Excitation-induced dynamics of external pH pattern in Chara corallina cells and its dependence on external calcium concentration
Alexey
Eremin
a,
Alexander
Bulychev
*b,
Natalia A.
Krupenina
b,
Thomas
Mair
a,
Marcus J. B.
Hauser
a,
Ralf
Stannarius
a,
Stefan C.
Müller
a and
Andrei B.
Rubin
b
aOtto-von-Guericke-Universität, Institute of Experimental Physics, Universitätsplatz 2, 39016, Magdeburg, Germany
bLomonosov Moscow State University, Faculty of Biology, 119992, Moscow, Russia. E-mail: bulychev@biophys.msu.ru; Fax: +7 495 9391115; Tel: +7 495 9393503
First published on 24th November 2006
Abstract
The influence of cell excitation and external calcium level on the dynamics of light-induced pH bands along the length of Chara corallina cells is studied in the present paper. Generation of an action potential (AP) transiently quenched these pH patterns, which was more pronounced at 0.05–0.1 mM Ca2+ than at higher concentrations of Ca2+ (0.6–2 mM) in the medium. After transient smoothing of the pH bands, some alkaline peaks reemerged at slightly shifted positions in media with low Ca2+ concentrations, while at high Ca2+ concentrations, the alkaline spots reappeared exactly at their initial positions. This Ca2+ dependency has been revealed by both digital imaging and pH microelectrodes. The stabilizing effect of external Ca2+ on the locations of recovering alkaline peaks is supposedly due to formation of a physically heterogeneous environment around the cell owing to precipitation of CaCO3 in the alkaline zones at high Ca2+ during illumination. The elevation of local pH by dissolving CaCO3 facilitates the reappearance of alkaline spots at their initial locations after temporal suppression caused by cell excitation. At low Ca2+ concentrations, when the solubility product of CaCO3 is not attained, the alkaline peaks are not stabilized by CaCO3 dissolution and may appear at random locations.
Introduction
The distribution of ion transport systems over the surface of animal and plant cells is often nonuniform owing to clustering of ion channels or their attribution to specific morphological structures (e.g., flagella).1,2 Membrane regions with particular properties can also arise in the initially uniform plasma membrane due to complex regulatory interactions.3 For example, in darkened cells of characean algae, the properties of plasma membrane are uniform along the internode. Upon illumination, these cells create alternating regions of proton uptake and release, with a periodic length of about 7 mm and pH shifts of up to 3 units.4,5 In young cells, these pH patterns and corresponding patterns of extracellular current are subject to changes during growth6 and are highly dynamic after dark–light transitions: at first, small alkaline spots (patches) originate and then the patches are transformed to regular alkaline bands.7 In a series of dark–light cycles, the steady-state pH profiles display spatially stable alternating alkaline and acidic zones. Taken together, the formation of plasma membrane domains can be considered as a self-organization process5,8 influenced by factors fixing the spatial position of alkaline zones.7In mature and senescent cells, the alkaline regions are less dynamic and usually, though not always, located in the area of calcium depositions (mainly as CaCO3) on the cell walls.9–12 Biogenic calcification is closely coupled to photosynthesis, because of the shift in the carbonate system by CO2 uptake and the concomitant pH increase, which enhances the calcium carbonate precipitation.13 The extracellular calcium dynamics closely follows the pH dynamics. Although young cells are devoid of calcium incrustations, the initial stages of mineralization, i.e., formation of CaCO3 crystallites suspended in the aqueous phase, can occur in the alkaline regions of pH 9.5–10, where high concentrations of CO32− are produced.10 The role of external Ca2+ in pH banding can be verified by studying the formation of alkaline zones in illuminated Chara cells at different concentrations of Ca2+ in the medium. It is anticipated that the increased [Ca2+] in the medium should favor CaCO3 crystallization and, thereby, promote the restoration of alkaline regions at their initial position in consecutive light cycles. Conversely, low Ca2+ levels insufficient for CaCO3 crystallization should raise the probability of displacement of the recovering alkaline region from its initial location.
The ability of a single electrical excitation (action potential, AP) to affect the pH pattern14 represents a convenient tool for temporal elimination of pH differences between alkaline and acidic regions. The effect of AP on external pH consists of long-term inactivation of H+-conducting pathways mediated presumably by the transient rise in the cytosolic Ca2+ level.14 Electric stimulation ensures repeated decay and restoration of the pH pattern under constant illumination conditions and is a proper means to study the influence of external and internal factors on pattern formation.
The aim of this study is to assess the role of external heterogeneities in the formation of the pH pattern along Chara cells. We examined AP-induced dynamics of pH profiles adjacent to the cell surface at different concentration levels of external Ca2+ using pH microelectrodes and digital imaging of a suitable pH indicator. It was found that elevated concentrations of Ca2+ in the medium promote a faster recovery of alkaline bands at their initial locations, while low external Ca2+ concentrations facilitate the displacement of re-arising alkaline peaks and prolong the pH recovery in the initially alkaline area. The heterogeneity of the external environment seems to be involved in the restoration of pH bands.
Experimental
Plant material and solutions
Isolated internodal cells of Chara corallina Klein ex Willd. were used in experiments. The algae used in the MSU laboratory were grown under scattered daylight illumination at 20–22 °C as described before.15 The algae cultured in the laboratory in Magdeburg were a gift of Prof. G. Thiel (Technical University of Darmstadt). They were grown under 14/10 h dark/light cycles of illumination with fluorescent lamps (2.2 W m−2) at 22 °C. The results obtained with cells from these two cultures were similar. Most experiments were performed using young uncalcified or sparsely calcified cells. Prior to experiments individual internodes of about 6 cm length and 0.9–1 mm diameter were excised and kept under scattered daylight conditions for 3–7 d in artificial pond water (APW) containing 0.1 mM KCl, 1.0 mM NaCl, and 0.1 mM CaCl2, pH 6.8–7.2. During experiments, APW solutions containing 0.05, 0.1, 0.6 and 2.0 mM CaCl2 were used. Experiments were usually started at low Ca2+ concentration. Prior to elevation of Ca2+ level, several AP were triggered at intervals of 20–30 min to obtain reproducible pH responses. At the end of the experiment, high-Ca2+ APW was replaced with the initial solution.Imaging dynamics of pH band formation
The cell was placed into a two-sectioned transparent chamber with an insulating partition, which enabled electrical stimulation with external electrodes. The alkaline pH bands were induced by illumination with a KL-1500 cold light source (Schott, Germany) and were visualized with the pH indicator thymol blue (100 µM).16 This indicator is better suited to monitor the dynamics of alkaline bands because its pH response is shifted to higher pH range compared to the commonly applied phenol red. Thymol blue changes its color from yellow to blue upon a pH increase from 8.0 to 9.2 (compared to 6.8–8.0 for phenol red). The temporal changes of the pH around the cell were recorded with a color AxioCam HR CCD camera (Carl Zeiss, Germany) at a resolution of 2012 × 260 pixels per image. Color images were acquired by the camera sensor through a Bayer color filter mask using color co-site sampling. Image acquisitions were made before cell excitation and at regular intervals after cell excitation. The acquisition time varied depending on the actinic light intensity and was 0.4 s at the lowest intensities used. Images were taken at a frequency of 4 min−1 during the first 3 min after electric stimulation and at a frequency of 1 min−1 for the subsequent 20–30 min thereafter. They were analyzed using the AxioVision software (Carl Zeiss, Germany). The digital color camera output yielded separated red, blue and green (RGB) profiles along the cell wall. Since the green channel showed the largest response on the color change of the pH indicator, the spatial pH distribution was estimated from the intensity profiles in the green channel along a predetermined path. A color scale calibration was accomplished by imaging thymol blue solutions in the same experimental chamber at given pH values, adjusted with 10 mM Hepes–KOH and Tris-HCl buffers in the pH range from 7.5 to 9.5. Three-dimensional time–space plots were reconstructed using the Matlab program. Since the color response of the pH indicator is nonlinear, the method provides qualitative information on pH dynamics. Quantitative measurements of local pH were obtained with pH microelectrodes.17Microelectrode pH measurements
The experimental chamber with a C. corallina cell was mounted on a stage of an Axiovert-25 microscope (Carl Zeiss, Germany) supplemented with a micromanipulator.5,18 The alkaline and acidic regions were identified with glass-insulated antimony pH microelectrodes having a tip diameter of 10–20 µm. The potential of the pH microelectrode as a function of pH was calibrated relative to an Ag/AgCl reference electrode with buffer solutions. The electrode function of the antimony pH electrode was linear with the slope of about 58 mV per pH unit.17 The terms “alkaline” and “acidic” cell regions denote the cell parts adjacent to alkaline and acidic zones of the outer medium, respectively. The pH changes were recorded with a VAJ-51 electrometric amplifier (RFT, Germany) and on a computer with a CED-1401plus analog-to-digital converter (Cambridge Electronic Design, UK) and WinWCP software (Strathclyde Electrophysiology Software). The scanning with a pH microprobe along the cell (scanning rate 185 µm s−1) was accomplished as previously described.5,7The actinic light was directed to the cell from above. The upper illuminator of the Axiovert 25 microscope with a 25 W halogen lamp was used as a light source. The maximum photon flux density of photosynthetically active radiation (white light) was 150 µmol m−2 s−1. Incident light intensities were measured with the MC-MQS Micro Quantum Sensor (Walz, Germany). Neutral glass filters were used for the attenuation of the actinic light.
Chlorophyll fluorescence measurements
The light response curves of photosynthetic linear electron flow were obtained by measuring chlorophyll fluorescence with the saturation pulse method on small portions of a chloroplast layer (a circular area of 100 µm in diameter) using a Microscopy-PAM fluorometer (Walz).15 The electron transport rates were calculated by multiplying the photon flux density and the effective quantum yield of electron flow in photosystem II for each light intensity and were expressed in arbitrary units. Light response curves were measured with more than 30 cells.Electrical stimulation
Cells were stimulated by external electrodes independent of the measuring circuit. The electrodes were placed in two compartments separated either by silicone oil (Baysilone) or air gaps. The cell was excited with single pulses of electric current (about 10 µA, 150–200 ms). The generation of AP was detected from temporal cessation of the cytoplasmic streaming as well as from a short artifact signal reflecting the extracellular recording of the propagating AP.Results
Imaging of excitation-induced dynamics of the pH pattern
Time-lapse imaging was applied for the first time to monitor the dynamics of the pH pattern along C. corallina cells. Fig. 1 shows an example of the typical dynamics of the pH profile after electric excitation of the plasma membrane. Zero time corresponds to the moment when an AP was induced by an electric stimulus. Other coordinates represent the distance along the cell length and the pH value in the immediate vicinity of the cell surface. It is evident that the alkaline peaks were suppressed after AP, with the most pronounced suppression occurring at ∼20 min after the action potential. After a transient flattening of the pH profile, a recovery of the alkaline peaks was observed. | ||
| Fig. 1 Spatiotemporal dynamics of the pH profile along the surface of an illuminated C. corallina cell after generation of a single action potential, visualized with thymol blue. The cell was placed in the medium containing 0.1 mM KCl, 1.0 mM NaCl, 0.05 mM CaCl2, and 100 µM thymol blue. Light intensity 1.022 W m−2. Zero time corresponds to the moment of cell excitation. The color coding shows an approximate pH scale. | ||
The extent and duration of AP-induced pH changes depended inversely on light intensity. The pH in alkaline zones of resting cells was almost constant at the light intensities used, but AP-induced pH responses were larger at low fluence rates (Fig. 2A). At elevated light intensities, the recovery of alkaline peaks started comparatively fast, prior to full dissipation of alkaline peaks. Consequently, the amplitudes of pH changes in the alkaline regions were smaller at high-intensity light than at reduced light intensities (Fig. 2B). The faster recovery of alkaline bands at higher fluence rates and the respective decrease in the extent of AP-induced pH shift suggest that photosynthesis mitigates the effect of AP on the pH pattern. This view is supported by the increase in photosynthetic linear electron transport as a function of light intensity (Fig. 2C). The light dependence is similar to well-known light-response curves of photosynthesis in green plants. Low fluence rates (4–12 µmol m−2 s−1) were found optimal for these experiments because they were sufficient for the maintenance of the pH bands and ensured almost complete smoothing of external pH after AP in cells bathed with the standard medium.
 | ||
| Fig. 2 Effect of light intensity on AP-induced pH changes and photosynthesis of C. corallina cell in the medium with 0.1 mM Ca2+. (A) pH changes in the alkaline zone induced by AP at various light intensities: (1) 150, (2) 60, (3) 20, and (4) 9 µmol m−2 s−1. Zigzag arrow marks the moment of AP generation. (B) The amplitude of AP-induced pH shift in the alkaline region (pH difference between the initial and minimum levels) as a function of fluence rate of actinic light. Bars indicate standard errors of the mean with n ranging from 3 to 15. (C) Representative light response curve of photosynthetic linear electron transport reconstructed from chlorophyll fluorescence parameters of C. corallina chloroplasts in an intact cell. | ||
At low Ca2+ concentrations in the medium, some alkaline spots reappeared at positions that were shifted with respect to their locations before and during peak dissipation (Fig. 1). For example, the alkaline peak in the region of 25 mm along the cell axis initially reappeared at a different place compared to that of the pre-existent peak. This feature is also evident in the original images of the cells (Fig. 3A), in the profiles of color intensity (Fig. 3B), and in the time–space diagram (Fig. 3C). During subsequent evolution of the recovering band, the peak shifted back to its initial position. The shift in locations of disappearing and reappearing peaks was only observed at low Ca2+ concentrations in the medium (0.05–0.1 mM) and did not occur at elevated Ca2+ levels in the medium (0.6–2.0 mM).
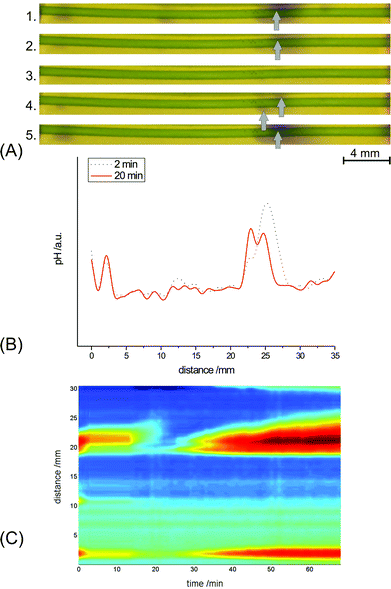 | ||
| Fig. 3 (A) Individual images for distribution of the pH-indicating dye thymol blue along an illuminated C. corallina cell under resting conditions and at various stages after AP generation: (1) before excitation; (2) 2 min after cell excitation; (3) 11 min after excitation; (4) 20 min after excitation; (5) 40 min after excitation. Arrows indicate the centers of the alkaline bands (bluish color) at different measurement times. (B) Longitudinal profiles of green color intensity at various stages after cell excitation: (1) 2 min; (2) 20 min. (C) Time-space diagram corresponding to the 3-d time-space plot of Fig. 1. The color map in (C) is the same as in Fig. 1. | ||
Fig. 4 shows the evolution of the pH profile measured with a pH microelectrode. The suppression of the pH profile and the beginning of its restoration are seen. The results of both the digital imaging and microelectrode methods are similar.The imaging technique is far superior to the scanning probe method in temporal resolution but has the drawback that the indicator response is confined to a relatively narrow pH range. In experiments with pH microelectrodes, small displacements of the initial and renewed alkaline peaks after AP generation could be readily distinguished based on the kinetics of local pH changes (see below).
 | ||
| Fig. 4 Excitation-induced dynamics of the pH profile along an C. corallina cell, measured with a scanning pH microelectrode. The cell was bathed in the solution with 0.1 mM Ca2+. Light intensity 2.9 W m−2. Zero time corresponds to the moment of cell excitation. Measurement of an individual pH profile at a scanning rate of 185 µm s−1 took 2 min per scan. | ||
Effect of the external Ca2+ concentration on the recovery of alkaline peaks after cell excitation
As external Ca2+ concentration was increased, the AP-induced pH shifts in the alkaline bands became smaller (Fig. 5A), similar to what was observed upon the increase in fluence rate. By contrast to variations in light intensity, variations in external [Ca2+] had no significant influence on the photochemical efficiency of photosystem II and linear electron transport in chloroplasts (data not shown). | ||
| Fig. 5 Effects of external Ca2+ concentrations on AP-induced pH changes in alkaline regions. (A) Acceleration by Ca2+ of pH recovery after AP-induced suppression of the alkaline peak. Numbers near the curves designate the order of treatments applied to the same cell: (1) 0.5 mM Ca2+, (2) 0.05 mM Ca2+, (3) 2 mM Ca2+. (B) Excitation-induced pH changes in the alkaline region indicating the shifted position of the reforming band at low Ca2+ (0.1 mM) and its unchanged location at high Ca2+ (0.6 mM) concentrations in the external medium (curves 1 and 2, respectively). Curves 1 and 2 were obtained by averaging four measurements made before elevation of Ca2+ concentration in the medium and four measurements obtained after the replacement of low Ca2+ medium with high Ca2+ solution. Curve 3 is an individual trace recorded after transferring the cell from a high-Ca2+ medium to a low-Ca2+ solution. At the end of measurement 3 (at t = 28 min), the pH electrode was displaced by a fraction of a millimeter and detected a fully developed shifted band. Light intensity 13.0 W m−2. Arrows indicate the moments of cell excitation. Bars represent standard errors of the mean. | ||
Fig. 5B displays AP-induced pH changes in a narrow alkaline region at Ca2+ concentrations of 0.1 and 0.6 mM. At 0.1 mM Ca2+ and observation periods of 25 min, the pH drop after AP was either irreversible (curve 1) or partly and slowly reversible. This apparent irreversibility of local pH changes after AP was caused by the shift in the position of the newly formed alkaline zone from the location of the pre-existent peak. Indeed, at the end of a 25–30 min period after AP, a fully developed alkaline spot was observed upon moving the electrode tip around the center of the previous spot. The pH kinetics shown by curve 1 in Fig. 5B is consistent with imaging data and provides independent evidence that, at low external [Ca2+], alkaline bands recovering after AP-induced suppression are often shifted from their initial location.
The replacement of medium or low Ca2+ concentration solutions (i.e. 0.1 mM Ca2+) with a 0.6 mM Ca2+ solution substantially modified the AP-induced pH changes. At elevated Ca2+ levels, the local pH values in the same alkaline region were rapidly restored after AP (Fig. 5B, curve 2). The reversibility of the alkaline peaks at high Ca2+ concentrations proves that the alkaline region was restored after AP at its initial place. This effect of [Ca2+] on the pH band dynamics was reversible: when the cell was washed repeatedly and placed into a medium with low Ca2+ concentration, the AP-induced pH changes were similar to those observed at low [Ca2+] at the beginning of the experiment (curve 3). After completing the observation period, the electrode was moved around its basic position, and a shifted alkaline region was detected at a distance of less than 1 mm from the initial location (terminal part of curve 3).
Discussion
The time-resolved digital imaging introduced in this work is a promising tool for studying the dynamics of the external pH pattern along illuminated C. corallina cells. The method is advantageous compared to pH scanning as it ensures high temporal and spatial resolution. The pH-indicating color profiles along the cell length can be sampled at a high repetition rate, whereas recording of the pH profiles by electrode scanning requires at least two minutes per scan. On the other hand, the color changes of pH indicators are limited to narrow pH intervals, such that single pH indicators are not suitable for monitoring large pH changes. Since most of the pH indicators change their colors depending on pH and not simply their brightness, the precision of the time-resolved digital imaging method can be improved by measuring and calibrating the apparent color by means of processing the information from all three RGB channels. Although the imaging method does not ensure quantitative pH measurements, it is perfectly suited to detecting changes in the number and position of alkaline peaks as well as monitoring the dynamics of band broadening or disappearance. This method is complementary to the pH microelectrode scanning technique, which allows quantitative measurements of local pH changes at lower temporal resolution.The kinetics of local pH changes measured with microelectrodes was very sensitive to small displacements of the alkaline peaks that reemerge after the AP-induced decay. At a determined position, the shift of the alkaline peak was manifested as an irreversible or poorly reversible pH decrease after AP generation. At low [Ca2+] (0.05–0.1 mM) the alkaline peak in Fig. 5B did not reappear in the observation time range (curves 1 and 3), indicating the displacement of the peak from its initial location. The reappearance of alkaline peaks at a shifted position indicates that the position of re-arising alkaline peaks is fixed less rigidly at low [Ca2+] than at higher Ca2+ level.
The processes behind the origin of fixed or shifted locations of recovering alkaline regions can be explained by currently accepted ideas about band formation including the interactions between photosynthesis and electrical properties of the plasma membrane.3 Photosynthetic fixation of CO2 is known to elevate external pH, while elevated pH augments the plasma membrane conductance up to 5–8 times when the external pH reaches values normally found in basic regions.19,20 This rise reflects a dramatic increase in H+ (OH−) conductance, which is negligibly small at neutral pH (compared to the predominant K+ conductance) but becomes dominant at high pH.19 Considering the cytoplasmic pH in illuminated characean cells of about 7.621 and the membrane potential in the alkaline cell regions around −160 mV,22 it is evident that the driving force for protons is directed inward in a broad pH range, up to pH ∼10.3. The activation of “high-pH channels”16 promotes the passive H+ movement along the electrochemical gradient into the cytoplasm, which additionally elevates the external pH and further activates the high pH channels. The inward H+ flux ceases to grow at high pH when the effect of increasing conductance is counterbalanced by the decrease in the driving force.
The above scheme predicts an essential role for photosynthesis and local pH in the regulation of H+ fluxes and pattern formation. It is important in this connection that dissipation of alkaline bands after AP was expressed stronger at low Ca2+ than at high Ca2+ concentrations (Fig. 3 and 5), while photosynthetic rates were nearly identical as judged from chlorophyll fluorescence data at 0.1–2 mM Ca2+. The residual heterogeneity in the pH profiles observed at high [Ca2+] might serve as a template for the recovery of alkaline bands in their initial positions, while the strong flattening of the pH pattern at low [Ca2+] increases the likelihood of band reappearance at shifted positions.
At elevated Ca2+ concentrations (0.6–2.0 mM), the alkaline peak reappeared at the same place that it was located before AP generation (Fig. 5B, curve 2). An important factor here is that large differences in pH between acidic and alkaline zones create an inhomogeneous distribution of CO32−, which gives rise to precipitation of poorly soluble CaCO3 at high (but not at low) Ca2+ concentrations. Kiyosawa23 studied the composition of salts leached from C. corallina cell walls and the solubility of CaCO3 in pure aqueous solutions. He found that the Ca2+ activity in the solution increases with the CaCO3 content reaching saturation at 0.3–0.4 mM and that the pH in solutions with a CaCO3 total content of 0.2 and 0.7 mM (below and above the solubility limit, respectively) was shifted to 8.5 and 9.2, respectively. Hence, CaCO3 crystallization is likely to occur in air-equilibrated alkaline solutions with a total CaCO3 concentration ≥0.5 mM, and the dissolution of these crystallites should retard pH lowering when the light-dependent H+ fluxes across the plasma membrane are arrested by darkness or cell excitation. The calcification is attributed to alkaline zones (pH ∼10) where the equilibrium between CO2, HCO3− and CO32− (pK1 = 6.25 and pK2 = 10.33) is shifted toward CO32− and the concentration of the CO32− anion reaches the highest values. The calcium deposition has been implicated in the explanation of correlated light-induced changes in [H+] and [Ca2+] near the surface of another calcifying alga.13
We suppose that submicrometer size crystallites of CaCO3 occurring in the alkaline zones create a comparatively stable physicochemical heterogeneity in the medium, which may affect the process of band formation after the AP-induced pH-band decay. The existence of external heterogeneity in the absence of visible calcification was noted by Dorn and Weisenseel.6 They observed that, after exposing the characean cell under atmosphere in a horizontal position, the inward current (alkaline) regions retained an adhering film of medium while the outward current (acid) regions did not. The CaCO3 particles formed under illumination in the alkaline zones ensure higher local pH values compared to pH in uncalcified regions.23 Therefore, light-induced alkalization of the medium caused by photosynthetic CO2 fixation should promote the activation of H+ conductance of the plasma membrane exactly in the regions where CaCO3 crystals are localized; i.e., the position of alkaline zones should be stabilized. The CaCO3 particles, produced under light in the alkaline zones, act as an immobile, slowly dissolving buffer and become the sites where new alkaline regions reemerge after the excitation-induced dampening of the pH pattern. Conversely, if the CaCO3 concentration is below the saturation concentration, the pH pattern is not fixed by the immobile buffer. Consequently, the alkaline zones may, in principle, reappear after AP randomly at any place. However, the displacements actually observed were relatively small (fractions of a millimeter), implying that, apart from external factors, internal heterogeneity (e.g., banding of photosynthetic activity) may impose strong restrictions on the location of re-arising bands. This issue is under current investigation and will be presented in a forthcoming article.
Our interpretation is consistent with the well-known correlation between the distribution of alkaline zones and calcium depositions at the C. corallina cell walls.10,12 The supposed accumulation of CaCO3 may also account for the retention of alkaline bands in plasmolyzed cells submerged in a medium with 10 mM Ca2+.24 The presence in the alkaline zones of CaCO3 particles acting as a slowly dissolving buffer is also indicated by an apparent asymmetry of alkaline peaks measured with a scanning pH probe (Fig. 4). This asymmetry cannot be ascribed to electrode properties since calibration tests proved equally fast (∼1 s) electrode responses to the increase and decrease in pH. In our view, the electrode tip drags a thin layer of solution with crystallites from the alkaline to acid zone, which delays the pH drop owing to dissolution of these crystals.
As an alternative to the proposed explanation of Ca2+ influence on band positioning, one can think about nonuniform Ca2+ fluxes during AP in the acidic and alkaline regions. Beilby and Bisson25 supposed from voltage clamp experiments that the cytosolic Ca2+ rise during AP in C. corallina cells proceeds more slowly at high pH than at neutral pH of the medium. Spatial variations of the Ca2+ level in the immobile ectoplasm may affect the conductance of ion channels, their mobility within the membrane, and may act indirectly through changes in functional activity of chloroplasts. Nevertheless, such alternative interpretations are presently more speculative than the main explanation based on physicochemical laws that are unavoidably valid for solutions and biological systems.
We conclude that local variations in physicochemical properties of the medium, promoted by elevation of the external Ca2+ concentration level, affect the formation of heterogeneous distribution of H+ fluxes across the plasma membrane of C. corallina cells. At the same time, the heterogeneity of the external medium determines the location of reemerging alkaline regions to a limited extent. The narrow range for displacements of re-arising alkaline peaks compared to pre-existent peaks (at low external Ca2+ levels) indicates that internal (intracellular) factors impose additional restrictions on the location of reemerging alkaline zones.
Acknowledgements
We are grateful to Prof. G. Thiel (Technical University of Darmstadt) for the kind gift of Chara corallina cells. This work was supported by the Russian Foundation for Basic Research and the Deutsche Forschungsgemeinschaft.References
- J. W. Shuai and P. Jung, Optimal ion channel clustering for intracellular calcium signaling, Proc. Nat. Acad. Sci. U. S. A., 2003, 100, 506–510 CrossRef CAS.
- E.-M. Holland, F.-J. Braun, C. Nonnengasser, H. Harz and P. Hegemann, The nature of rhodopsin-triggered photocurrents in Chlamydomonas, Biophys. J., 1996, 70, 924–931 CrossRef CAS.
- A. A. Bulychev, P. W. J. v. d. Wijngaard and A. H. De Boer, Spatial coordination of chloroplast and plasma membrane activities in Chara cells and its disruption through inactivation of 14-3-3 proteins, Biochemistry (Moscow), 2005, 70, 55–61 CAS.
- W. J. Lucas, D. W. Keifer and D. Sanders, Bicarbonate transport in Chara corallina: Evidence for cotransport of HCO3− with H+, J. Membr. Biol., 1983, 73, 263–274 Search PubMed.
- A. A. Bulychev, A. A. Polezhaev, S. V. Zykov, T. Y. Pljusnina, G. Y. Riznichenko, A. B. Rubin, W. Jantoss, V. S. Zykov and S. C. Müller, Light-triggered pH banding profile in Chara cells revealed with a scanning pH microprobe and its relation to self-organization phenomena, J. Theor. Biol., 2001, 212, 275–294 CrossRef CAS.
- A. Dorn and M. H. Weisenseel, Growth and the current pattern around internodal cells of Nitella flexilis L., J. Exp. Bot., 1984, 35, 373–383 Search PubMed.
- A. A. Bulychev, S. V. Zykov, A. B. Rubin and S. C. Müller, Transitions from alkaline spots to regular bands during pH pattern formation at the plasmalemma of Chara cells, Eur. Biophys. J., 2003, 32, 144–153 Search PubMed.
- K. Toko, H. Chosa and K. Yamafuji, Dissipative structure in the Characeae: Spatial pattern of proton flux as a dissipative structure in characean cells, J. Theor. Biol., 1985, 114, 125–175 CrossRef.
- M. Borowitzka, Morphological and cytological aspects of algal calcification, Int. Rev. Cytol., 1982, 74, 127–162 Search PubMed.
- T. A. McConnaughey and R. H. Falk, Calcium-proton exchange during algal calcification, Biol. Bull., 1991, 180, 185–195 Search PubMed.
- K. A. Serikawa, D. M. Porterfield, P. J. S. Smith and D. F. Mandoli, Calcification and measurement of net proton and oxygen flux reveal subcellular domains in Acetabularia acetabulum, Planta, 2000, 211, 474–483 CrossRef CAS.
- C. Brownlee and A. R. Taylor, Algal calcification and silification, in Encyclopedia of Life Sciences, John Wiley & Sons, Ltd, Chichester, 2002, pp. 1–6 Search PubMed.
- D. De Beer and A. W. D. Larkum, Photosynthesis and calcification in the calcifying algae Halimeda discoidea studied with microsensors, Plant, Cell Environ., 2001, 24, 1209–1217 CrossRef CAS.
- A. A. Bulychev, N. A. Kamzolkina, J. Luengviriya, A. B. Rubin and S. C. Müller, Effect of a single excitation stimulus on photosynthetic activity and light-dependent pH banding in Chara cells, J. Membr. Biol., 2004, 202, 11–19 Search PubMed.
- A. A. Bulychev and W. J. Vredenberg, Spatio-temporal patterns of photosystem II activity and plasma-membrane proton flows in Chara corallina cells exposed to overall and local illumination, Planta, 2003, 218, 143–151 CrossRef CAS.
- M. J. Beilby, T. Mimura and T. Shimmen, The proton pump, high pH channels, and excitation: voltage clamp studies of intact and perfused cells of Nitellopsis obtusa, Protoplasma, 1993, 175, 144–152 Search PubMed.
- D. Remis, A. A. Bulychev and G. A. Kurella, The electrical and chemical components of the protonmotive force in chloroplasts as measured with capillary and pH-sensitive microelectrodes, Biochim. Biophys. Acta, 1986, 852, 68–73 CAS.
- A. A. Bulychev, A. A. Cherkashin, A. B. Rubin, W. J. Vredenberg, V. S. Zykov and S. C. Müller, Comparative study on photosynthetic activity of chloroplasts in acid and alkaline zones of Chara corallina, Bioelectrochemistry, 2001, 53, 225–232 CrossRef CAS.
- M. A. Bisson and N. A. Walker, Control of passive permeability of the Chara plasmalemma, J. Exp. Bot., 1982, 33, 520–532 Search PubMed.
- J. R. Smith and N. A. Walker, Membrane conductance of Chara measured in the acid and basic zones, J. Membr. Biol., 1983, 73, 193–202 Search PubMed.
- M. Tazawa, Cell physiological aspects of the plasma membrane electrogenic H+ pump, J. Plant Res., 2003, 116, 419–442 Search PubMed.
- S. A. Frost, Shartzer, J. Fisahn and W. J. Lucas, Simultaneous measurement of extracellular current and membrane potential of Chara corallina internodal cells during light-induced modulation of H+ transport, C. R. Acad. Sci., Ser. III, 1992, 315, 247–254.
- K. Kiyosawa, Ca2+ and phosphate releases from calcified Chara cell walls in concentrated KCl solution, J. Exp. Bot., 2001, 52, 223–229 Search PubMed.
- T. Shimmen, S. Yonemura, M. Negoro and W. J. Lucas, Studies on alkaline band formation in Chara corallina: Ameliorating effect of Ca2+ on inhibition induced by osmotic shock, Plant Cell Physiol., 2003, 44, 957–960 CrossRef CAS.
- M. J. Beilby and M. A. Bisson, Chara plasmalemma at high pH: Voltage dependence of the conductance at rest and during excitation, J. Membr. Biol., 1992, 125, 25–39 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2007 |
