. She is the author of more than one hundred papers and fourteen patents on bioluminescence. |
1 Introduction
Redox reactions are a part of vital metabolic cycles such as breathing, photosynthesis and others. They include the reactions of enzymatic systems of luminous bacteria, which comprise two reactions:NAD(P)H:FMN–oxidoreductase catalyzes the reduction of FMN at the expense of NAD(P)H
 | (1) |
Bacterial luciferase catalyzes the oxidation of FMNH2 and a long chain aldehyde by molecular oxygen to yield the corresponding acid, H2O, FMN, and light (490 nm)
 | (2) |
This system is extensively used as the basis for constructing assay systems for ecological monitoring.1–6 The main principle of the bioluminescent bioassay is to correlate toxicity of the samples under study and changes in the kinetic parameters of the bioluminescent reaction. The response of bioluminescent systems to the action of pollutants is of an integral character, resulting from the multiple effects of exogenous compounds on bioluminescence.7 With the development of recombinant DNA technology, genetically engineered bioluminescent bacteria have been used as sensitive elements in bacterial bioluminescent biosensors for environmental monitoring.8
It is important to review the investigations of the mechanisms of the action of various chemical compounds on bioluminescence. The discussion will be focused on the experimental approach based on the use of homologous model compounds. This is the way to find correlations between the kinetic parameters of bioluminescence and physicochemical properties of the exogenous compounds. These correlations are related to the bioluminescence mechanism and, hence, provide a physicochemical basis for using bioluminescent enzymatic systems in ecological monitoring, forecasting the sensitivity of bioluminescent assay systems to different pollutants.
Of special interest is the study of bioluminescent assay systems under the action of redox compounds. The redox potential of the environment is one of the main indicators of chemical processes occurring in natural water. It also plays a vital role in the physiology of living organisms. Changes in the redox potential of the environment may be caused by the action of redox pollutants (e.g. quinones and phenols as sewage components6). Bioluminescent assay systems that are sensitive to redox compounds can be effective tools for the ecological monitoring of the redox state of the environment. As the bioluminescent system of the coupled enzyme reactions includes redox reaction (1), it can be used as an assay which is specific to a group of oxidants.
This paper considers the influence of organic redox compounds (quinones and phenols) on the bioluminescent system of the coupled enzymatic reactions catalyzed by bacterial luciferase and NADH:FMN–oxidoreductase (reactions (1) and (2)). It has long been known that redox compounds affect the kinetics of bioluminescence of the enzyme system.8,9 However, the mechanism of their action on elementary physicochemical processes in a bioluminescent system has not been comprehended yet. There may be a number of ways in which redox compounds influence a bioluminescent system: (1) inhibition of redox reactions, and (2) interaction with the enzymes, leading to a loss of enzymatic activity. Additionally, (3) luminescence quenching as a result of the acceptance of excitation energy cannot be excluded. Evaluation of the contributions of these mechanisms to changes in bioluminescence kinetic parameters would provide a basis for the interpretation and prediction of the bioluminescent assay response to the redox compounds.
2. Mechanisms of redox compounds’ influence on the bioluminescent coupled enzyme system
Three possible mechanisms for the influence of exogenous redox compounds on the NAD(P)H:FMN–oxidoreductase–bacterial luciferase bioluminescent enzyme system10,11 such as (1) effects on NADH-dependent redox reactions, (2) interactions with the enzymes of the coupled enzyme system, and (3) intermolecular energy migration are under discussion.2.1. Influence of quinones on redox processes in the bioluminescent system
Enzymatic oxidation of NADH (reaction (1)) serves as a source of FMNH− for the bioluminescent reaction (2). Two electrons and one proton are transferred from NADH to FMN in reaction (1). Exogenous oxidants (quinones) compete with FMN in binding these electrons and proton according to reaction (3):| NADH + oxidant → NAD+ + oxidant·H− | (3) |
The efficiency of competition in a quantitative aspect is determined by the surplus of the reaction (3) redox potential, ΔE3
| ΔE3 = Equinone/quinone·H− − ENAD+/NADH |
| ΔE1 = EFMN/FMN·H− − ENAD+/NADH |
To analyze the action of quinones in the bioluminescent system, the rates of NADH oxidation in reaction (3) (Vm) and bioluminescent kinetic parameters (inhibition coefficient K, bioluminescence delay −tmax) of the system of coupled reactions (1) and (2) were compared with the redox potentials of the quinones.9
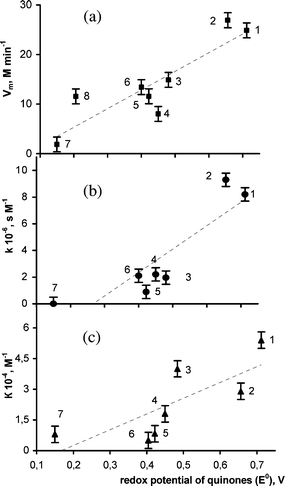 | ||
| Fig. 1 Maximal rate of the NADH oxidation in the presence of NADH:FMN-oxidoreductase (reaction (3)) Vm, (a); bioluminescence delay parameter of the coupled enzyme system k, (b); bioluminescence inhibition coefficient, K, (c), versus the standard redox potentials of quinones (E°): (1) 1,4-benzoquinone; (2) methyl-1,4-benzoquinone; (3) 1,4-naphtoquinone; (4) 1,4-anthraquinone; (5) 2-methyl-1,4-naphtoquinone; (6) tetramethyl-1,4-benzoquinone; (7) 9,10-anthraquinone-β-monosulfonic acid; (8) FMN. | ||
Evidently, the Vm of NADH oxidation by quinones (molecules 1–3 and 6) exceeds the rate of NADH oxidation by FMN (molecule 8).
 | ||
| Fig. 2 Bioluminescence kinetics of the coupled enzyme system NADH:FMN–oxidoreductase–luciferase under different concentrations of methyl-1,4-benzoquinone:9 (1) 0; (2) 12 µM; (3) 15 µM; (4) 20 µM; (5) 25 µM. | ||
The k values were chosen to characterize the luminescence delay and calculated from the plots of tmaxversusC as k = tgα. It was shown that the k-value increased with the growth of the standard redox potential of the quinone (Fig. 1b). A similar relationship was also observed for the NADH oxidation rate (Vm) in reaction (3) (Fig. 1a). The correlation coefficient between the k value and Vm is 0.94. This fact suggests that the participation of quinones in NADH oxidation determines the changes in the bioluminescence kinetic parameter tmax of the coupled enzyme system.
The efficiency of the action of redox compounds on the maximal bioluminescence intensity was defined by the inhibition coefficient K11 calculated using the equation
| ln(I/Icontrol) = −KC |
Fig. 1(c) shows the relationship between the bioluminescence inhibition coefficient K and the standard redox potential of the quinones. It can be concluded that the bioluminescence intensity (I) is also determined by the redox properties of quinones. So, the revealed relationship between the kinetic parameters of bioluminescence and the standard redox potential of the quinones (Fig. 1) proved that the inhibition of redox reactions was the key mechanism by which the quinones decreased light emission intensity.
It is worth noting that the influence of ferricyanide K3[Fe(CN)6] on the bioluminescence of the coupled enzyme system, similarly to the influence of some quinones (Fig. 2), results in the delay of luminescence tmax. The value of k for K3[Fe(CN)6] (k = 1.5 106 s M−1) is less than k for all quinones (Fig. 1), though the standard redox potential of K3[Fe(CN)6] (E° = 0.777 V) is higher than that of the quinones.
To study specific features of the effect of this compound, the rate of NADH oxidation (reaction (3)) with K3[Fe(CN)6] was measured in the presence and the absence of NAD(P)H:FMN–oxidoreductase. The results show that K3[Fe(CN)6] takes part in non-enzymatic oxidation only, in contrast to the organic oxidants (quinones), which participate in both enzymatic and non-enzymatic oxidation reactions. Thus, the luminescence delay tmax of the coupled enzyme system occurs under the action of not only the organic but also the inorganic oxidant.
We compared the influence of the members of the organic redox pairs (quinones and their corresponding reduced forms, diphenols) on bioluminescence.9 The inhibition coefficients of diphenols (Kred) were shown to be an order of magnitude lower than those of the corresponding quinones (Kox) (Table 1).
| Oxidized form | K ox 10−4/M−1 | Reduced form | K red 10−4/M−1 |
|---|---|---|---|
| 1,4-Benzoquinone | 5.4 ± 1.0 | Hydroquinone | 0.09 ± 0.02 |
| 1,4-Naphthoquinone | 2.6 ± 0.3 | 1,4-Naphtolendiol | 0.45 ± 0.09 |
| 1,4-Anthraquinone | 1.8 ± 0.4 | 1,4-Anthracendiol (95%) | 0.4 ± 0.2 |
| 9,10-Anthraquinone-β-monosulfonic acid | 0.8 ± 0.03 | 9,10-Anthracendiol-β-monosulfonic acid (60%) | 0.36 ± 0.03 |
| K3[Fe(CN)6] | 14 ± 3 | K4[Fe(CN)6] | 0.28 ± 0.09 |
A similar dependence was obtained for the inorganic redox pair ferricyanide K3[Fe(CN)6] and ferrocyanide (K4[Fe(CN)6]).
As the redox potentials of quinones are higher than those of phenols, the action of quinones is more efficient. The dependence of the bioluminescent kinetic parameters of the coupled enzyme system upon the redox potentials of exogenous compounds accounts for the difference in the effects of quinones and phenols on bioluminescence in vitro.
2.2. Interaction between quinones and enzymes
The changes in the bioluminescence intensity of a coupled enzyme system in the presence of exogenous compounds are due to their interaction with the enzymes. To study the interactions of quinones with luciferase and NAD(P)H:FMN–oxidoreductase, the time-resolved fluorescence technique was used.13–18 In these experiments we used the fluorescence of endogenous flavin in the enzyme samples. It has been reported in the literature that the non-covalently bound endogenous flavin in the preparation of NAD(P)H:FMN–oxidoreductase isolated from Vibrio fischeri is present as a cofactor19,20 and that the luciferases of luminous bacteria can have affinity to flavins.21,22The decay of polarized fluorescence of FMN (10−6 M) and flavin in the enzyme preparations was recorded in the presence of 1,4-benzoquinone (5 × 10−2 M) or 2,3-dimethoxy-5-methyl-1,4-benzoquinone (5 × 10−3 M) and without them.13
The anisotropy r(t) decay curves were fitted by the sum of exponential terms16,17
We compared characteristics of enzyme-bound flavin fluorescence anisotropy, such as fluorophore rotational correlation time (ϕbound) and its fractional contribution (fbound), in the presence and in the absence of exogenous nonfluorescent compounds, to understand the dynamics of the enzyme in different molecular environments and the process of interaction between exogenous quinones and enzymes.
Characteristics of the fluorescence anisotropy decay of endogenous flavin of the enzymes in the presence and in the absence of quinones are listed in Table 2.
| Sample | ϕ free/ns | β free | f free (%) | ϕ bound/ns | β bound | f bound (%) |
|---|---|---|---|---|---|---|
| FMN | 0.19 ± 0.01 | 0.34 | 100 | — | — | — |
| R | 0.26 ± 0.01 | 0.27 | 13 | 10 ± 1 | 0.05 | 87 |
| R + 1,4-benzoquinone | 0.15 ± 0.01 | 0.36 | 28 | 3.3 ± 0.4 | 0.04 | 72 |
| R + 2,3-dimethoxy-5-methyl-1,4-benzoquinone | 0.16 ± 0.01 | 0.32 | 100 | — | — | — |
| L | 0.24 ± 0.01 | 0.22 | 2.2 | 36 ± 4 | 0.063 | 98 |
| L + 1,4-benzoquinone | 0.14 ± 0.01 | 0.37 | 100 | — | — | — |
| L + 2,3-dimethoxy-5-methyl-1,4-benzoquinone | 0.16 ± 0.01 | 0.33 | 100 | — | — | — |
These data show that the anisotropy decay in the enzymes includes short and long components. The rotational correlation time of the short component (ϕfree) is comparable to that of free FMN (Table 2).
The intrinsic rotational correlation times of the enzymes were estimated using the empiric formula23ϕ = 3.84 × 10−4Mr (Mr is a molecular weight of the enzyme, kDa) as 10 ns for NAD(P)H:FMN–oxidoreductase and 32 ns for luciferase. In ref. 24 the intrinsic rotational correlation time of luciferase was estimated experimentally as 34 ± 4 nm. These values are similar to experimentally obtained ϕbound values in the corresponding enzymes (Table 1). So it might be concluded that the long components (ϕbound values) correspond to those of the enzyme-bound flavin.
The anisotropy decay of endogenous flavin in the NAD(P)H:FMN–oxidoreductase solution in the presence of 1,4-benzoquinone was described by two components. In the presence of quinone, the long component (ϕbound) dropped to 3.3 ns and its contribution decreased, while that of the short component (ffree) increased, indicating weaker binding18 of the endogenous flavin to NAD(P)H:FMN–oxidoreductase and a rise in free FMN concentration. When 1,4-benzoquinone was added to the solution of luciferase, the anisotropy decay of endogenous flavin fluorescence was described by a short component (ϕfree) only (Table 2).
Changes in the anisotropy decay of endogenous flavin were observed in the solutions of both enzymes on the addition of 2,3-dimethoxy-5-methyl-1,4-benzoquinone (Table 1). These results show the absence of the bound flavin in the luciferase and NAD(P)H:FMN–oxidoreductase solutions after addition of quinones.
The polarized fluorescence of endogenous flavin under steady-state conditions was registered at different 1,4-benzoquinone concentrations. The fluorescence steady-state anisotropy was calculated from the formula18
| 〈r〉 = (I‖ − G*I⊥)/(I‖ + 2G*I⊥), |
The dependence of fluorescence steady-state anisotropy of endogenous flavin, 〈r〉, on quinone concentration are shown in Fig. 3.
 | ||
| Fig. 3 Steady-state fluorescence anisotropy 〈r〉 of the endogenous flavin in luciferase (L) and NAD(P)H:FMN–oxidoreductase (R) versus concentration of 1,4-benzoquinone. | ||
Values of 〈r〉 in the absence of 1,4-benzoquinone correspond to flavin bound to enzymes (0.15 and 0.10 for luciferase and NAD(P)H:FMN–oxidoreductase, respectively). As 1,4-benzoquinone concentration grows, the fluorescence anisotropy of endogenous flavin decreases, tending to the free FMN anisotropy. This is indicative of changes in binding of flavin to the enzymes.
These results are suggestive of the interaction of quinones with luciferase and NAD(P)H:FMN–oxidoreductase, which causes a weaker flavin binding to the enzymes.18 Similar effects may lead to changes in catalytic activity of the enzymes, eventually decreasing the bioluminescence intensity in the presence of quinones.
2.3. Bioluminescence quenching
The intermolecular migration of energy onto electron-excited states of exogenous molecules (due to energy resonance transfer and/or the light absorption) is one way of the influence of exogenous compounds on the bioluminescence intensity. It is known that two types of energy transfer mechanisms are possible (trivial absorption, resonance energy transfer), both depending on spectral overlaps. Efficiency of these transfers was evaluated25 using integral approach: the efficiency of energy acceptance by the redox compounds was evaluated by comparing the overlapping areas (Sq) of the quinone absorption spectra, and the bioluminescence spectrum. We applied normalized overlapping areas calculated as S = Sq/Snq for quinones of the same concentration (C = 10−4 M). Here Snq is the maximal overlapping area of 1,4-naphthoquinone, which was chosen as a standard for normalization.The efficiency of the energy transfer was evaluated by overlapping the absorption spectra of the redox compounds (Sabs) with bioluminescence spectrum (SBL). The relative areas of spectral overlapping (S) and the inhibition coefficient of bioluminescence (K values) for the studied quinones are shown in Fig. 4.
 | ||
| Fig. 4 Bioluminescence inhibition coefficient, K, and relative area of spectral overlapping (S) of the quinones absorption spectra and the bioluminescence spectrum for the redox compounds: (1) hydroquinone; (2) 9,10-anthraquinone-β-monosulfonic acid; (3) 1,4-benzoquinone; (4) methyl-1,4-benzoquinone; (5) 1,4-anthraquinone; (6) 1,4-naphtoquinone. | ||
Although some of the quinones have spectral overlapping with bioluminescence spectrum, it is obvious that S and K values are not interrelated, indicating that the intermolecular energy transfer played a minor role in the effect of quinones on the bioluminescence of the coupled enzyme system. This conclusion is in accordance with conventional opinion that energy transfer processes are not effective in biological systems without specific binding.18 However, under the conditions of the experiment, the collision-type interactions cannot be excluded as a mechanism of bioluminescence quenching.
Conclusion
• Three mechanisms of the quinones effect on the NAD(P)H:FMN–oxidoreductase–luciferase coupled enzyme system were studied:• The contribution of the redox reactions of the quinones to the inhibition of bioluminescence was considerable.
• The changes in fluorescence anisotropy decay of endogenous flavin in the luciferase and NAD(P)H:FMN–oxidoreductase preparations under the quinones influence indicated that the quinones interact with the enzymes.
• The intermolecular energy migration was not the principal mechanism determining changes of bioluminescent intensity.
The high sensitivity of the bacterial bioluminescent enzyme system to the toxic redox compounds is of great importance to environmental monitoring. The dependence of bioluminescence kinetic parameters on redox characteristics of exogenous compounds is an essential feature of the coupled enzyme system: NAD(P)H:FMN–oxidoreductase–luciferase and this makes this system suitable for bioassays.
It should be noted that the mechanisms discussed in this paper have only been proven for the bacterial bioluminescent enzyme-based systems. Due to the complex structure of luminous bacteria cells the effects of oxidizers should be more complicated.9
Acknowledgements
The work was financially supported by Award No. REC-002 and Y1-B-02-12 of the U.S. Civilian Research & Development Foundation (CRDF) and RF Ministry of Education, BRHE Programs; “Molecular and Cellular Biology” program of the Russian Academy of Science; grant of President of Russian Federation MK-1950.2005.4.References
- S. H. Choi and M. B. Gu, Phenolic toxicity-detection and classification through the use of recombinant bioluminescent Escherichia coli cells, Environ. Toxicol. Chem., 2001, 20, 248–255 CrossRef CAS.
- R. J. Mitchell and M. B. Gu, An Escherichia coli biosensor capable of detecting both genotoxic and oxidative damage, Appl. Microbiol. Biotechnol., 2004, 64, 46–52 Search PubMed.
- V. A. Kratasyuk, E. V. Vetrova and N. S. Kudryasheva, Bioluminescent water quality monitoring of salt lake Shira, Luminescence, 1999, 14, 193–195 CrossRef CAS.
- V. A. Kratasyuk, E. N. Esimbekova, M. I. Gladyshev, E. B. Khromichek, A. M. Kuznetsov and E. A. Ivanova, The use of bioluminescent biotests for study of natural and laboratory aquatic ecosystems, Chemosphere, 2001, 42, 909–915 CrossRef CAS.
- D. I. Stom, T. A. Geel, A. E. Balayan, A. M. Kuznetsov and S. E. Medvedeva, Bioluminescent method in studing the complex effect of sewage components, Arch. Environ. Contam. Toxicol., 1992, 22, 203–208 CrossRef CAS.
- E. V. Vetrova, V. A. Kratasyuk and N. S. Kudryasheva, Bioluminescent characteristics map of Shira lake water, Aquat. Ecol., 2002, 36, 309–315 Search PubMed.
- N. S. Kudryasheva, E. V. Shalaeva, E. N. Zadorozhnaya, V. A. Kratasyuk, D. I. Stom and A. E. Balayan, Patterns of inhibition of bacterial bioluminescence in vitro by quinones and phenols-components of sewage, Biofizika, 1994, 39, 455–464 Search PubMed.
- S. F. D'Souza, Microbial biosensors, Biosens. Bioelectron., 2001, 16, 337–353 CrossRef CAS.
- N. Kudryasheva, E. Vetrova, A. Kuznetsov, V. Kratasyuk and D. Stom, Bioluminescent assays: Effects of quinones and phenols, Ecotoxicol. Environ. Saf., 2002, 53, 221–225 CrossRef CAS.
- N. A. Tyulkova, Purification of bacterial luciferase from Photobacterium leiognathi with the use of FPLC-system, in Luminescence, ed. B. Jezowska-Trzebiatowska, B. Kochel, J. Stawinski and W. Strek, ,Biological World Scientific, Singapore, 1990, pp. 369–374 Search PubMed.
- N. S. Kudrysheva, V. A. Kratasyuk and P. I. Belobrov, Bioluminescent analysis. The action of toxicants: Physical-chemical regularities of the toxicants effects, Anal. Lett., 1994, 27, 2931–2938 CAS.
- E. V. Vetrova and N. S. Kudryasheva, Mechanism of quinones' influence on bioluminescent enzyme system NAD(P)H:FMN-oxidoreductase-luciferase, in Bioluminescence & chemiluminescence: progress & current applications, ed. P. Stanley and L. Kricka, World Scientific Publishing, Singapore, 2002, pp. 101–104 Search PubMed.
- E. V. Vetrova, N. S. Kudryasheva, A. J. Visser and A. von Hoek, Characteristics of endogenous flavin fluorescence of Photobacterium leiognathi luciferase and Vibrio fischeri NAD(P)H:FMN-oxidoreductase, Luminescence, 2005, 20, 205–209 CrossRef CAS.
- van Hoek and A. J. W. G. Visser, Pulse selection system with electro-optic modulators applied to mode-locked cw lasers and time-resolved single photon counting, Rev. Sci. Instrum., 1981, 52, 1199–1205 CrossRef.
- J. C. Brochon, Maximum entropy method of data analysis in time-resolved spectroscopy, in Methods in Enzymology, 240, Numerical Computer Methods, ed. B. M. L. Johnson and L. Brand, 1994, pp. 262–311 Search PubMed.
- E. G. Novikov, A. van Hoek, A. J. W. G. Visser and J. W. Hofstraat, Linear algorithms for stretched exponential decay analysis, Opt. Commun., 1999, 166, 189–198 CrossRef CAS.
- J. M. Beechem, E. Gratton, M. Ameloot, J. R. Knutson and L. Brand, The global analysis of fluorescence intensity and anisotropy decay data: Second-generation theory and programs, in Topics in Fluorescence Spectroscopy, Principles, ed. J. R. Lakowicz, Plenum, New York, 1991, vol. 2 Search PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum, New York, 1999 Search PubMed.
- S. Inouye, NAD(P)H-flavin oxidoreductase from Vibrio fischeri ATCC-7744 is a flavoprotein, FEBS Lett., 1994, 347, 163–168 CrossRef CAS.
- S. C. Tu, Reduced flavin: donor and acceptor enzymes and mechanisms of channeling, Antioxid. Redox Signaling, 2001, 3, 881–97 Search PubMed.
- C. J. Wei, B. Lei and S. C. Tu, Characterization of the binding of Photobacterium phosphoreum p-flavin by Vibrio harveyi luciferase, Arch. Biochem. Biophys., 2001, 396, 199–206 CrossRef CAS.
- E. V. Vetrova, N. S. Kudryasheva, A. J. Visser and A. von Hoek, Characteristics of endogenous flavin fluorescence of Photobacterium leiognathi luciferase and Vibrio fischeri NAD(P)H:FMN-oxidoreductase, Luminescence, 2005, 20, 205–209 CrossRef CAS.
- R. Leenders, A. van Hoek, M. van Iersel, C. Veeger and A. J. W. G. Visser, Flavin dynamics in oxidized Clostridium beijerinckii flavodoxin as assessed by time-resolved polarized fluorescence, Eur. J. Biochem., 1993, 218, 977–984 CrossRef CAS.
- N. S. Kudryasheva, E. V. Nemtseva, A. J. W. G. Visser and A. van Hoek, Interaction of aromatic compounds with Photobacterium leiognathi luciferase: fluorescence anisotropy study, Luminescence, 2003, 18, 156–161 CrossRef CAS.
- E. V. Vetrova, Mechanisms of influence redox active compounds on coupled enzyme system NADH:FMN–oxidoreductase–luciferase, Teethes of dissertation, Institute of Biophysics SB RAS, 2002 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |