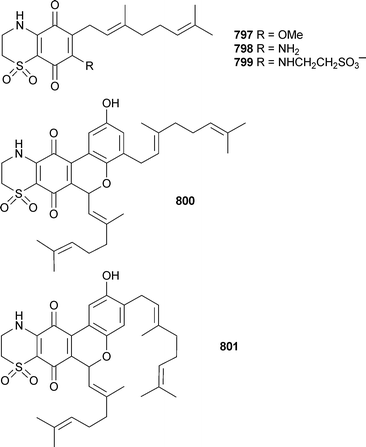
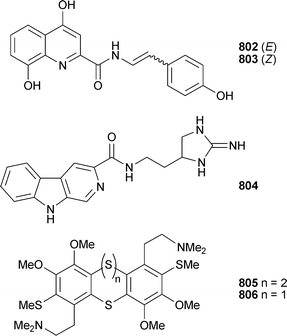
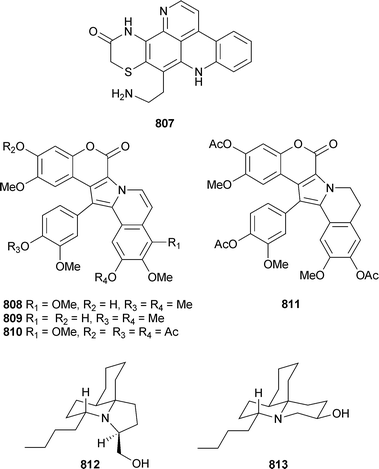
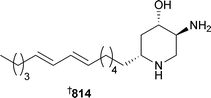
Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Wan-Ping
Hu
a,
Murray H. G.
Munro
a,
Peter T.
Northcote
c and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bDepartment of Chemistry, University of Auckland, Auckland, New Zealand
cSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 11th January 2007
Abstract
Covering: 2005. Previous review: Nat. Prod. Rep., 2006, 23, 26
This review covers the literature published in 2005 for marine natural products, with 704 citations (493 for the period January to December 2005) referring to compounds isolated from marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates and echinoderms. The emphasis is on new compounds (812 for 2005), together with their relevant biological activities, source organisms and country of origin. Biosynthetic studies, first syntheses, and syntheses that lead to the revision of structures or stereochemistries, have been included.
 John W. Blunt John W. Blunt | John Blunt obtained his BSc (Hons) and PhD degrees from the University of Canterbury, followed by postdoctoral appointments in Biochemistry at the University of Wisconsin-Madison, and with Sir Ewart Jones at Oxford University. He took up a lectureship at the University of Canterbury in 1970, where he is now a Professor. His research interests are with natural products, and the application of NMR techniques to structural problems. |
 Brent R. Copp Brent R. Copp | Brent Copp received his BSc (Hons) and PhD degrees from the University of Canterbury, where he studied the isolation, structure elucidation and structure–activity relationships of biologically active marine natural products under the guidance of Professors Blunt and Munro. He undertook postdoctoral research with Jon Clardy at Cornell and Chris Ireland at the University of Utah. 1992–93 was spent working in industry as an isolation chemist with Xenova Plc, before returning to New Zealand to take a lectureship at the University of Auckland, where he is currently a Senior Lecturer. |
 Wan-Ping Hu Wan-Ping Hu | Wan-Ping Hu received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied molecular beams with Professor Peter Harland. She carried out postdoctoral research with Professor Stephen Price at University College London before returning to New Zealand where she is currently a research associate at the University of Canterbury working on breath testing projects using SIFT-MS for clinical studies, in addition to maintenance of the MarinLit database. |
 Murray H. G. Munro Murray H. G. Munro | Murray Munro, University of Canterbury, Christchurch New Zealand, has worked on natural products, mainly of New Zealand origin, right through his career. Following a sabbatical with Ken Rinehart at the University of Illinois in 1973, interest in marine natural products developed with a particular focus on bioactive compounds. In recent years his research interests have widened to include terrestrial and marine fungi and actinomycetes as well as marine invertebrates. |
 Peter T. Northcote Peter T. Northcote | Peter Northcote received his BSc and PhD degrees from the University of British Columbia, Canada where he was a member of R. J. Andersen's marine natural products research group. He carried out postdoctoral research with Professors Blunt and Munro at the University of Canterbury before taking a position as a senior research scientist at Lederle Laboratories, American Cyanamid Co. He joined the faculty of the Victoria University of Wellington in 1994 where he is currently an Associate Professor in organic chemistry. |
 Michèle R. Prinsep Michèle R. Prinsep | Michèle Prinsep received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied the isolation and structural elucidation of biologically active secondary metabolites from sponges and bryozoans under the supervision of Professors Blunt and Munro. She undertook postdoctoral research on cyanobacteria with Richard Moore at the University of Hawaii before returning to New Zealand to take up a lectureship at the University of Waikato, where she is currently a Senior Lecturer. |
1 Introduction
This review is of the literature for 2005 and describes 812 new compounds from 283 articles, an increase of ∼13% from the number of compounds reported for 2004. As in previous reviews, we show structures only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described, are referenced, but separate structures are generally not shown. In a departure from past practice, no reference is made in the text to the establishment of absolute stereochemistry when standard methods (e.g. Mosher, Marfey, X-ray crystallography) have been used, unless there are some unusual features associated with the use of these techniques. However, where such determinations have been made for a compound, that compound's identifying number in the diagrams is distinguished by addition of a †. Stereochemistries shown for compounds not labelled with † should be assumed to be relative.It is with great sadness that for a third year we note the passing of a chemist eminent in the field. On 3 February 2006 our colleague Pierre Potier, Professor of Chemistry at the Muséum National d'Histoire Naturelle in Paris, Member of the French Academy of Sciences and former Director of the Institut de Chemie des Substances Naturelles, passed away. Professor Potier was noted for his inspirational work on anticancer drugs and in the marine area for his pioneering marine natural product work in the New Caledonia area including the advancement of girolline towards clinical trials.
2 Reviews
There continues to be a steady stream of reviews on specific topics within marine natural products, but with only one review1 giving overall coverage of the 2003 literature. Several reviews explore the development of marine compounds as drugs.2–6 There have been reviews on aspects of the chemistry and bioactivity of compounds from soft corals,7 cyanobacteria and microalgae,8 cyanobacteria and macroalgae,9 sponges,10,11 cnidaria,12 hoplonomertea, platyhelminthes, polychaetes, bryozoans and hemichordata,13 echinoderms,14–16 ascidians and fish,17 the gorgonian Pseudopterogorgia elisabethae,18 ascidians of the genus Aplidium,19 the sponge genus Halichondria,20 and terpenes from the soft coral genus Sinularia.21 Specific types of bioactivity associated with marine natural products have been reviewed in articles on anticancer drugs,22 agents for treating tuberculosis, malaria, osteoporosis and Alzheimer's disease,23 antifoulants,24 treatments for neurological disorders,25 anti-inflammatory agents,26,27 anti-HIV compounds,28 and topoisomerase inhibitors.29 Growing interest in marine microorganisms is reflected in several reviews.30–35 Reviews on several aspects of the chemistry,36–38 toxicology,39–41 assay,42 and risk factors43,44 of marine toxins have appeared. Particular classes of compounds from marine organisms continue to be well reviewed, with offerings on pyrroloiminoquinone alkaloids,45,46 polymethyleneamine alkaloids,47fatty acids from lipids ,48 indole alkaloids,49,50 guanidine derivatives,51 bromopyrrole alkaloids,52 phenylpyruvic acid oxime derivatives,53triterpenoids ,54 imidazole, oxazole and thiazole alkaloids,55 sponge alkaloids,56 iminium alkaloids,57 diterpene-containing adenine alkaloids,58 unusual sterols from sponges,59 and briarane-related diterpenoids .60 There have been several reviews for different classes of compounds from all natural sources, but where the contributions from marine sources are relatively minor, such reviews have not been noted here. Biosynthesis of marine natural products in microorganisms61 and molluscs62 has been reviewed, and the use of microbial genetics to ensure adequate supplies of marine compounds for development as drugs is surveyed.63 The role of symbiotic bacteria in metabolite production and the analysis of the appropriate biosynthesis gene clusters has been reviewed.64,65 In the first of what is expected to be a continuing series, a broad review on synthetic aspects of marine natural products covering publications in 2003 has appeared.66 More specific reviews that appeared in 2005 relating to the synthesis of marine natural products will be referenced in the third of that series. There has been a useful review of the methods used for the isolation of marine natural products.67 The MarinLit database68 continues to be updated and has again been used as the basis for the preparation of this present review.3 Marine microorganisms and phytoplankton
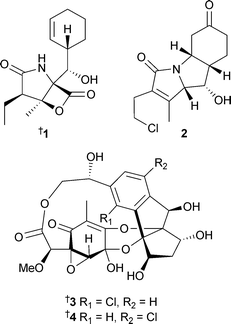
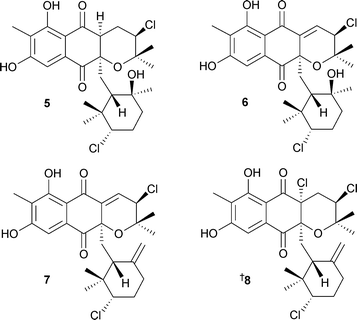
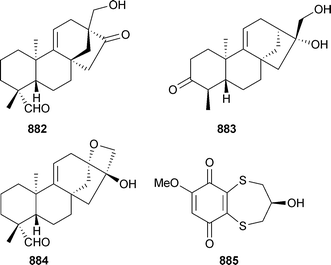
Microorganisms continue to be a productive and successful focus for much marine natural products research. The actinomycete Salinispora tropica, that had previously yielded salinosporamide A,69 also contained several related metabolites, salinosporamides B1 and C270 and the unprecedented chlorinated macrolides sporolides A3 and B4.71The structures of the sporolides are remarkable in that 23 of the 24 carbons of the skeletons are either oxygenated or sp2 hybridised.71 Salinosporamides A and B1 inhibited human colon carcinoma HCT-116 cells with salinosporamide A being the most potent. Salinosporamide A also exhibited extreme potency against several non-small cell lung, CNS and breast cancer cell lines at the NCI.70 A new genus of actinomycete from deep water sediment (La Jolla, California) has yielded three chlorinated dihydroquinones 5–7. Two known analogues were also isolated,72,73 with the absolute configuration of one of these analogues established recently74 and that of the other, 8, reported in 2005.
Compounds 5–8 displayed significant activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VREF), and were cytotoxic to human colon carcinoma HCT-116 cells.75Thallusin9, isolated from an epiphytic marine bacterium obtained from the surface of the green alga Monostroma oxyspermum (Japan),76 is a potent differentiation inducer of M. oxyspermum and induced germination in other green macroalgae.77 A Thermoactinomyces species isolated from mud (Mecherchar, Republic of Palau) was the source of the cyclic peptide mechercharmycin A10 as well as the linear congener mechercharmycin B11.78
Mechercharmycin A10 was active against A-549 lung and Jurkat human leukaemia cell lines, and was also reported as a cytotoxic compound in a patent also published in 2005.79 A yellow pigment 12, an analogue of the tambjamine alkaloids,80–82 has been isolated from the marine bacterium Pseudoalteromonas tunicata83 (source not given).84 Cell-free culture supernatant of the psychrophilic aerobic bacterium P. haloplanktis obtained from seawater (Dumont d'Urville station, Antarctica) contained a diketopiperazine 13. Two further diketopiperazines, known synthetic compounds,85,86 were reported for the first time from a natural source. Two linear peptides 14 and 15, stable to bacterial proteolytic enzymes, were also isolated.
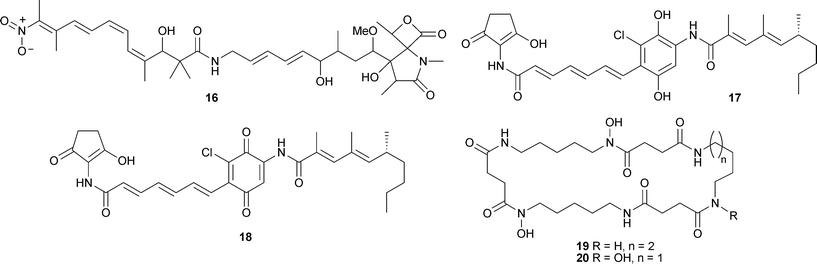
Peptide 15 displayed antioxidant activity in the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay.87Streptomyces nodosus, isolated from marine sediment (Scripps Canyon, California), yielded the antibiotic lajollamycin16 active against both drug-sensitive and drug-resistant Gram positive microorganisms and inhibited growth of the murine melanoma cell line B16-F10.88 The chlorine-containing manumycin derivatives, chinikomycins A17 and B18 were isolated from a Streptomyces species obtained from sediment (Jiaozhou Bay, China). Both 17 and 18 displayed antitumour activity against a number of human cancer cell lines.89
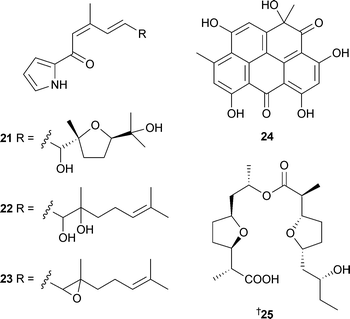
A Streptomyces species isolated from an unidentified sponge (Jaeju Island, Korea) was the source of the cyclic peptides dehydroxynocardamine19 and desmethylenylnocardamine20, weak inhibitors of the recombinant enzyme sortase B.90 The pyrrolosesquiterpenes, glaciapyrroles A–C21–23 were obtained from a Streptomyces species isolated from marine sediment (Alaska). Glaciapyrrole A21 exhibited activity against HT-29 and B16-F10 cell lines.91 A resistomycin derivative, 1-hydroxy-1-norresistomycin24, has been independently isolated from different cultures of sediment-derived Streptomyces species, one from Laguna de Terminos, Gulf of Mexico92 and the other from Streptomyces chibaensis (Bay of Bengal, India).93 Compound 24 was active against E. coli, S. aureus and Streptomyces viridochromogenes92 and cytotoxic to HMO2 (gastric adenocarcinoma) and HePG2 (hepatic carcinoma) cell lines.93Feigrisolide C25, isolated originally from Streptomyces griseus,94 was also isolated from a marine Streptomyces species.95
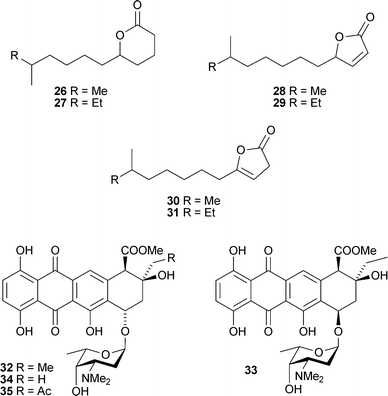
Structures proposed in each instance have been corrected by chemical reactions of natural feigrisolide C and confirmed by total synthesis.96 From a North Sea strain of Streptomyces, new methyl branched γ- and δ-lactones 26–31 were obtained along with two previously reported lactones 97,98 isolated for the first time from a natural source.99 A Streptomyces species from marine driftwood (West Coast, New Zealand) gave four new cytotoxic anthracycline derivatives (7S*,9R*,10R*)-pyrromycin32, (7R*,9R*,10R*)-pyrromycin33, 1-hydroxyauramycin T34 and 1-hydroxysulfurmycin T35.100
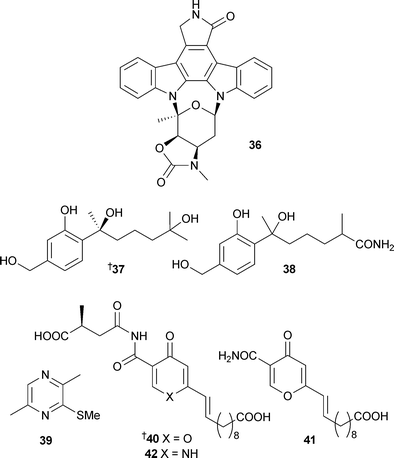
An Actinomadura species from sea sediment (Jiaozhou Bay, China) contained the staurosporine analogue ZHD-050136, an inhibitor of the proliferation of a variety of murine and human cancer cell lines.101 The phenolic bisabolane sesquiterpenoids (+)-curcutetraol37 and (–)-curcutriolamide38 were isolated from a co-culture of unidentified bacterium and fungus separated from marine sediment (Bahamas). Total synthesis of (±)-curcutetraol was achieved in seven steps from o-hydroxy-p-methylacetophenone.1022,5-Dimethyl-3-(methylsulfanyl) pyrazine39, one of several 2,5-dialkylpyrazines identified in extracts of the Alphaproteobacteria Loktanellasp. (North Sea), has been previously reported as a flavouring agent103 but this was the first isolation from a natural source.104Himeic acids A–C40–42 were isolated from an Aspergillus species separated from the mussel Mytilus edulis (Toyama Bay, Sea of Japan).
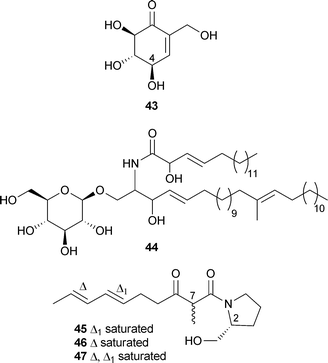
Himeic acid A40 inhibited the ubiquitin-activating enzyme E1.105Aspergillus varians (Sakhalin Island, Russia) yielded the known compound patulin106 and 43, the 4-epi-isomer of a known compound.107 This is the first report of patulin from the marine environment. Both compounds were active against the sea urchin Strongylocentrus intermedius, cytotoxic to embryos and spermatoxic to sperm.108 The cerebroside asperiamide A44 has been reported from a fungus described as Asperillussp. (presumably Aspergillussp. ) isolated from seawater (Mei-Zhou Gulf, China).109Scalusamides A–C45–47, pyrrolidine alkaloids, originated from Penicillium citrinum separated from the gastrointestine of the parrot fish Scalus ovifrons (Hedo Cape, Okinawa).
The absolute configuration of C-2 was established as (R), but all three compounds were shown to be epimeric mixtures at C-7. Scalusamide A45 showed weak antifungal activity against Cryptococcus neoformans and antibacterial activity against Micrococcus luteus.110 The same fungus also yielded the tetracyclic alkaloid , perinadine A48, weakly active against L1210 cells and was antibacterial against M. luteus and B. subtilis.111P. citrinum, separated from the red alga Actinotrichia fragilis (Hedo Cape, Okinawa), was the source of citrinadin B49 and the previously isolated citrinadin A50.112
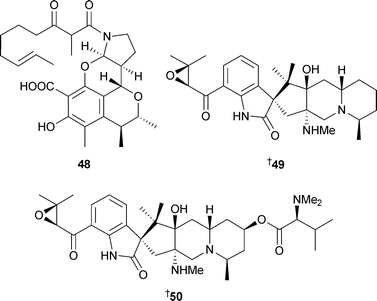
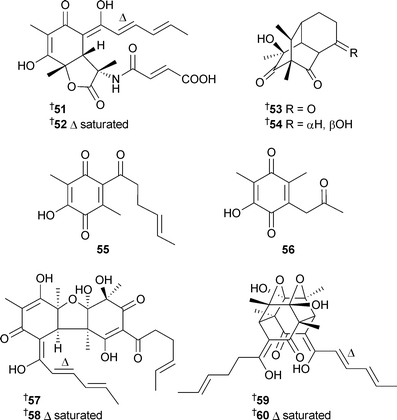
Citrinadin B49 was modestly cytotoxic against L1210 cells.113 Two sorbicillin-derived alkaloids, the sorbicillactones A51 and B52, were isolated from a saltwater culture of P. chrysogenum, separated from the interior of the sponge Ircinia fasciculata (Bight of Fetovaia, Sicily). The biosynthesis of sorbicillactone A51 was examined with 13C-labelled precursors and found to arise via an unprecedented pathway from acetate, S-adenosyl methionine, alanine and a biosynthetic equivalent of fumaric acid. Sorbicillactone A51 was active against HIV, had neuroprotective potential and was selectively active against L5178y cells.114Penicillium terrestre from marine sediment (Jiaozhou Bay, China) generated a number of new metabolites; the polyketides penicillones A53 and B54,115 two benzoquinone derivatives 55 and 56, two bisorbicillinoids 57 and 58116 and the bisorbicillinoids dihydrotrichodimerol59 and tetrahydrotrichodimerol60.117
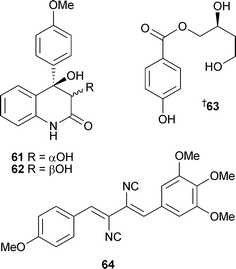
Trichodimerol118–120 was isolated from a marine source for the first time.117 Against the P388 and A-549 cell lines, dihydrotrichodimerol 59 and tetrahydrotrichodimerol 60 were cytotoxic,117 bisorbicillinoids 57 and 58 were antiproliferative,116penicillone A53 displayed weak cytotoxicity while penicillone B54 was weakly cytotoxic to A-549 cells only.115 The diastereoisomeric quinolinones 61 and 62 were isolated from P. janczewskii derived from surface water (German Bight, Helgoland Island). Both compounds were cytotoxic to a range of human tumour cell lines, with 62 strongly cytotoxic to SKOV-3 cells (human ovarian carcinoma).121P. auratiogriseum, derived from the sponge Mycale plumose (Qingdao, China), was the source organism for the benzoate 63, a cytotoxic agent against tsFT210 cells.122 A new xanthocillin 64 and three known xanthocillins,123 all thrombopoietin mimics, were isolated from a marine Basipetospora species (source not given).124
On fermentation, the fungus Clonostachys rogersoniana from the sponge Halichondria japonica (Numazu, Japan), gave the cyclic nonapeptides, clonostachysins A65 and B66, motility inhibitors of the dinoflagellate Prorocentrum micans.125
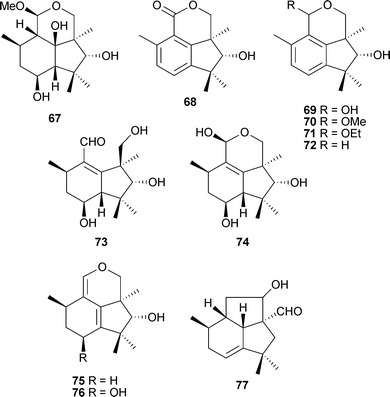
The mitosporic fungus Geniculosporumsp. associated with a red algal Polysiphonia species (Ahrenshoop, Baltic Sea) was cultured to yield eleven new botryane compounds 67–77, some of which displayed modest growth inhibition of alga, bacteria and fungi.126
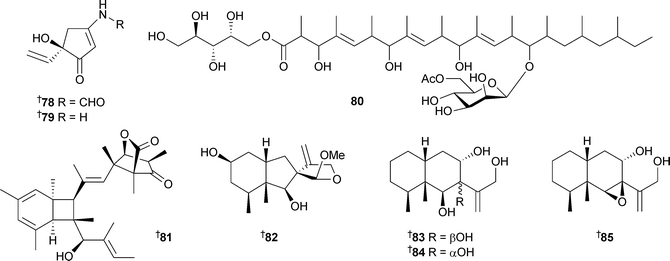
Myrothenones A78 and B79, new amino-5-ethenylcyclopentenones, were obtained from the fungus Myrotheciumsp. isolated from the surface of the green alga Enteromorpha compressa (Busan, Korea). Myrothenone A78 inhibited tyrosinase.127 The polyketide glycoside , cladionol A80, isolated from the fungus Gliocladiumsp. , separated from the sea grass Syringodium isoetifolium (Maeda Cape, Okinawa), was moderately cytotoxic to L1210 and KB cells.128Emericella variecolor, isolated from marine sediment (Gokasyo Gulf, Japan), yielded the polyketide shimalactone A81, a compound which induced neuritogenesis in neuroblastoma neuro-2a cells at low concentration, but was cytotoxic at higher concentration.129Peribysins E–G82–84, from Periconia byssoides, separated from the sea hare Aplysia kurodai (Osaka, Bay of Japan), were inhibitors of adhesion of human leukaemia HL-60 cells to human umbilical vein endothelial cells (HUVEC).130 The absolute configuration of the known peribysin A85131 was established.
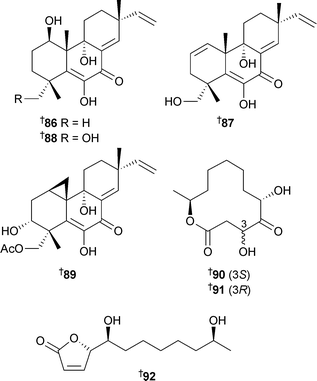
Four novel diterpenoids , libertellenones A–D86–89, were isolated from a co-culture of a marine α-proteobacterium and an established culture of the fungus Libertellasp. separated from an ascidian collected in the Bahamas. The metabolites were not found in pure cultures and are proposed to be of fungal origin. The bacterium used was isolated originally as a contaminant of a fungal culture and was the same strain which induced pestalone production in another marine fungus.132 The libertellenones exhibited cytotoxicity against HCT-116 cells with 89 being the most potent.133 A Cladosporium species associated with the Red Sea sponge Niphates rowi (Gulf of Aqaba) was the source of a hexaketide pandangolide 1a90, and the known diastereomer pandangalide 191,134 along with iso-cladospolide B92.134
Absolute configurations of 90–92 were determined by Riguera's method135 and circular dichroism , and confirmed the absolute configuration of 92 suggested previously.136,137Phomopsis asparagi, from the sponge Rhadphidophlus juniperina (U.S. Virgin Islands), produced chaetoglobosin-51093, chaetoglobosin-54094 and chaetoglobosin-54295 when challenged with the known F-actin inhibitor138 jasplakinolide139/jaspamide140 obtained from Jaspis sponges.
Chaetoglobosin-54295 had antimicrofilament activity and was cytotoxic towards L1210 and C38 cell lines.141Xyloketal G96 was isolated from the marine-derived mangrove fungus Xylariasp. separated from seeds of an angiosperm (Mai Po, Hong Kong),142 while a Xylaria species isolated from seeds of the mangrove Avicenna marina (Hong Kong) produced the dimeric xyloketal F97. Xyloketal F was a potent L-calcium channel blocker while xyloketals A and B143 were also blockers, but less potent.144Diaporthelactone98, and the known compounds 7-methoxy-4,6-dimethyl-3H-isobenzofuran-1-one145 and mycoepoxydiene,146 were isolated from a Diaporthe species, a marine fungus growing in submerged rotten leaves of the mangrove Kandelia candel (Fugong, China), and were cytotoxic against the KB and Raji cell lines.147
Lyngbya majuscula (Alotau Bay, Papua New Guinea) yielded five lyngbyabellin analogues, lyngbyabellins E–I99–103, which were cytotoxic to NCI-H460 and neuro-2a cell lines.148
Lyngbya semiplena (Wewak Bay, Papua New Guinea) was the source of four new depsipeptides , wewakpeptins A–D104–107, cytotoxic to brine shrimp and to the NCI-H460 and neuro-2a cell lines.149Ankaraholides A108 and B109, glycosylated swinholides, were isolated from the cyanobacterium Geitlerinemasp. (Nosy Mitso-ankaraha Island, Madagascar) while swinholide A (previously isolated from the sponge Theonella swinhoei150) was isolated from a cyanobacterium collected in the Fiji Islands. These two findings represent the first direct isolations of swinholides from cyanobacteria, establishing unequivocally that cyanobacteria can produce these metabolites (previously isolated from sponges but localised to heterotrophic bacteria). Ankaraholide A possessed comparable activity to swinholide A, inhibiting proliferation in NCI-H460, neuro-2a and MDA-MB-435 cell lines and inhibiting cytokinesis.151 From the filamentous mat-forming cyanobacterium Phormidiumsp. (Bise, Okinawa) the 2-alkylpyridine alkaloids phormidinines A110 and B111 were isolated.152Majusculoic acid112, a brominated cyclopropyl fatty acid with modest antifungal activity, was isolated from a cyanobacterial mat assemblage (Sweetings Cay, Bahamas).153 When the coastal cyanobacterium Synechococcussp. (Woods Hole Oceanographic Institution) was cultured under iron-limiting growth conditions, the synechobactins A–C113–115 were produced.154
The new amphidinol AM7116, from the dinoflagellate Amphidinium klebsii obtained from a surface wash of a seaweed (Aburatsubo Bay, Japan), is sulfated, has a truncated polyhydroxyl chain, and has the shortest carbon backbone of any of the known amphidinols. AM7116 was active towards Aspergillus niger and also possessed haemolytic activity.155 Five amphidinol analogues, amphidinols 9–13117–121 were isolated from Amphidinium carterae (Kauauroa, New Zealand). All were haemolytic against mouse erythrocytes, cytotoxic against P388 cells and antifungal towards Aspergillus niger to varying degrees. Those containing a sulfate ester at C-1, namely amphidinols 11–13119–121, had much reduced haemolytic and antifungal activities.156Luteophanol D122, a weakly antibacterial polyhydroxy compound, was obtained from an Amphidinium species, isolated from the marine acoel flatworm Pseudaphanostoma luteocoloris (Okinawa).157
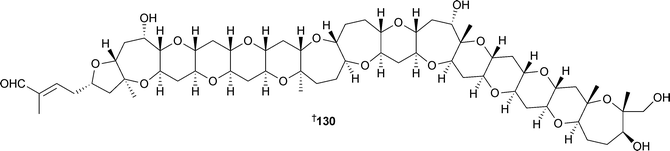
The 26-membered macrolides , amphidinolides B4123 and B5 124, obtained from an Amphidinium species, isolated from a marine acoel flatworm Amphiscolopssp. (Ma'eda Cape, Okinawa), were potently cytotoxic against L1210 and KB cell lines.158 From A. carterae (Hainan Island, China), an unsaturated glycoglycerolipid 125 was isolated,159 while a glycosylated yessotoxin analogue, glycoyessotoxin A126 originated from Protoceratium reticulatum from a culture collection at the Centro Oceanográfico de Vigo.160 Two further yessotoxin analogues, 127 and 128, trihydroxylated amides of 41a-homoyessotoxin and 9-methyl-41a-homoyessotoxin respectively, have been isolated from P. reticulatum, isolate CAWD40161 (Cawthron Institute Culture Collection).16220-Methyl spirolide G129 was isolated concurrently from the microalga Alexandrium ostenfeldii and from the mussel Mytilus edulis (Skjer, Norway).163 The polyether gymnocin-B130, isolated from the red tide dinoflagellate Karenia mikimotoi (Kushimoto Bay, Japan),164 possesses the greatest number (15) of contiguous ether rings known to date.
The absolute configuration was not initially determined, as preparation of MTPA esters was unsuccessful, probably due to steric hindrance,164 but the full absolute configuration was subsequently determined using a variety of approaches including conformational analysis of derivatives and chirality determination based on porphyrin–porphyrin circular dichroism exciton -coupled interactions over distance.165 Gymnocin-B was cytotoxic to P388 cells, but only weakly ichthyotoxic.164K. brevis (Wilson's 58 clone) contained a polyether compound, brevenal131, which competitively displaces brevetoxin from the binding site in rat brain synaptosomes and can antagonise toxic effects of brevetoxins in fish, but is itself non-toxic to fish.166 Artificial cultures of Prorocentrum lima and P. belizeanum from strains obtained from the IEO (Vigo) produced new ester derivatives of okadaic acid: P. lima was the source of 132–134 and P. belizeanum135.167 The polyketide prorocentrin136, an inhibitor of DLD-1 and RP-MI7951 cell lines, was isolated from P. lima (northern coast of Taiwan).168Neosymbioimine137, in addition to the previously reported symbioimine138,169 originated from a dinoflagellate Symbiodinium species separated from the acoel flatworm Amphiscolopssp. (Sesoko Island, Okinawa). Symbioimine138 had anti-resorptive activity,57 and inhibited cyclooxygenase-2 (COX-2) activity.170 An inseparable mixture of polyhydroxylated 61–66-membered macrolides , termed the zooxanthellamide Cs (ZAD-C1 to ZAD-C5) 139–143 was obtained from a Symbiodinium species (a sand beach in Hawaii).171
Detailed NMR spectroscopic analyses determined that the mixture consisted of five macrolactonised analogues of zooxanthellamide A, previously isolated from the same microalgae.172 The mixture displayed vasoconstrictive activity on rat blood vessels.173 From the diatom Thalassiosira rotula (source not given) a series of C16 oxylipins 144–151 were isolated.
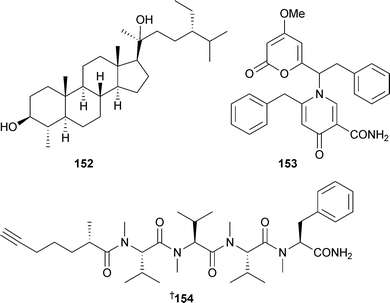
These are formally derived by the unprecedented enzymic oxidation of C16 fatty acids . In support of this, a network of oxygenase-mediated transformations that require C16 fatty acids as substrates was demonstrated.174 A dihydroxysterol 152 was obtained from a phytoplankton Diacronema species (Sampayo, Portugal).175 Aspernigrins A and B were originally isolated from a sponge-derived culture of Aspergillus niger.176 The structure of aspernigrin B153 has now been revised177 based on X-ray analysis of aspernigrin A reisolated from another source.178 The first total synthesis of dragonamide154, a lipopeptide from Lyngbya majuscula,179 was reported, and led to a reassignment of the configuration at the stereogenic centre in the alkyne -bearing fragment.180
Total synthesis of a thiomarinol derivative isolated from Alteromonas rava originally separated from Darwinella rosacea181 has been reported.182 A short stereocontrolled sequence starting from p-methoxyphenol was employed in the total synthesis of yanuthones A–C and 22-deacylyanuthone A,183 originally isolated from Aspergillus niger separated from an Aplidiumsp. ascidian.184 An asymmetric synthesis of abyssomicin C, a polyketide from the actinomycete Verrucosisporasp. ,185 has been reported,186 as has the total synthesis of the heptapeptide cyclomarin C187 from a Streptomyces species.188 Syntheses of semiplenamide C,189 a fatty acid amide isolated from Lyngbya semiplena190 and pitipeptolide A,191 a cyclodepsipeptide originally isolated from L. majuscula have appeared.192 Tryptoquivaline J,193 from Aspergillus fumigatus, has been isolated from a marine strain of the fungus for the first time.194 The known microbial pigment metacycloprodigiosin195 was isolated from the actinomycete Saccharopolysporasp. associated with the sponge Mycale plumose (Qingdao coast, China) and displayed significant cytotoxicity against five human cancer cell lines.196 Bacillomycin D, first isolated from a terrestrial strain of Bacillus subtilis,197 has been isolated from a strain separated from the sponge Stelletta validissima (Kuril Islands, Russia) and showed pH-dependent cytotoxic activity against Ehrlich carcinoma cells198 and was also cytotoxic towards murine erythrocytes.199 A marine-derived Aspergillus species yielded the polyketides (+)-epoxydon,200 (+)-epoxydon monoacetate,201 gentisyl alcohol,202 3-chlorogentisyl alcohol202 and methylhydroquinone203 as potent antibacterial agents against MRSA and multi-drug-resistant Staphylococcus aureus (MDRSA). The first four metabolites also exhibited significant radical scavenging activity against DPPH.204 Gymnodimine, originally isolated from a Gymnodinium species,205 sensitised the neuro-2a cell line to the apoptotic effects of another algal toxin , okadaic acid, raising the possibility that algal blooms involving the production of both of these classes of metabolites may generate greater cytotoxicity and pose more severe public health problems.206Biosynthesis of pentabromopseudilin,207,208 originally isolated from Pseudomonas bromoutilis but also produced by other marine bacteria such as Alteromonas, has been studied. Feeding studies with A. luteoviolaceus demonstrated that the pyrrole ring is derived from proline.209 Biosynthetic studies on Symbiodiniumsp. , the dinoflagellate symbiont of the Caribbean gorgonian Pseudopterogorgia bipinnata, using 3H-labelled geranylgeranyl diphosphate indicated that this alga was capable of biosynthesising diterpenes previously ascribed210 solely to the host coral.211 A fluorescent system has been described that profiles the connection between biosynthetic production and autotoxicity using complementary okadaic acid producing and nonproducing strains of the dinoflagellate genus Prorocentrum.212
4 Green algae
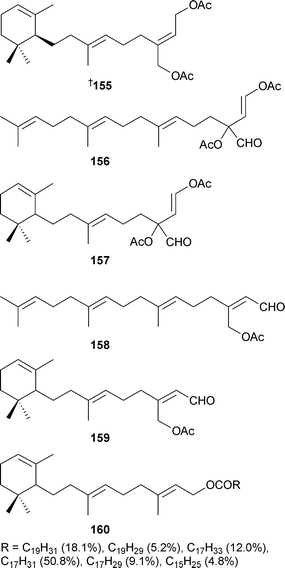
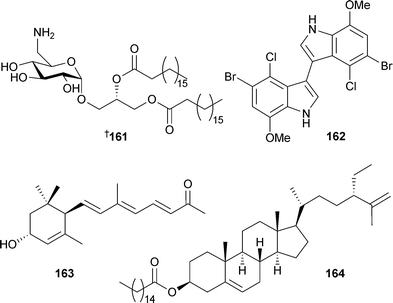
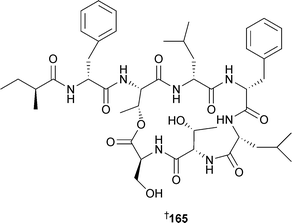
Relatively few new secondary metabolites have been reported from green algae but the number still represents an increase from the 2004 review.213Caulerpa brownii (Tasmania, Australia) occurs in branched and unbranched forms, each of which has yielded a number of novel diterpenoids . Unbranched material was the source of 155–159 and another diterpenoid reported previously as a synthetic compound.214 The branched form yielded the terpenoid ester 160.215Avrainvillea nigricans (Portsmouth, Dominica), yielded the glycoglycerolipid avrainvilloside161 which contains the very rare 6-deoxy-6-aminoglucose moiety.216 A halogenated biindole 162 and an apo-carotenone 163 were isolated from Chaetomorpha basiretorsa (Naozhou Island, China),217 while clerosterol palmityl ester 164 was obtained from Codium fragile (Qingdao coast, China).218 A solid-phase total synthesis of kahalalide A165, isolated from a Bryopsis species and its predator Elysia rufrescens,219,220 established the stereochemistry of the methylbutyrate sidechain as (S).221 The mild antimycobacterial activity of 165 and related analogues was also reported. The long-chain aldehyde (8Z,11Z,14Z)-8,11,14-heptadecatrienal, a component of the essential oil of Ulva pertusa,222 has been synthesised.223
5 Brown algae
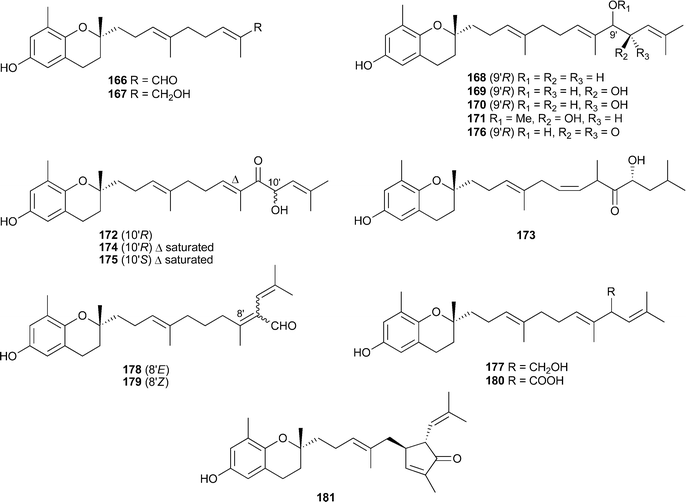
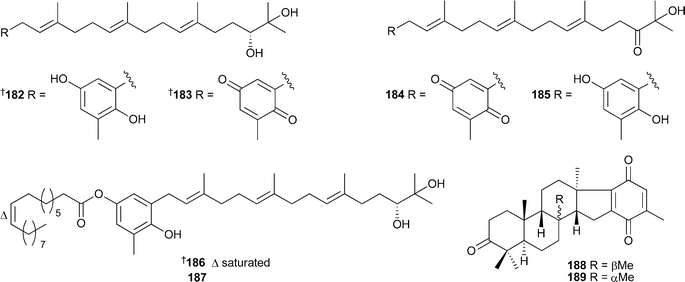
Terpenes and polyphenols continue to be the predominant classes of compounds reported from brown algae. The sargachromanols A–P166–181, meroterpenoids of the chromene class, were isolated from Sargassum siliquastrum (Jaeju Island, Korea).The compounds all exhibited significant activity in the DPPH assay while compounds 172 and 180 were also inhibitors of butylcholine esterase.224 The known plastoquinones 182 and 183225 were isolated from S. micracanthum. Compound 182 displayed significant antioxidant activity while in contrast, 183 was potently active against human cytomegalovirus (HCMV) in vitro.226S. micracanthum (Toyama Bay, Japan) was the source of the strongly antioxidant plastoquinones 184–187, while compounds 185–187 also showed antiproliferative effects against 26-L5 cells.227Taonia atomaria (Serifos Island, Central Aegean Sea), was the source of the meroditerpenes atomarianones A188 and B189, cytotoxic agents against the NSCLC-N6 and A-549 cell lines.228(Z)-Sargaquinone190, the more saturated analogue 191, and the known sargaquinone229 were also isolated from T. atomaria, and were anti-inflammatory agents by inhibition of leukotriene biosynthesis.230
Five meroditerpenoids, 2β,3α-epitaondiol192, flabellinol193, flabellinone194, stypotriolaldehyde195stypohydroperoxide196 and the previously reported stypoldione231 were isolated from Stypopodium flabelliforme (Long Island, Papua New Guinea) and were variously active in a range of bioassays including cytotoxicity, sodium channels as well as modulation of intracellular calcium concentrations.232
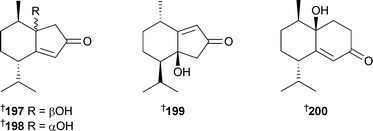
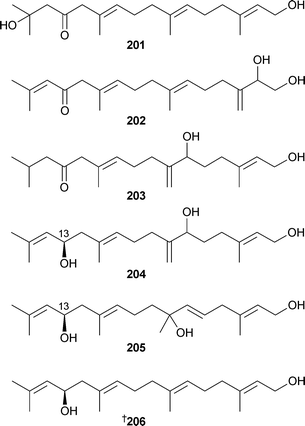
Dictyopteris divaricata (Qingdao, China) was the source of the bisnorsesquiterpenes 197–199 and the norsesquiterpene 200.233 The linear diterpenoids 201–205 were isolated from Bifurcaria bifurcata (Southern Brittany, France),234 and the absolute configuration of C-13 determined for 204 and 205, requiring revision of the stereochemistry of eleganediol206.235
Sargochromenol, originally isolated from Sargassum serratifolium,236 was shown to promote neurite outgrowth and survival in PC12D cells via distinct signalling pathways.237Phloroglucinol238 and the phloroglucinol derivatives eckstolonol,239 eckol,240 phlorofucofuroeckol A241 and dieckol242 were isolated from the brown alga Ecklonia stolonifera as hepatoprotective agents.243 (±)-Yahazunol244 and cyclozonarone245 were cytostatic and cytotoxic agents against several human tumour cell lines, while zonarol, zonarone and isozonarol246 also displayed cytotoxicity against various human tumour cell lines.247
6 Red algae
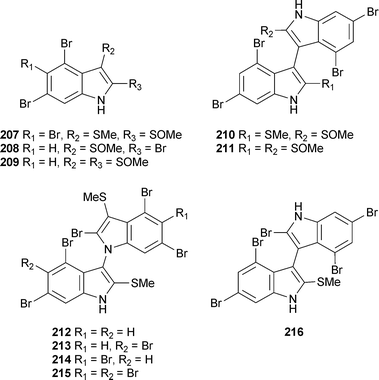
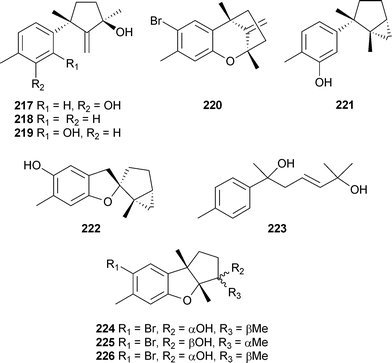
Reports of red algal metabolites have increased from the previous review but the field is still dominated by terpene and halogenated polyphenol chemistry. Five sulfur-containing polybromoindoles 207–211 were isolated from Laurencia brongniartii (Ken-Ting National Park, South Taiwan), of which 210 and 211 were active against P388 cells and 210 against HT-29 cells.248 From L. brongniartii (Kikai Island, Japan), five sulfur-containing polybrominated bisindoles 212–216 were isolated.249A number of sesquiterpenes were isolated from L. tristicha (Naozhou Island, China). These included seven new compounds 217–223250 along with 10-hydroxyepiaplysin224, 10-hydroxyaplysin225 and 10-hydroxybromoepiaplysin226, all previously reported as synthetic intermediates251,252 but now isolated as natural products.253
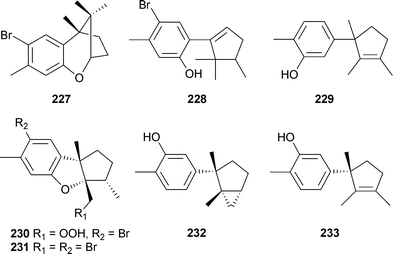
Compound 222 has a novel rearranged skeleton while compound 220 was previously reported as a synthetic racemate.254 Laurebiphenyl, a dimeric sesquiterpene reported previously from L. nidifica,255 was also isolated and exhibited moderate cytotoxicity against several human cancer cell lines.250 The cuparene sesquiterpenes 227–229, isolated from L. microcladia (Chios Island, North Aegean Sea), were cytotoxic against the NSCLC-N6 and A-549 cancer cell lines.256Laureperoxide230, 10-bromoisoaplysin231, isodebromolaurinterol232 and 10-hydroxyisolaurene233 are cuparene-derived sesquiterpenes isolated from L. okamurai (Nanji Island, China).257
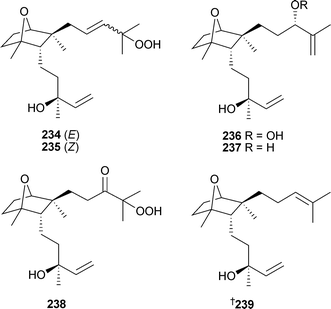
Six diterpene compounds representing a new skeletal type, the dactylomelanes, were isolated from a Laurencia species (Paraíso Floral, Canary Islands). The compounds were the relatively unstable E-dactylohydroperoxide A234 and Z-dactylohydroperoxide A235, dactylohydroperoxide B236, the alcohol 237, dactylohydroperoxide C238 and puctatene239.258
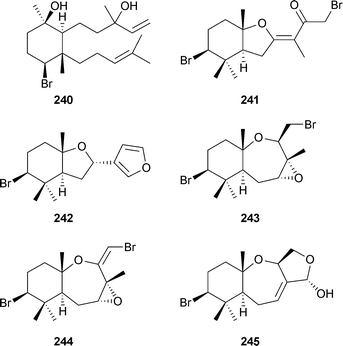
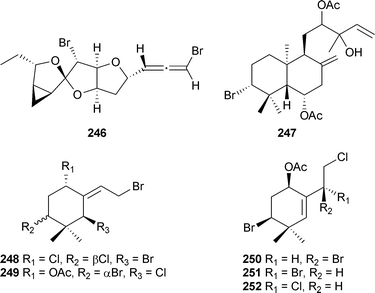
L. luzonensis (Kudaka Island, Okinawa) was the source of the diterpene luzodiol240 and five snyderane sesquiterpenes 241–245.259 A brominated C15 polyketide allene chinzallene246, and a brominated diterpene 247 originated from another Laurencia species (Hatomisaki, Chinzei, Japan). The diterpene was also isolated from a collection made at Hachijo-jina Island, Tokyo.260 From Carpopeltis crispata (Johgashima Island, Japan) the ochtodene derivatives 248–252 were isolated.
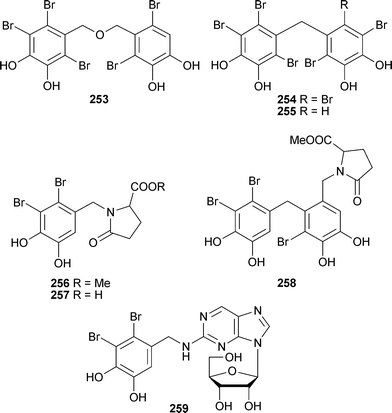
All are regioisomers or stereoisomers of known compounds261,262 from an Ochtodes species.263 Three new bromophenols 253–255 and two bromophenols known previously only as synthetic compounds,264–267 came from Symphyocladia latiuscula (Dalian, China). All of the bromophenols were significant aldose reductase inhibitors.268 Three bromophenols coupled to pyroglutamic acid derivatives 256–258 and a bromophenol coupled with deoxyguanosine 259 were isolated from Rhodomela confervoides (Qingdao, China).
Compound 257 is a possible artifact since in aqueous methanol256 converts to 257 on standing.269 The polyhalogenated monoterpenes , plocoralides A–C260–262 have been isolated from the red alga Plocamium corallorhiza (Kalk Bay, South Africa).
Plocaralides B261 and C262, in addition to three other compounds previously isolated from a Plocamium species270,271 and Aplysia californica,272 displayed moderate activity against the human oesophageal cancer cell line WHCO1.273 From a Galaura species (Xisha Island, South China Sea), the cycloartane triterpenes galaxaurols A–E263–267 were isolated,274 while from Ptilonia magellanica (Straits of Magellan, Chile), three pyranosyl-like polyhalogenated metabolites, the pyranosylmagellanicus A–C268–270, and the putative biogenetic precursor 271 were discovered.275Corallinafuran272 and corallinaether273, a brominated dibenzofuran and diphenyl ether respectively, were isolated from crustose coralline red algae (Yomitan, Okinawa). Both were toxic to larvae of the scleractinian coral Pseudosiderastrea tayamai.276Callophycus serratus collected from several Fijian sites was the source of three antibacterial and antifungal diterpene-benzoate compounds, bromophycolides A274 and B275, and a non-halogenated compound 276. Bromophycolide A274 was also anti-HIV active and cytotoxic to several human tumour cell lines by specific induction of apoptosis.277 Synthesis of agardhilactone277, an eicosanoid isolated from the red alga Agardhiella subulata,278 has been reported and resulted in a revised structure.279
The isomeric conjugated trienes (5E,7E,9E,14Z,17Z)-eicosapentaenoic acid and (5Z,7E,9E,14Z,17Z)-eicosapentaneoic acid, originally isolated from Ptilota filicina,280 have been synthesised from methyl 5-oxopentanoate and found to be cytotoxic to DLD-1 cells by induction of apoptosis.281 A stereoselective and concise total synthesis of (–)-isoprelaurefucin from Laurencia nipponica282 has been completed283 and the total synthesis of (–)-isodomoic acid C, originally isolated from Chondria armata,284 has been reported.285 Free-radical chemistry was utilised in the total syntheses of several terpenoids with 7-membered heterocycles including laukarlaol286 from Laurencia karlae.287 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether,288 isolated from Symphyocladia latiuscula (Pusan, Korea), was active against wild type HSV-1, as well as APr HSV-1 and TK-HSV-1 and significantly delayed the appearance of lesions in infected mice without toxicity.289
7 Sponges
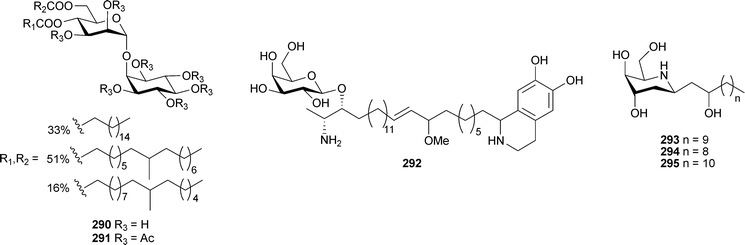
Sponges continue to attract considerable attention from both synthetic and natural product chemists. New ceramides and sphingolipids continue to be isolated from sponges, and the use of tandem MS to detect and determine the structure of these ceramides has been described.290Ircinia fasciculata (China) contained ircisulfamide278 and ircicerebroside279.291Haliclonacerebroside280 was isolated from a Haliclona species and reported in 2004.292 Vesparioside 281 was obtained from Spheciospongia vesparia (Bahamas),293 while an Amphimedon species (Japan) yielded the amphimelibiosides A–F282–287.294The immunostimulatory glycosphingolipid damicoside 288 was isolated from Axinella damicornis (SW coast of Italy).295Rhizochalina incrustata (Seychelles Islands) contained rhizochalin A289.296 The glycolipid discoside 290, isolated as the peracetate derivative 291, was obtained from Discodermia dissoluta (Bahamas).297 A hybrid glycolipid –tetrahydroisoquinoline, oceanalin A292, obtained from an Australian Oceanapia species, was antifungal against Candida glabrata.298 A series of iminosugar-like N-cyclised sphingolipids, batzellasides A–C293–295, obtained from a Batzella species (Madagascar), were inhibitors of Raf kinase.299
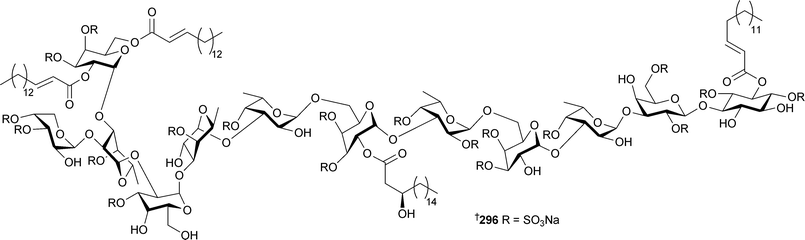
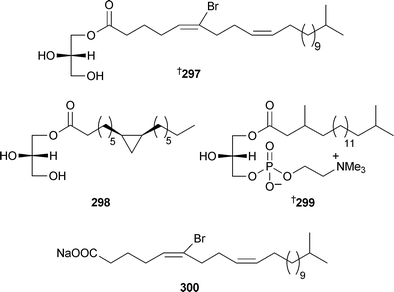
A remarkably complex polysulfated glycolipid , axinelloside A296 with telomerase inhibitory activity, was isolated from Axinella infundibula (Japan).300 Two monoglycerides, homaxinolins A297 and B298, a phosphatidyl choline, homaxicholine A299 and a cytotoxic fatty acid 300 were obtained from a Korean Homaxinella species.301
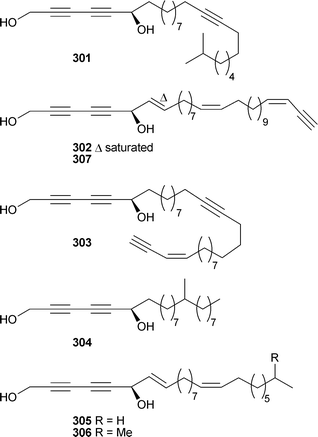
Untenone A302 and plakevulin A,303 originally isolated from Plakortis species, have been synthesised.304 Syntheses of hachijodine A and B,305 originally isolated from Xestospongia and Amphimedon species respectively,306 and three irciniasulfonic acids,307 originally isolated from an Ircinia species,308 have been reported. Two groups have independently reported the synthesis309,310 of pachastrissamine (jaspine B), originally isolated from a Pachastrissa species311 and subsequently from a Jaspis species.312 Caminoside A, from Caminus sphaeroconia,313 has been synthesised.314 A series of acetylenic fatty acid derivatives, strongylodiols D–J301–307, were obtained as non-racemic mixtures of enantiomers from an Okinawan Petrosia (Strongylophora).315
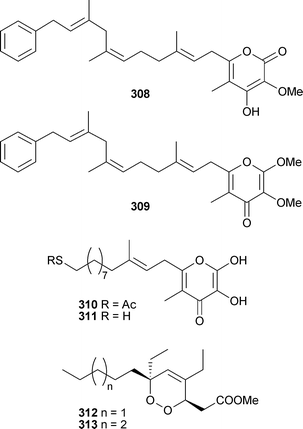
Diplynes C and E, isolated from a Diplastrella species316 have been synthesised asymmetrically allowing tentative assignment of the relative stereochemistries of the series.317 The syntheses of (–)-siphonodiol and (–)-tetrahydrosiphonodiol, isolated from Siphonochalina truncata,318,319 have been reported.320 Methyl peroxyacarnoates A and D, isolated from Acarnus cf. bergquistae321 and bicladotylota322 respectively, have been synthesised.323 Four branched chain polyketide-derived metabolites, lehualides A–D308–311, were isolated from a Plakortis species (Hawaii). Compounds 309 and 311 were cytotoxic to cancer cell lines.324Plakortis simplex (Norway), collected from 280 m by manned submersible, yielded two cycloperoxy-methyl esters 312 and 313 of which 313 was moderately cytotoxic.325
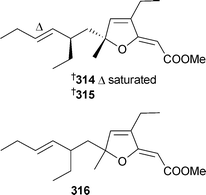
Two furanylidenic methyl esters, 314 and 315, from P. angulospiculatus (Brazil), were cytotoxic to brine shrimp.326Spongosoritin A316 was isolated from a Spongosorites species (Fiji).327
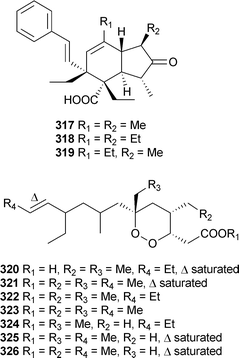
The reported structure of 316 is identical to that of 315 without relative stereochemistry specified. Although the optical rotations were similar, the reported NMR data show some significant differences; these compounds may be diastereomeric. Plakortis zyggompha (Martinique, Caribbean Sea) contained dinor-spiculoic acid317, and the weakly cytotoxic iso-318 and nor-spiculoic acids A319.328 From what appears to be the same collection of P. zyggompha, a series of cytotoxic cycloperoxides 320–326 were also isolated.
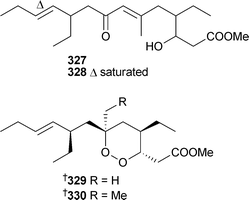
Plakortide Q320 was isolated as an acid while 321–326 were isolated as methyl esters prepared with TMSCHN2.329 Interestingly, a sample of the crude extract of the sponge left standing in methanol for one year yielded the methyl esters directly; this finding may go some way to accounting for the prevalence of methyl esters as reported metabolites of Plakortis species. P. simplex (Bahamas) yielded two enone-containing branched chain metabolites, 327 and 328, and two anti-malarial cycloperoxides, 329 and 330.330
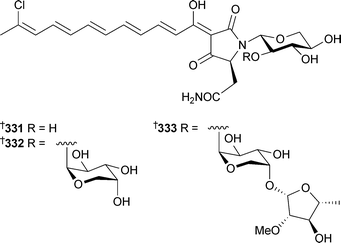
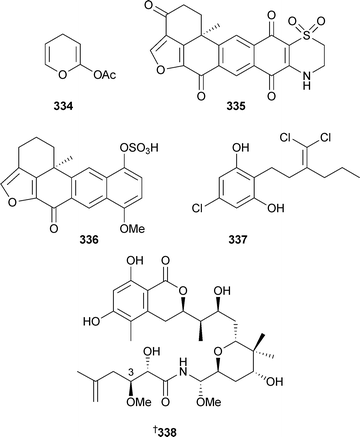
Unaware of the previous report, 329 was named plakortide Q, while 330 has previously been isolated from P. halichondroides without complete relative stereochemistry.331 Methyl (2Z,6R,8R,9E)-3,6-epoxy-4,6,8-triethyl-2,4,9-dodecatrienoate, originally isolated from P. halichondrioides,123 has been synthesised as a racemic mixture.332 Three new aurantosides G–I331–333 have been isolated from Theonella swinhoei (Papua New Guinea).333Ircinia felix (Brazil) was the source of 4H-pyran-20-ol acetate334.334 An inhibitor of the protein-tyrosine phosphatase CDC25A, 335, was isolated from a Xestospongia species (Sulawesi, Indonesia), along with another polycyclic quinone 336.335 A Hyrtios species (Hawaii) yielded a trichloro metabolite poipuol337.336Psymberin338 (irciniastatin A), independently isolated from a Psammocinia species337 and Ircinia ramosa,338 has been synthesised establishing the identity and absolute stereochemistry, the relative stereochemistry at C-3 and confirming the originally proposed stereochemistry for psymberin.339
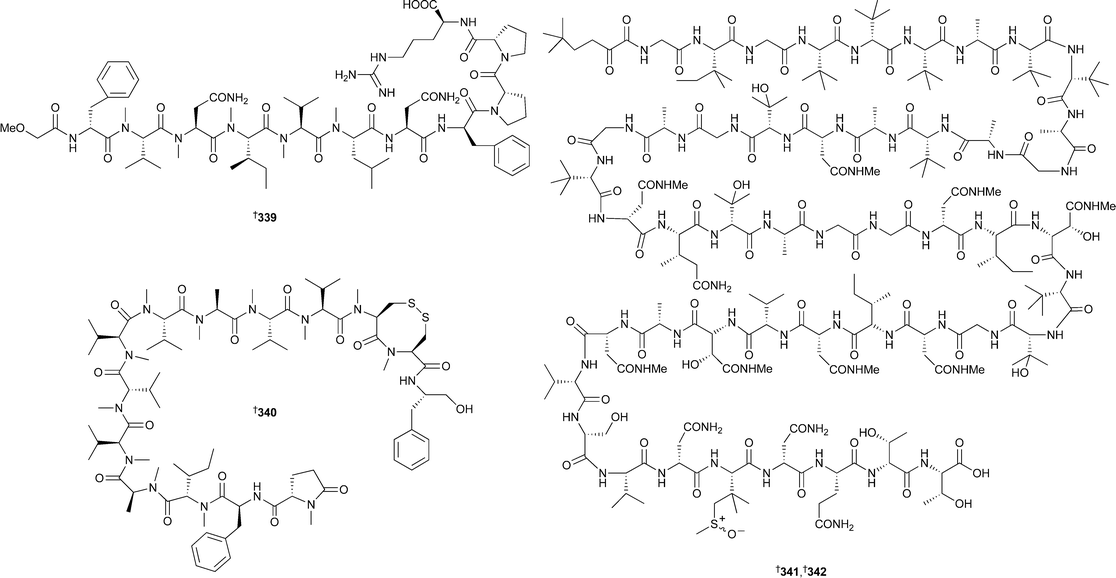
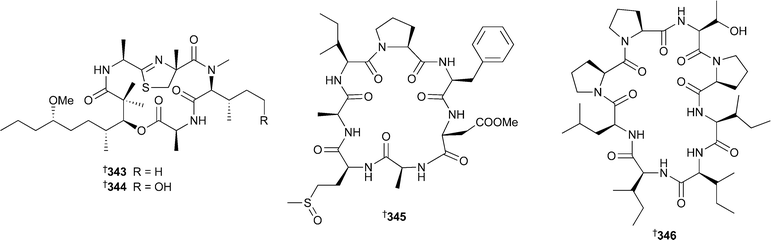
The relative and absolute stereochemistry of the C-1 to C-6 side chain was independently established by synthesis of a degradation product.340 Cylindramide, isolated from Halichondria cylindrata,341 has been synthesised enantiomerically.342 13-Deoxytedanolide, isolated from Mycale adhaerans,343 binds to the 60S large ribosome subunit, thereby inhibiting protein synthesis.344 Two independent groups have shown that pateamine, originally isolated from Mycale hentscheli,345 inhibits eukaryotic protein synthesis initiation.346,347 Potently bioactive peptides of non-ribosomal origin are a feature of sponge chemistry and continue to attract the attention of natural product and synthetic researchers alike. The moderately cytotoxic koshikamide A2339 was isolated from the same Japanese Theonella species that yielded koshikamide A1.348 A Haliclona species contained kendarimide A340. By synthesising a series of diastereoisomeric tetrapeptides corresponding to the region in 340 containing the 8-membered disulfide ring system it was concluded that the two contributing cysteine residues had an L configuration.349 The picomolar active cytotoxins polytheonamides A341 and B 342, originally isolated from Theonella swinhoei,350,351 have been re-isolated from the same sponge, and from spectroscopic and chemical analyses the structures have been revised to those of the sulfoxide epimers .352
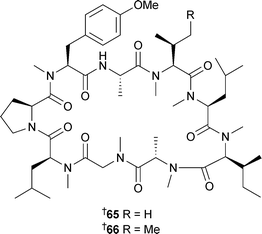
Halipeptin D343 has been isolated from Leiosella cf. arenifibrosa (Philippines),353 while halipeptins A354344 and D343 have been synthesised, confirming the presence of the thiazoline ring and establishing the relative stereochemistry of the side chain and absolute configuration . Intriguingly, the reported potent biological activity of halipeptins A and D was not confirmed by the synthesis, as the synthetic samples both showed weak cytotoxicity only.355 A Phakellia species (Chuuk, Micronesia) yielded the cytotoxic peptide phakellistatin 14345.356Dominicin346 was isolated from Eurypon laughlini (Dominica).357
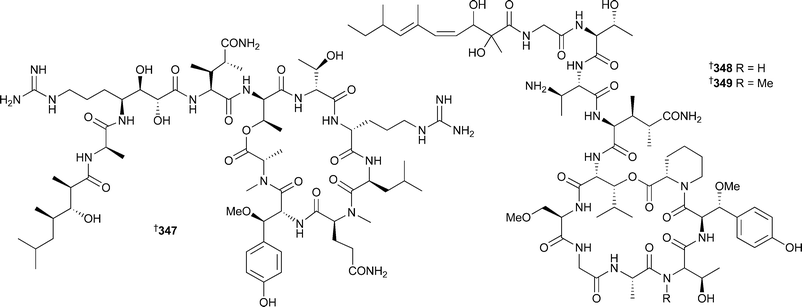
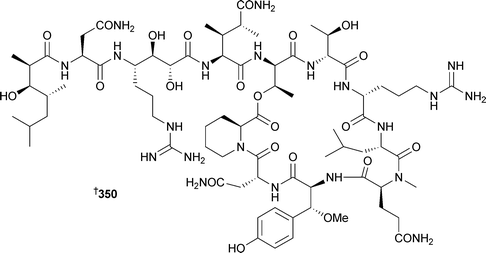
Syntheses of phakellistatin 7 and the proline-rich phakellistatins 8 and 9, all isolated from Phakellia costata,358 have been reported.359 The synthetic phakellistatin 7 was spectroscopically identical to the natural compound while data for the phakellistatins 8 and 9 differed, again highlighting the difficulties associated with proline cis –trans isomerism . Also, all compounds were only weakly bioactive, in contrast to the potent activities originally reported.358 The determination of the stereochemistry of β-methoxytyrosine (β-MeOTyr) found in several sponge-derived cyclic peptides has received considerable attention. Callipeltin A347, originally isolated from a Callipelta species (New Caledonia), was reported without stereochemistry at the β-MeOTyr residue due to degradation during acid hydrolysis.360 All four possible stereoisomers of β-MeOTyr were synthesised and subjected to oxidative ozonolysis to yield the corresponding β-methoxyaspartates. Callipeltin A was then subjected to ozonolysis and acid hydrolysis. Marfey's analysis of the synthetic and callipeltin derived residues established the β-MeOTyr residue as (2R,3R).361 The same general technique of hydrolysis and oxidation was used to determine a (2R,3R) configuration for the β-MeOTyr of papuamide B348,362 isolated from a Theonella species.363 In a similar study, the stereochemistry of β-MeOTyr in papuamide A349, originally isolated from two species of Theonella,363 was determined.
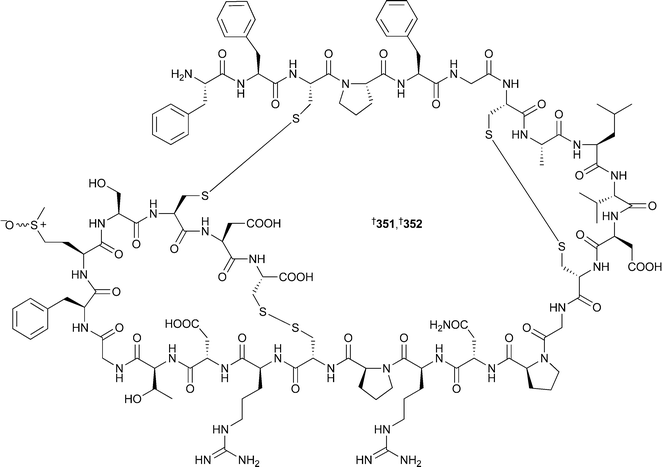
In this case all four stereoisomers of β-MeOTyr were synthesised and incorporated into a tripeptide corresponding to the β-MeOTyr tripeptide sequence originally recovered from partial hydrolysis of 349. Comparison of the NMR spectra led to the assignment of this residue as (2R,3R) as above.364 Rather surprisingly, the β-MeOTyr of neamphamide A350, isolated from Neamphius huxleyi,365 was (2S,3R) in contrast to the above examples. In the same study, the remaining stereochemical uncertainties were determined: 4-amino-7-guanidino-2,3-dihydroxyheptanoic acid was assigned as (2R,3R,4S), and 3-hydroxy-2,4,6-trimethylheptanoic acid as (2R,3R,4R), establishing the full stereochemistry of the molecule.362 Two epi-sulfoxides, neopetrosiamides A351 and B 352, found to inhibit amoeboid invasion by human tumour cells, were isolated from a Neopetrosia species (Papua New Guinea).366
Macrolides and related uncyclised PKS-derived molecules feature strongly in sponge metabolite chemistry. A series of potently cytotoxic 1,3-polyols, the mycapolyols A–F353–358, have been obtained from Mycale izuensis (South Japan).367 Another Japanese Mycale species yielded secomycalolide A359, an inhibitor of proteasome activity.368
Spongistatin 1, isolated from a Spongia species,369 was found to be an effective antifungal agent in vitro as well as in vivo in mice against Candida albicans and Cryptococcus neotormans.370 The structure of swinholide A, isolated from Theonella swinhoei,150 bound to actin has been determined, revealing a similar binding site to the trioxazole sponge metabolites.371 A synthesis of haterumalide NA, isolated from an Ircinia species,372 has been reported.373 The original structure of clavosolide A, from Myriastra clavosa (Philippines),374 has been synthesised and on the basis of the very similar, but not identical spectroscopic data, it was proposed that the natural compound was a diastereomer of the synthetic compound with inversion of the stereogenic centres at the cyclopropyl rings.375 A pentabrominated diphenyl ether 360 has been isolated from Dysidea herbacea (Great Barrier Reef, Australia),376 and two other diphenyl ethers, 3,5-dibromo-2-(2′,4′-dibromophenoxy)phenol and 4,6-dibromo-2-(2′,4′-dibromophenoxy)phenol, previously isolated from Dysidea herbacea,377 are inhibitors of the Tie2 kinase involved in angiogenesis.378 A general synthetic strategy for the bastadins, macrocyclic brominated phenyl ethers typical of Ianthella basta,379–382 has been reported with syntheses of bastadins 5, 10, 12, 16, 20 and 21 achieved.383 The importance of marine sponges as a source of novel alkaloids, in particular those with antitumour activity, has been recognized and reviewed.56 A series of pyrrolidinones, dysidamides D–G361–364, epi-G365 and H366, and the related seco compounds 367 and 368 were isolated from Lamellodysidea herbacea (formerly Dysidea) (Red Sea).384
The relative stereochemistry of dysithiazolamide369, from a Dysidea species (Sulawesi, Indonesia), was determined by an extension of the J-based Murata method to include nitrogen substituents.385 The nematocidal agents, echinobetaines A370 and B 371, were isolated from a species of Echinodictyum (S. Australia), and syntheses reported.386,387 In the same study, norzooanemonin372 was reported from a variety of Australian sponges.387 An adrenaline analogue, S1319 originally isolated from a Dysidea species,388 has been synthesised.389 An alkyl pyridinium dimer 373 with agonist activity against chemokine receptor CXCR3 was isolated from an unidentified sponge (Bahamas).390
The battle over determining the true structure of pyrinodemin A rages on.12,13Alkene positional isomers have been synthesised and the NMR and MS data compared with data from the natural product, originally isolated from an Amphimedon species.391 The closest match was found to be 374, but the authors concede that until more natural material is available for a complete spectral analysis, some doubt remains.392 In a separate study the same alkene isomers were synthesised asymmetrically and compared by chiral HPLC analysis to a sample of the natural substance and were found to co-elute with a racemic mixture of 375.393Makaluvic acid C376 and the cytotoxic N-1-β-D-ribofuranosylmakaluvic acid C377 were both isolated from Strongylodesma aliwaliensis (E. coast of South Africa).394 A Latrunculia species (New Zealand) contained the cytotoxic dimeric discorhabdin W378.395
Zyzzya fuliginosa (E. Australia) yielded the moderately cytotoxic zyzzyanones B–D379–381.396 A series of bromoindole metabolites 382–385 were isolated from a Thorectandra species (Papua New Guinea).397 In the same study, two further related metabolites 386 and 387 were described from a Smenospongia species (Papua New Guinea).
A range of metabolites from both sponges displayed activity against Staphylococcus epidermis. A Psammoclemma species (New Caledonia) yielded the cytotoxic echinosulfonic acid D388.398Dendridine A389, a bis-indole alkaloid with antimicrobial activity, was obtained from a Dictyodendrilla species (Okinawa).399 (6R)-6″-Debromohamacanthin A390, (6R)-6′-debromohamacanthin A391, (+)-(S)-6″-debromohamacanthin B392 and dibromodeoxytopsentin393 were isolated from a Spongosorites species (Korea).400 In an independent study, (–)-(R)-6′-debromohamacanthin B394 was obtained from what appears to be the same Korean Spongosorites species.
Although the structure illustrated is the 6′-debromo derivative, it is referred to in the text of this study as the 6″-debromo derivative. Since the NMR solvents used in the two studies were different, a direct comparison of data cannot be made. In this second report, a series of related known metabolites were also isolated, several of which (deoxytopsentin, bromodeoxytopsentin and bromotopsentin) were inhibitors of bacterial sortase A.401 Three moderately cytotoxic indole alkaloids, hyrtioerectines A–C395–397, were isolated from Hyrtios erectus (Red Sea, Egypt).402 An Okinawan unidentified member of the Thorectidae family contained the antibacterial imidazole-β-carboline alkaloid gesashidine A398.403
Three new manzamine-type alkaloids 399–401 were isolated from an Acanthostrongylophora species (Indonesia) and reported in 2004. Compound 401 had antimalarial and antituberculosis activity.404 The synthesis of the (–) antipode of cis -dihydrohamacanthin B, originally isolated from Rhaphisia lacazei,405 was reported.406 From Axinella damicornis (Corsica), the alkylpyridinium-pyrrole alkaloid daminin402 was isolated and synthesised. While lacking cytotoxicity, the compound was found to inhibit Ca2+ uptake by neurons.407Petrosamine B403, an inhibitor of the Helicobacter pylori enzyme aspartyl semialdehyde dehydrogenase, was isolated from an Oceanapia species (Gulf of Carpentaria, Australia).408
Variolin B, originally isolated from Kirkpatrickia variolosa,409 and known to be a potent antitumour agent, inhibits cyclin dependent kinase (CDK) activity causing p53-independent apoptosis.410 An enantiospecific synthesis of cribrostatin IV, isolated from Cribrochalina vasculum,411 has been achieved.412 Renieramycin G, originally isolated from Xestospongia caycedoi,413 has been independently synthesised in both racemic414 and enantiospecific415 forms. The polyphenolic alkaloid dictyodendrin B, isolated from Dictyodendrilla verongiformis,416 has been synthesised.417Laughine404, a guanidine-bromopyrrole alkaloid , was isolated from the same Dominican sponge, Eurypon laughlini, as dominicin346 described earlier in this review.357Didiscus oxeata (Jamaica) was found to contain mukanadin D405.418
Cyclooroidin, originally isolated from Agelas oroides,419 has been synthesised.420 Enantiospecific syntheses of (S)-(–)-longamide B, (S)-(–)-hanishin and (S)-(–)-longamide B methyl ester have been reported establishing the configuration of the laevorotary enantiomers of these compounds.421 Two ptilomycalin/crambescidin-type guanidine alkaloids, batzelladine J406 and crambescidic acid407 were obtained from Monanchora unguifera (Caribbean coast of Panama).422 A total synthesis of crambescidin 431, originally isolated from a Monanchora species,423 has been achieved.424(–)-Crambidine408, originally isolated as the tetraacetate from Crambe crambe,425 has been synthesised establishing the relative stereochemistry.426 A bromotyrosine derived carbamic acid methyl ester 409 was isolated from Psammaplysilla purpurea (Southern India).42711-N-Methylmoloka'iamine410, 11-N-cyano-11-methylmoloka'iamine411 and kuchinoenamine412, all with antibacterial activity against the fish pathogen Aeromonas hydrophila, were obtained from a Hexadella species (Japan).428
A FOXO1a inhibition assay was used to isolate psammaplysenes A413 and B 414 from a Psammaplysilla species (Indian Ocean),429 while in a separate paper, the syntheses are reported.430Pseudoceratina purpurea (Gulf of Thailand) contained purpuroceratic acids A415 and B416.431 The C-11 stereochemistries of fistularin-3417 (from Aplysina fistularis,432) and 11-epi-fistularin-3418 (from Agelas oroides433) were determined as (11S) and (11R) respectively. The variability of the C-11 stereochemistry of these, and related compounds from different organisms, was examined.434 Synthesis of the proposed structure of calafianin, originally isolated from Aplysina gerardogreeni,435 and the 6,6′ diastereomer 419 confirmed that 419 was the correct structure of the natural compound.436
A species of Erylus (S. Australia) was the source of N3,5′-cycloxanthosine420, the first naturally occurring cyclonucleoside, but already known as a synthetic product.437 Agelasine E, originally isolated from an Agelas species,438 has been synthesised.439 A synthesis of the proposed structure of epi-agelasine C, originally isolated from Agelas mauritiana,440 led to a structural revision of epi-agelasine C to 421 and of agelasine C, also isolated from an Agelas species,441 to 422.442 Four merosesquiterpenes, 20-O-acetyl-21-hydroxy-ent-isozonarol423, 20-O-acetylneoaverol424, ent-yahazunol425 and dysienone426 were obtained from two Dysidea species (Gulf of California).
Compounds 423 and 426 were cytotoxic.443Dysidea cf. cristagalli (New Zealand) yielded the previously described 20-O-acetyl-21-hydroxy-ent-isozonarol423, as well as 20-O-acetylneoaverol424 and the deacetylquinone analogue 427. Both new compounds inhibited superoxide production by human neutrophils.444Hatarumadienone428, a nor-puupehenone type meroterpenoid isolated from a Dysidea species (Okinawa), inhibited the division of fertilised sea urchin eggs.445
A racemic synthesis established that smenochromene D (Smenospongia species446) and likonide B (Hyatella species447) were enantiomers.448Strongylophorines 13–19429–435, isolated from Strongylophora strongylata (Okinawa), inhibited the maturation of starfish oocytes.449
Two polyprenylated hydroquinones 436 and 437, inhibitors of leucotriene production in porcine leukocytes, were isolated from Ircinia spinosula (Greece).450
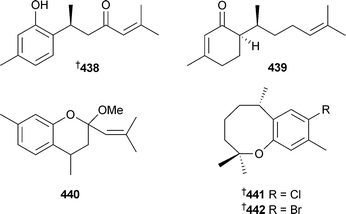
Pelorol, originally isolated from Dactylospongia elegans451 and Petrospongia metachromia,451 was found to be a Src homology inositol-5-phosphatase (SHIP) activator, and was synthesised from (+)-sclareolide confirming the previous assignment of absolute stereochemistry.452 Similarly, (–)-15-oxopuupehenol, originally isolated from a Hyrtios species,453 was synthesised from (–)-sclareol.454 A microwave-mediated Claisen rearrangement was used to synthesise panicein, originally isolated from Halichondria panicea.455,456 Three bisabolene-type sesquiterpenoids 438–440 were reported from a Myrmekioderma species (Vanuatu).457 Two halogenated derivatives of helianane 441 and 442 were obtained from Spirastrella hartmani (Martinique).458
An ethanolic extract of Dysidea arenaria (New Caledonia) yielded an ethyl ether, 9-hydroxyfurodysinin-O-ethyl lactone 443, which the authors suggest was an artifact of the ethanolic extraction process .459 A Halichondria species (Okinawa) yielded a series of antimicrobial nitrogen-containing eudesmane sesquiterpenoids , halichonadins A–D444–447.460 A new curcuphenol analogue 448, several dimeric dicurcuphenols A–E449–453 and dicurcuphenol ether F454 were obtained from Didiscus aceratus (Papua New Guinea).
The dimers were synthesised from curcuphenol using the enzyme laccase, and the absolute stereochemistries of the biphenyl axes of 450 and 451 were determined using vibrational (IR) CD spectra.461 Both (+)-(8S)-12-nordrimane-8,11-diacetate and (+)-11-hydroxy-12-nordrim-9-en-8-one, originally isolated from a Dysidea species,462 have been synthesised.247 The isocopalane diterpenoid mycgranol 455 was isolated from Mycale aff. graveleyi (Kenya).463Axinyssa tethyoides (Okinawa) contained two 13-epi-neoverrucosanyl sulfates 456 and 457.464 Two rearranged spongane diterpenoids , omriolides A458 and B459 were obtained from Dictyodendrilla aff. retiara (Kenya).465
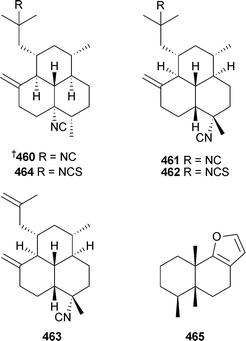
A re-isolation of amphilectene diterpenoids , originally isolated from a Caribbean Cribrochalina species,466 has led to a reassignment of the absolute stereochemistry of 460 and the relative stereochemistries of 461–463. A new compound 464 was also described in the same study.467 A re-isolation of microcionin-1465 from Fasciospongia cavernosa, but originally isolated from Microciona toxystila,468 established the relative stereochemistry with the unusual cis-fused ring A/B system.469
(–)-Marginatone, from Aplysilla glacialis,470 has been synthesised confirming the absolute stereochemical assignment.471 Three partially cyclised sesterterpenoids 16-oxoluffariellolide466, 16-hydroxyluffariellolide467, and 468, all with phosphatase Cdc25B inhibitory activity, were isolated from a Thorectandra species (Papua New Guinea).47219-Methoxyscalarin469, from Hyrtios erectus (China), was reported in 2004.473
The hyrtiosins A–E470–474, scalarane type sesterterpenoids , were isolated from two separate collections of Hyrtios erecta (Hainan Island, China).474Hyrtios erecta (Maldives) yielded the cytotoxic sesterstatin 6475,475 while a collection of the same sponge from the Egyptian Red Sea yielded sesterstatin 7476.476
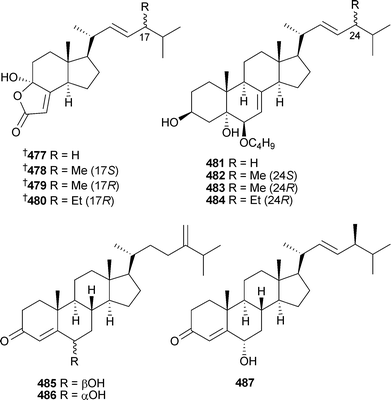
Ircinin-1, originally isolated from Ircinia oros,477 has been found to induce cell cycle arrest and apoptosis.478 Enantiospecific syntheses of luffolide, isolated from a Luffariella species,479 16,25-dihydroxycheilantha-13(24),17-dien-19,25-olide and 25-hydroxycheilantha-13(24),15,17-trien-19,25-olide, isolated from an Ircinia species,480 have been reported.481 A series of cytotoxic, highly degraded steroids , demethylincisterols A1–A4477–480 were isolated from a Homaxinella species (Korea). In the same study, four butoxy-derivatised sterols , homaxisterols A1–A4481–484, were also isolated from the same sponge.482Iotrochoto birotulata (Hainan Island, China) contained three 4-en-3-one sterols 485–487.483
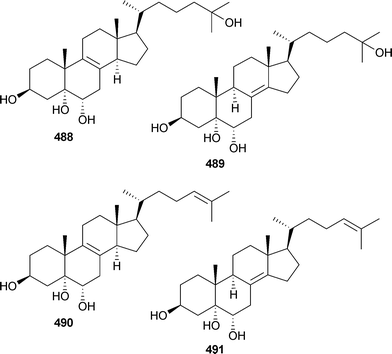
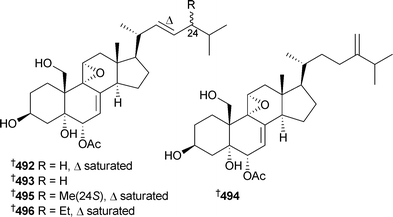
Several polyhydroxylated sterols 488–491 were isolated from Lamellodysidea (Dysidea) herbacea (Red Sea), and 490 and 491 were active against Candida albicans.484 Another series of polyhydroxylated sterols , dysideasterols A–E492–496, were isolated from a Dysidea species (Hainan Island, China).485
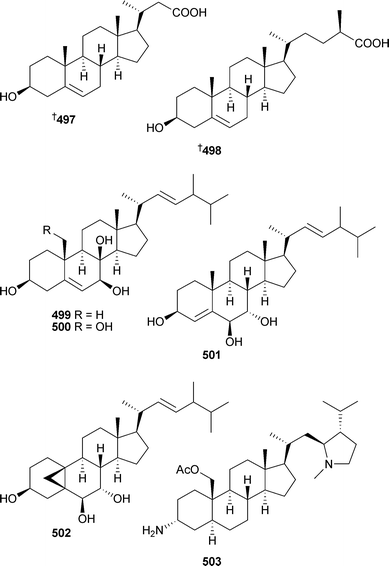
Two moderately cytotoxic steroids 497 and 498 were obtained from a new species of Euryspongia (Vanuatu).486Stylisterols A–C499–501 and the unusual ring-fused cyclopropane-containing hatomasterol502 were obtained from a Stylissa species (Okinawa).487 The cytotoxic steroidal alkaloid plakinamine I503 was isolated from a Corticium species (Vanuatu).488
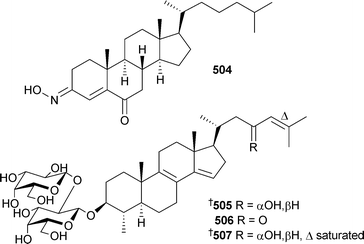
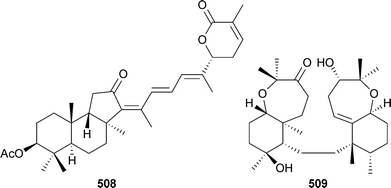
An unusual oxime containing steroid , (3E)-cholest-4-en-3,6-dione-3-oxime504 was isolated from Cinachyrella australiensis (China).489 Two steroidal glycosides , erylosides K505 and L 506 from Erylus lendenfeldi (Red Sea), were selectively active against a yeast strain defective in double stranded DNA repair. The absolute stereochemistry of eryloside A507, originally isolated from the same sponge, has also been established.490 A Jaspis species (Hainan Island, China) yielded 22,23-dihydrostellettin D508.491 The cytotoxic triterpenoid sodwanone S509 was obtained from Axinella weltneri (Comoros Island, Indian Ocean).492
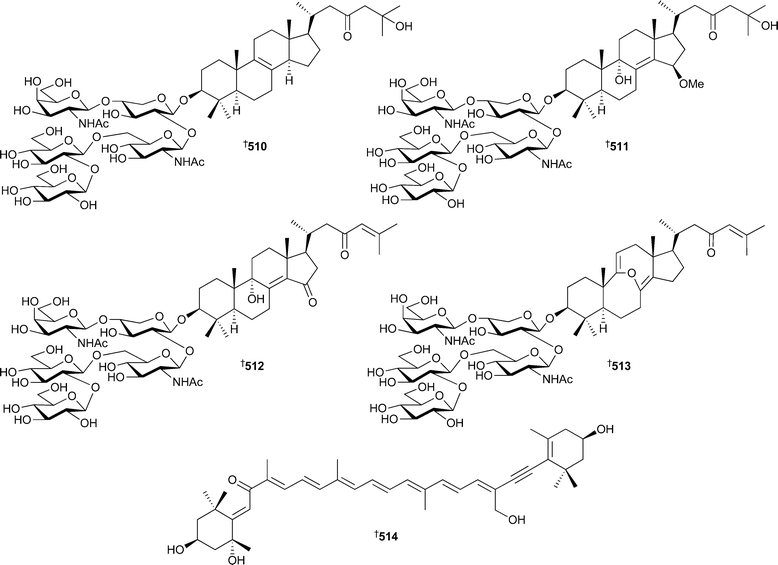
The sarasinosides J–M510–513, triterpene glycosides isolated from Melophlus sarassinorum (Sulawesi, Indonesia), were antimicrobial to B. subtilis and S. cerevisae.493Prianos osiros (Pohnpei, Micronesia) contained the unusual cytotoxic carotenoid 514.494
8 Coelenterates
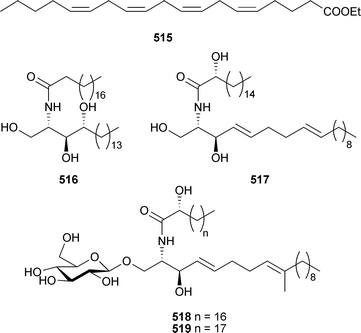
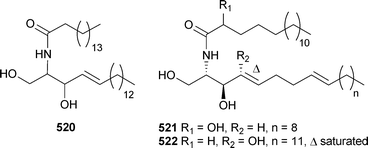
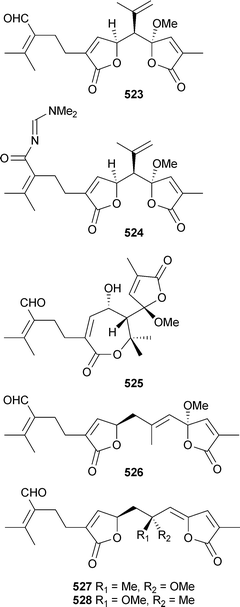
There has been a noticeable increase in the number of new metabolites reported from coelenterates over the 2002–2005 period. The fatty acid ethyl ester 515 was isolated from the soft coral Nephthea hainansis (China), along with a sesquiterpene metabolite (see later).495 The polyacetylene carboxylic acids montiporic acids A and C, previously reported from Montipora digitata,496,497 are potent feeding attractants of the coral predator mollusc Drupella cornus.498 (±)-1-Nonadecyloxy-2,3-propanediol, previously reported from the sponge Desmapsamma anchorata,499 was identified as a weakly cytotoxic constituent of Dendronephthya gigantea (Korea).500 Three sphingolipids 516–518 and a sterol (see later) were isolated from Lobophytumsp. (Indian Ocean).501 A homologue of 518, 519, was obtained from a Lobophytum species (South China Sea).502The dominant sphingolipid of Subergorgia suberosa (Indian Ocean) was 520,503 while confertamides A521 and B522 were identified in extracts of Sinularia conferta (China).504 A structurally-related sphingosine derivative,505 re-isolated from Sinularia crassa (Indian Ocean), exhibited anti-inflammatory activity.506Caucanolides A–F523–528 were isolated from Pseudopterogorgia bipinnata (Colombia),507 and while 523 and 526 exhibited mild activity towards Plasmodium falciparum, 523 also exhibited cytotoxicity towards a panel of tumour cell lines.
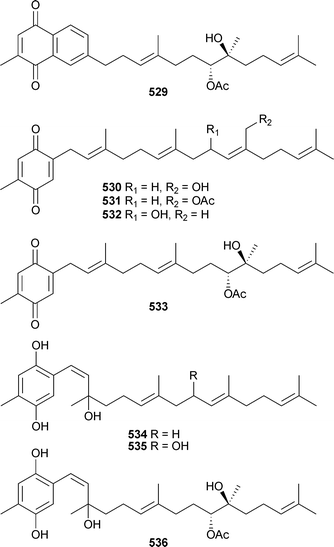
Meroditerpenoids chabrolonaphthoquinone B529, chabrolobenzoquinones E–H530–533 and chabrolohydroxybenzoquinones E–G534–536 were isolated as mildly cytotoxic constituents of Nephthea chabrolii (Taiwan).508
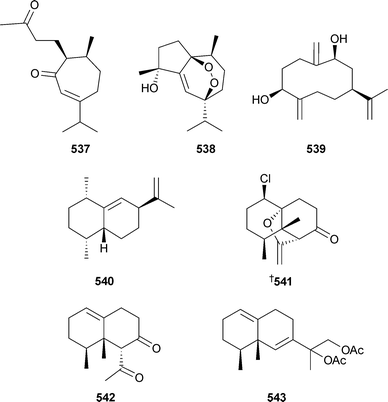
Oxygenated sesquiterpenes gibberodione537, peroxygibberol538 and sinugibberodiol539 were isolated from Sinularia gibberosa (Taiwan).509 Mild cytotoxicity was ascribed to 538. Bicyclic sesquiterpene 540 was reported from Nephthea hainansis (China).495 Chlorinated norsesquiterpene paralemnolin A541 and co-metabolites B542 and C543 were isolated from the soft coral Paralemnalia thyrsoides (Taiwan).510
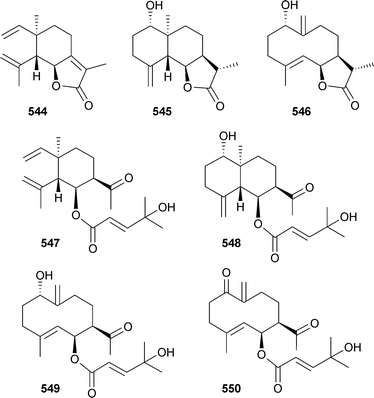
Seven sesquiterpenoid metabolites 544–550, comprising elemane, eudesmane and germacrane skeletal types, were reported as mildly antiplasmodial constituents of a Eunicea species (Caribbean).511
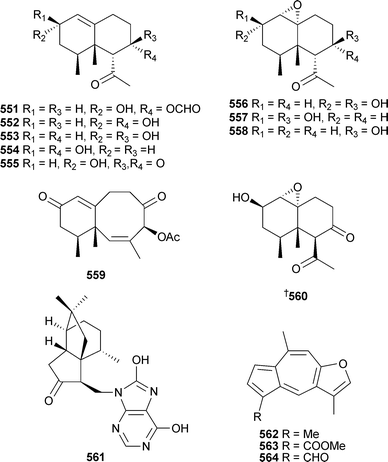
Laevinols A–H551–558, laevinone A559 and the known decalin 560 were isolated from Lemnalia laevis (Taiwan).512 The sesquiterpene alkaloid 561 was reported as a mildly cytotoxic metabolite of the gorgonian Subergorgia suberosa (China).513Linderazulene562 and two new congeners 563 and 564 were isolated as mildly cytotoxic constituents of a deep-sea collection of the gorgonian Paramuriceasp. (Curaçao).514
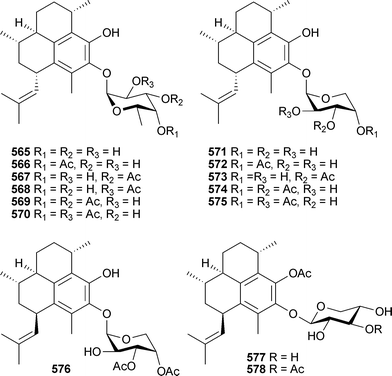
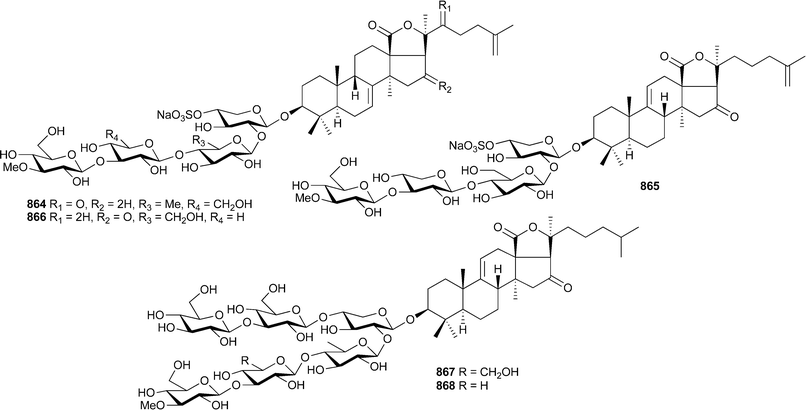
As noted in the 2004 review in this series,213 three publications presenting new pterosin diterpene glycosides from Pseudopterogorgia elisabethae unwittingly led to duplication in structures and trivial names.515–517 Communication with the authors of these studies has now led to the following clarification of trivial names associated with structures: pseudopterosin P565, Q566, R567, S568, 3′-O-acetyl-pseudopterosin Q569, 2′-O-acetyl-pseudopterosin Q570, pseudopterosin T571, U572, V573, 3′-O-acetyl-pseudopterosin U574, 2′-O-acetyl-pseudopterosin U575, pseudopterosin W576, X577 and Y578.518 No structural revisions are proposed.
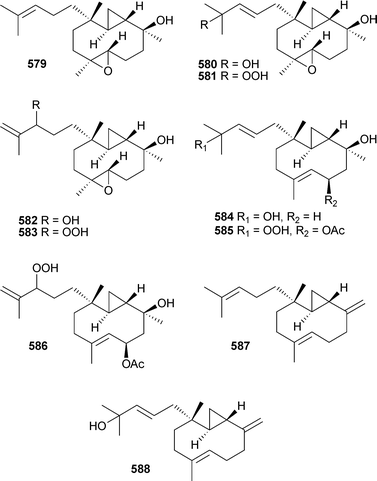
7-Hydroxyerogorgiaene and 7,8-dihydroxyerogorgiaene have been shown to be intermediates in the biosynthesis of the pseudopterosins.519 Of ten diterpenes, the pacificins A–J579–588, isolated from Nephthea pacifica (Taiwan), only 581 and 586 were cytotoxic (mild).520
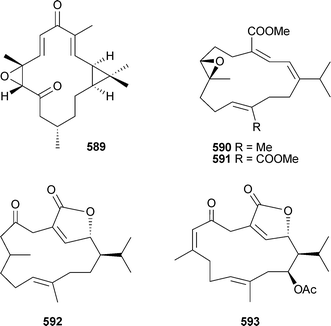
Microclavatin589 and the previously reported cembranolide capillolide,521 were isolated as cytotoxic components of the soft coral Sinularia microclavata (China).522Sarcophytonsp. (China) was the source of sarcophytonolides A–D590–593.523 The relative configuration of leptolide594, isolated from Leptogorgia alba (Panama), was determined by X-ray analysis.524 A number of known diterpenes were also isolated in the same study from L. alba or L. rigida; combinations of CD spectra and X-ray analyses led to the determination of absolute configuration of pukalide aldehyde595, lophotoxin596, pukalide597, lopholide598 and 13α-acetoxypukalide599.
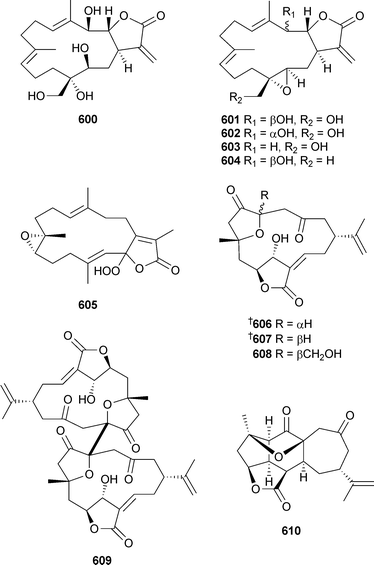
Sinularolides A–E600–604 were isolated from Sinularia gibberosa (China).525 Of these, 601–604 were mildly cytotoxic. 2-Hydroperoxysarcophine605 was reported as a natural product from the soft coral Lobophytum crassum (China).526 In addition to the known metabolites sinuleptolide606 and 5-epi-sinuleptolide607,527isocembranoid sinulochmodin B608, dimeric norcembranoid sinulochmodin A609, and yonarane diterpene sinulochmodin C610 were also reported from Sinularia lochmodes (Taiwan).528
The absolute configurations of 606 and 607 were determined and by comparison of [α]D values, the absolute configurations of 608 and 609 were implied. The known cembranoid sinulariol D, previously obtained from a Sinularia species,529 was reported as mildly cytotoxic.530 Two separate reports have described the bioassay -directed fractionation of an extract from Xenia umbellata (Taiwan) leading to the mildly cytotoxic diterpenoids xenibellols A611 and B612531 and umbellactal613.532 The architecturally-impressive pentacyclic diterpene intricarene614 was reported from Pseudopterogorgia kallos (Colombia), with structural confirmation by X-ray analysis.533
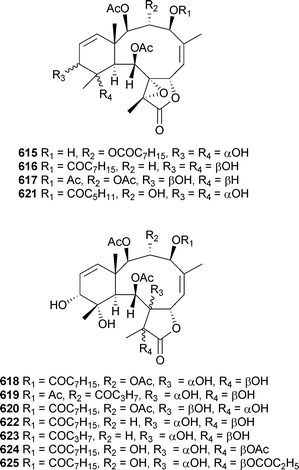
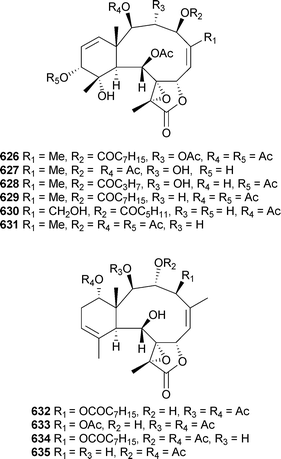
A number of new examples of briarane diterpenoids were presented in separate reports from Briareumsp. (Japan). Violides Q–V615–620 and related metabolites 621–625 were reported from a Briareum species (Bonotsu, Kagoshima Prefecture),534 while a Briareum species (Amami Oshima, Kagoshima Prefecture) yielded briarlides I–R626–635.535,536
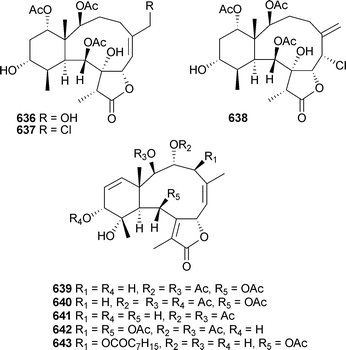
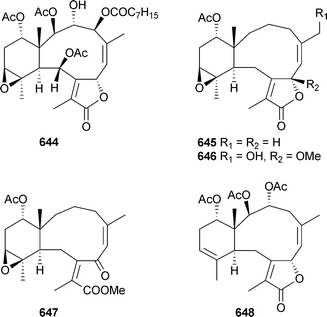
Mild cytotoxicity was observed for 615, 616, 618 and 622. In a separate report, further investigation of Briareumsp. (Bonotsu) led to the isolation of briviolides A–D636–639, 12-O-acetylbriviolide D640, 9-deacetoxybriviolide D641, 4-acetoxybriviolide D642 and briviolides E–I643–648.537,538
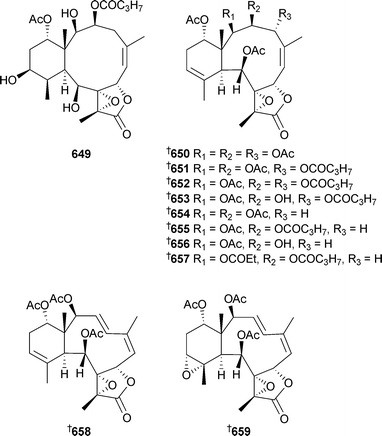
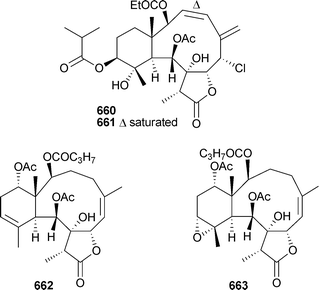
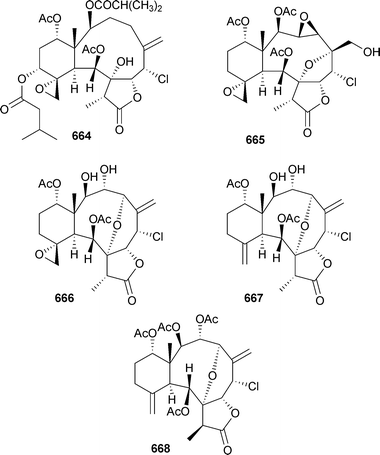
Mild cytotoxicity was observed for 637, 639–641 and 644. 8,17-Epoxybriarane diterpenes briarenol A649539 and briaranolides A–J650–659540 were isolated respectively from Taiwanese and Okinawan collections of Briareumsp. Renillins A–D660–663 were identified as feeding deterrent components of an extract of the sea pansy Renilla reniformis (North Carolina).541 Chlorinated briaranes juncenolides F664 and G665 were isolated from Junceella juncea (Taiwan),542 while Junceella fragilis (South China Sea) yielded junceellonoids C–E666–668.543
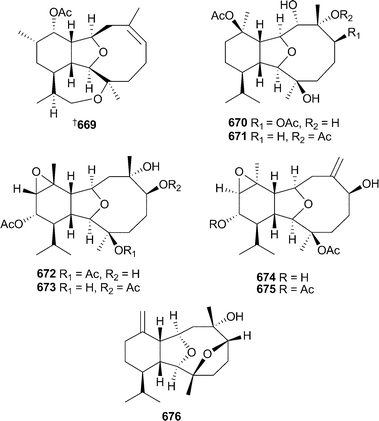
The absolute configuration of the eunicellin-class diterpenoid 11-acetoxy-4-deoxyasbestinin669, originally isolated from Briareum asbestinum,544 has been determined by stereoselective total synthesis.545 New eunicellins klyxumines A670 and B671 and epoxycladines A–D672–675 were reported from specimens of Klyxum flaccidum and Cladiella kashmani (Kenya).546Vigulariol676, previously known as a reaction intermediate in the stereoselective synthesis of the sclerophytins,547 was isolated from the sea pen Vigularia juncea (Taiwan).548
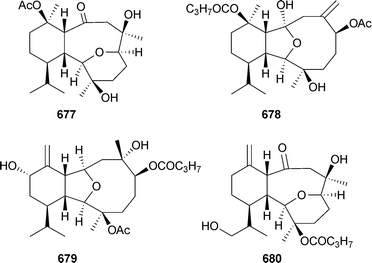
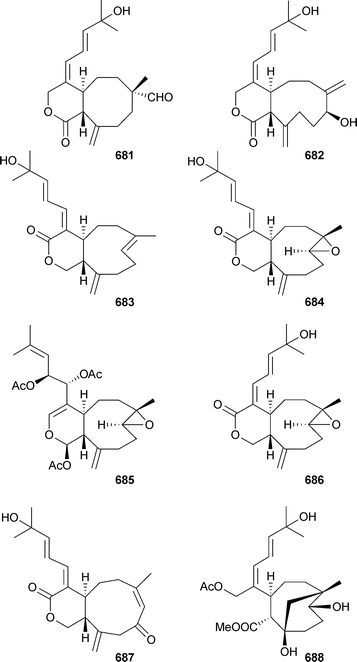
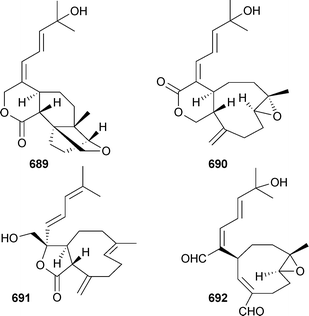
Australins A–D677–680 were isolated from Cladiella australis (Taiwan), but only 678 was mildly cytotoxic.549 Xenicane diterpenes 681–688 were reported from Xenia blumi (Taiwan)550 while Xenia florida (Taiwan) was the source of xeniolactones A–C689–691.551 Mild cytotoxicity was observed for 681–684, 686, 687 and 689. The nor-xenicane metabolite xenibellal692 was reported as a mildly cytotoxic component of Xenia umbellata (Taiwan).552
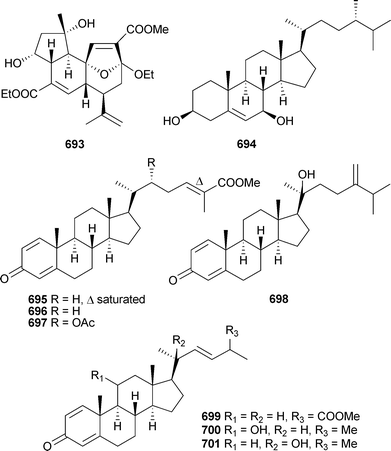
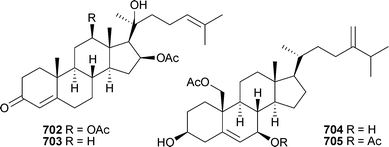
Confertdiate693 is a tetracyclic diterpenoid isolated from Sinularia conferta (China),504 while sterol 694 was reported from a Lobophytum species (India).501 3-Oxo-sterols 695–701 were reported as mildly cytotoxic metabolites of Anthomastus bathyproctus (Antarctica)553 and 702 and 703 were isolated from Nephthea bayeri (China),554 while the 19-acetoxy-sterols 704 and 705 were reported from a soft coral Sinularia species (China).555
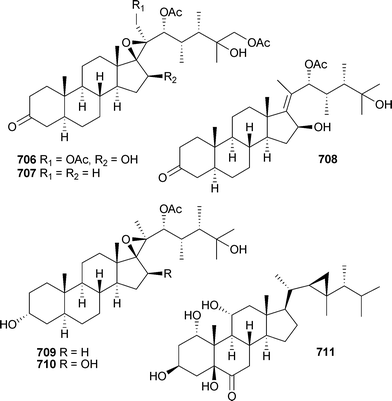
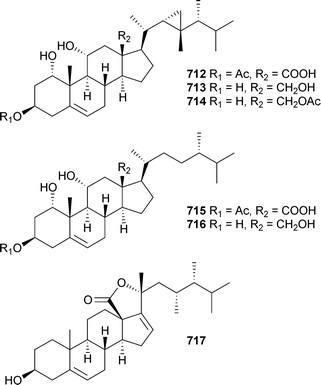
The polyoxygenated sterols hippuristerones J–L706–708, the hippuristerols E709 and F710 and the cyclopropyl-containing sterol 711 were isolated from Isis hippuris (Taiwan),556 while Sinularia dissecta (China) was the source of 712–717.557
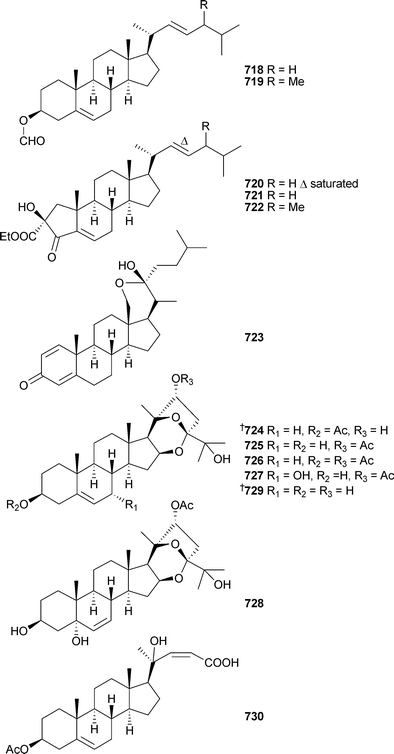
Rare examples of cholesterol-3-formate esters 718 and 719 and A-nor sterols 720–722 were reported from Dendronephthyasp. (China).558 The hemiketal steroid cladiellin A723, isolated from Cladiellasp. (China), along with two dehydration products were found to have radical scavenging properties.559 The 24-ketal steroids suberoretisteroids A–E724–728 were reported from Subergorgia reticulata (China).560 Structure elucidation, which included X-ray analysis of 724,561 necessitated a structural revision of 729 previously reported from Gorgonella umbraculum.562 Similar conclusions were made by another group working on the same species of gorgonian from the same locale.563 Their studies led to the determination of absolute configuration of 724 (which they named reticulatin) and 729. In addition, this study also reported the structure of reticulatic acid730.
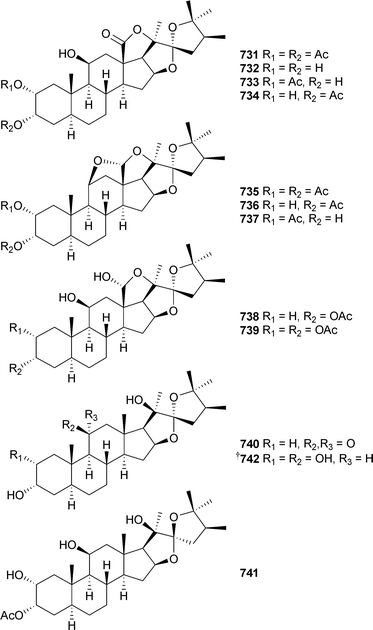
In addition to a number of known congeners , eleven new examples of hippuristanol sterols , 731–741, were reported from Isis hippuris (Taiwan).564 The absolute configuration of the known sterol 742565 was determined on biogenetic grounds and imputed to all but 741 of the compounds reported.
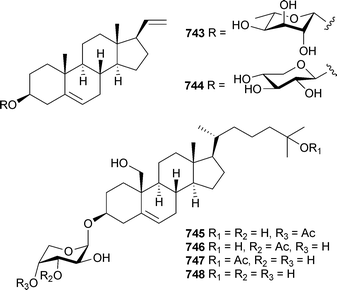
Steroidal glycosides 743 and 744 were obtained from Cladiellasp. (China),566 while 745–748 were isolated from Junceella juncea (China).567 Gorgosterol derivatives previously isolated from Eunicea laciniata568 and Plexaurella grisea,569 were reported to be agonists of Liver X receptors.570 A sea anemone of the order Actinaria was the source of diterpenoids actiniarins A–C749–751, moderate inhibitors of recombinant human cdc25B phosphatase.571 The usual class of natural products isolated from anemones, peptides and protein toxins , have been reviewed,572 and new examples reported. Antheopsis maculata (Japan) was the source of crab toxic peptides Am I–III,573 while acrorhagins I and II are 50 and 44 residue peptides respectively isolated from Actinia equina (Japan).574 Cytolytic toxins Or–A and Or–G were isolated from Oulactis orientalis (Japan) and found to form ion channels in bilayer lipid membranes.575 Cangitoxin, a 4958 Da peptide isolated from Bunodosoma cangicum (Brazil), acts on sodium channels.576
9 Bryozoans
Remarkably, there have been no reports of new bryozoan chemistry. Some analogues of amathamide A (originally obtained from Amathia wilsoni577) have been prepared and their antimicrobial activities studied.57810 Molluscs
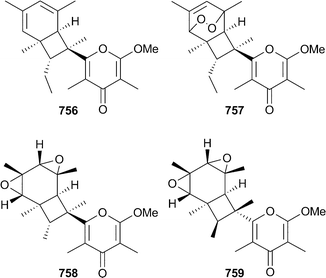
The number of new metabolites reported annually from molluscs has been somewhat cyclical over the 2002–2005 period, with a modest increase reported in 2005. A range of minor nonmethylene-interrupted di-, tri- and tetraenoic fatty acids were identified in extracts of the gonads of limpets Cellana grata and Collisella dorsuosa (Japan).579Turbostatins 1–4752–755 were isolated as cytotoxic components of the Asian topshell snail Turbo stenogyrus (Taiwan).58020-Methyl spirolide G129 was identified by MS/MS analysis of extracts of Mytilus edulis (Norway).163 Further biological testing of the hexacyclic alkaloid lamellarin D, first reported from Lamellariasp. in 1985581 has identified lamellarin D to be a pro-apoptotic agent which remains cytotoxic in multidrug resistant cell lines.582 Molluscs of the order Sacoglossa are a renowned source of polypropionate-derived metabolites.583 Pyrone 756, and the possibly artefactual peroxy derivative 757, were isolated from Placobranchus ocellatus (India).584 The bicyclo[4.2.0]octane core of 756 is analogous to that contained in the related metabolites elysiapyrones A758 and B759 reported from Elysia diomedea.585
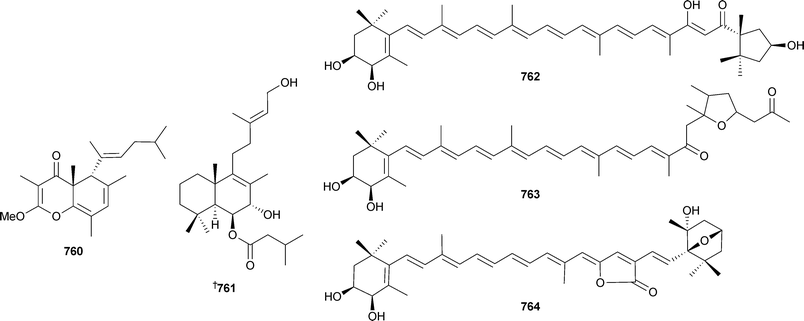
The proposed biogenesis of the bicyclic core is via an 8π–6π electrocyclic cascade. A biomimetic synthesis of elysiapyrones A and B utilised such a pathway586 and a biomimetic synthesis of (±)-9,10-deoxytridachione based upon an electrocyclisation step has also been reported.587 9,10-Deoxytridachione also reacts under triplet excited state sensitisation to afford photodeoxytridachione583 which has the ecological implications of polypropionates acting as natural sunscreening agents.588 Related pyrone, cyercene A,589 also undergoes photoisomerisation to yield alkene isomers and hydroperoxides which have been reported previously as natural products.590 The structure proposed for tridachiahydropyrone760, isolated from Tridachia crispata,591 has been synthesised but the spectroscopic data observed did not match that reported for the natural product. A revision of the structure of the natural product is required.592 The absolute configuration of the trans-decalin 761 isolated from the limpet Trimusculus reticulatus593 has been determined by stereoselective synthesis .594Carotenoids 762–764 were reported from the oyster Crassostrea gigas (Japan)595 while a 4265 Da defensin antimicrobial peptide was isolated from the American oyster Crassostrea virginica.596
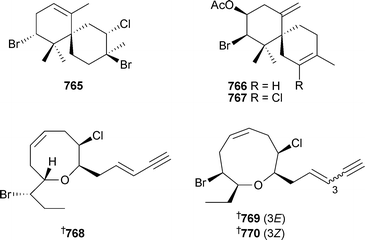
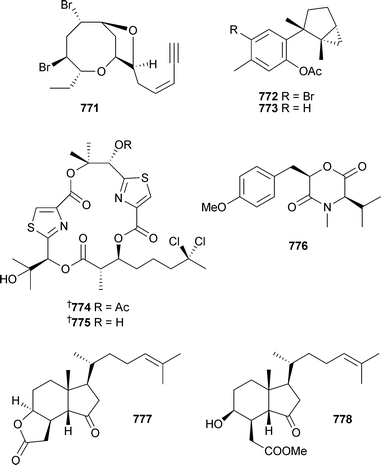
Two conotoxins, µ-conotoxins SIIIA and KIIIA, were characterised by cDNA analysis of Conus striatus and C. kinoshitai (Pacific) and subsequent solid-phase synthesis.597 A more classical approach led to the isolation of peptide de7a from the venom of C. delessertii (Mexico),598 and rg11a from C. regius (Brazil).599 The biological activities of both peptides have yet to be characterised. The solution structure of δ-Am2766, a highly hydrophobic conotoxin isolated from C. amadis600 has been studied by 3D NMR spectroscopy.601 Two reviews cover the chemical ecology of New Zealand opisthobranch molluscs,602 and the role played in ink secretions from the sea hare Aplysia californica.603 Sesquiterpenes 765–767, in addition to a number of known derivatives, were isolated from A. dactylomela (Canary Islands).604In vitro antitumour evaluation supported the hypothesis that acetylation leads to a decrease in metabolite toxicity. Three C15-halogenated compounds 768–770 were isolated from A. dactylomela (China).605 The structure and absolute configuration of 768 was established and it was concluded that 769 and 770 were enantiomers of previously reported natural products606 of defined absolute configuration .607
This study also necessitated the stereochemical revision of (3E)-epi-pinnatifidenyne, previously reported from Laurencia obtusa,608 to ent-768. The related metabolite (3Z)-bromofucin771 was isolated from extracts of Aplysia parvula (South Africa).609 Acetates of laurinterol 772 and debromolaurinterol773, previously known as semi-synthetic compounds, were reported as mildly bioactive natural products from Aplysia kurodai (Japan).610Bursatella leachii (Thailand) was the source of the known metabolite hectochlorin774, the deacetyl analogue 775 and the structurally-unrelated 776.611 Both 774 and 775 were potently cytotoxic. Degraded sterols aplykurodinone-1777 and -2778 were isolated from skin extracts of the sea hare Syphonota geographica (Greece).612
The stereochemical assignment of (+)-aurilol779, isolated from the sea hare Dolabella auricularia,613 has been achieved via stereoselective total synthesis.614 Analogues of dolastatin 11, also originally isolated from D. auricularia,615 have been synthesised and evaluated for antitumour activity and stimulatory effects on the polymerisation of actin.616 Two groups have reported the stereoselective synthesis of the nor-sesquiterpene (+)-austrodoral780, originally isolated from the Antarctic nudibranch Austrodoris kerguelenensis,617 starting from either (–)-sclareol618 or (+)-sclareolide.619 Furanosesquiterpenes alcohols pelseneeriols-1781 and -2782 were isolated from the mantle of the nudibranch Doriopsilla pelseneeri (Portugal).469 Syntheses of 5-methyl-2-((2E,6E)-3,7,11-trimethyl-2,6-dodecadien-9-onyl)benzo-1,4-quinone and the hydroquinone, isolated from the South African nudibranch Leminda millecra,620 have been reported.621 The absolute configurations of nudibranch-derived diterpenes norrisolide783,622 isolated from Chromodoris norrisi,623 and dorisenone C784624 from C. obsoleta,625 have been determined by stereoselective syntheses.
The stereoselective synthesis of (–)-jorumycin, isolated from the Pacific nudibranch Jorunna funebris,626 has confirmed the absolute configuration of the cytotoxic tetrahydroisoquinoline alkaloid and also provided related structures for preliminary examination of structure–activity relationships.415
11 Tunicates (ascidians)
The number of new metabolites reported annually from ascidians has been steadily declining over the 2002–2005 period. A didemnid ascidian (Okinawa) was the source of biselides A–E785–789,627 of which 785 and 787 were modestly cytotoxic towards a panel of tumour cell lines.The absolute configuration of bistramide C790, isolated from Lissoclinum bistratum,628 has been determined by computational analysis629 and total synthesis.630 Two long chain polyacetoxy acyl amino acids, sagittamides A791 and B792 were isolated from an unidentified ascidian (Pohnpei).631Aplidium aff. densum (Oman) was the source of the meroterpenes methoxyconidiol793 and didehydroconicol794,632 while the ubiquinone derivatives glabruquinones A795 and B796 were reported from Aplidium glabrum (Far East).633
The structures of 795 and 796 were confirmed by synthesis and 795 was found to exhibit in vitro cancer preventive activity in a tumour cell transformation assay. The structures of the demethoxy diprenylquinone analogues verapliquinones A and B, isolated from Brittany specimens of Aplidiumsp. ,634 have also been confirmed by synthesis.456Extracts of Aplidium conicum (Mediterranean) yielded 1,1-dioxo-1,4-thiazine containing aplidinones A–C797–799635 and thiaplidiaquinones A800 and B801.636
Bioassay evaluation of 800 and 801 demonstrated that the compounds had entered the cells, and induced the intracellular production of reactive oxygen species leading to disruption of mitochondrial potential and cell death by apoptosis. Perspicamides A802 and B803 are quinolinecarboxylic acid derivatives isolated from Botrylloides perspicum (Australia).637Synoicum macroglossum (India) was the source of a modestly α-glucosidase inhibitory alkaloid , tiruchanduramine804. The structure was confirmed by synthesis.638 A full account of the structure elucidation of lissoclibadin 1639 from Lissoclinum cf. badium (Indonesia) has been reported, along with two new congeners , lissoclibadins 2805 and 3806.640
All three metabolites exhibited varying degrees of cytotoxicity and antibiotic properties. Investigations of the chemistry of colour morphs of a Cystodytes species (Mediterranean) led to the isolation of deacetylshermilamine B807.561 In addition to a number of known congeners , new lamellarin alkaloids 808–811 were isolated from Didemnum obscurum (India).641 Potent cytotoxicity towards colorectal tumour cells was observed. An unidentified ascidian of the family Polycitoridae (Flores, Indonesia) contained two tricyclic alkaloids, polycitorols A812 and B813, closely related to the known cylindricines,642 together with the known compound lepadiformine.643,644
Two studies have reported that the biosynthetic genes for production of the patellamide cyclic peptides reside within Prochloronsp. , the cyanobacterial symbiont of Lissoclinum patella.645,646 The structures of cyclic peptides bistratamides F–I, isolated from Lissoclinum bistratum,647 have been confirmed by total synthesis,648,649 as were the structures of the Didemnum molle metabolites650 didmolamides A and B.651 The antimalarial activity of cyclic peptides ulithiacyclamide and patellamides A–C has been reported.652 The absolute stereochemistry of (+)-pseudodistomin D814, reported in 1997 from Pseudodistoma megalarva,653 has been established by total synthesis.654
Stereoselective synthesis of the Nephteis fasicularismetabolite 655 (–)-fasicularin has been reported.656 The original isolation report of fasicularin noted differential activity towards DNA repair-deficient yeast mutants. The DNA damaging ability of a racemic preparation of the alkaloid has now been studied in depth.657 The structurally related quinolizidine and decahydroquinoline alkaloids (–)-pictamine658 and (–)-lepadin B659 block neuronal nicotinic receptors.660 Ningalin D, an MDR reversing cytotoxic alkaloid originally reported from Didemnumsp. (Australia),661 has been synthesised and structure–activity relationships studied.662 The cyclodepsipeptide aplidine continues through clinical trials663 with a recent report detailing structures of human liver microsome induced metabolism.664 The in vivo activity profile of ET-743 has been summarised665 and the anti-inflammatory properties reported.666 The solution structure of vanabin2, a vanadium binding protein from Ascidia sydneiensis samea, has been studied by NMR spectroscopy.667
12 Echinoderms
The number of new metabolites reported annually from echinoderms has remained relatively constant over the 2002–2005 period. While echinoderms are a well documented source of biologically active saponins and glycolipids ,16,668 they also represent an important food source.15 The naphthoquinone metabolites echinamines A815 and B816 were isolated from the sea urchin Scaphechinus mirabilis (Japan Sea), and the structures confirmed by semi-synthesis from known urchin quinones .668 Radical scavenging properties were observed for both compounds. Ceramide lipids 817–819 were isolated from the starfish Distolasterias nipon (East Sea, Korea).669The sea cucumber Stichopus japonicus was the source of five glycolipid molecular species SJC-1 820, SJC-2 821, SJC-3 822, SJC-4 823 and SJC-5 824.670Linckiacerebroside A825 was identified as a new glycolipid from the starfish Linckia laevigata (Japan).671
Further attempts at purification of the components of the glucocerebroside molecular species HLC-2, previously reported from the sea cucumber Holothuria leucospilota,672 led to the characterisation of 826.673 The ante-iso type side chain configuration of 826 was determined by synthesis.674 A ganglioside molecular species LLG-5 827, containing an unusual trisialosyl moiety, was isolated from the Japanese starfish Linckia laevigata.675 In addition to a number of known compounds, the isopropylidenedioxy sterol 828 was reported from the starfish Asterina pectinifera (China).676 Further investigation of the constituents of the Far-Eastern starfish Ctenodiscus crispatus led to the isolation of 829 as a mixture of C-24 and C-25 stereoisomers , together with 830 and 831. The side chain stereochemistry of the known structurally-related sterol 832677 was defined as (24R,25R).678
The cyclopropane-containing hydroxysteroid phrygiasterol833 and steroid glycoside phrygioside B834 were isolated from the Far-Eastern starfish Hippasteria phrygiana.679 Mild cytotoxicity towards, and apoptotic induction of, Ehrlich carcinoma cells was observed. Two β-D-xylosides, minutosides A835 and B836, were reported from the starfish Anasterias minuta (Patagonia).680Minutoside B836 bears an unusual amidohypotaurine sidechain. Both 835 and 836 were modestly antifungal. Studies of two species of Far-Eastern starfishes, Henricia aspera and H. tumida led to identification of a number of new polyhydroxyl steroids and glycosides .681Henricia aspera was the source of asperosides A837 and B838, H. tumida the source of 839 and 840, while tumidosides A841 and B842 were isolated from both species.
All six metabolites exhibited moderate cytostatic activity towards sea urchin eggs. Bioassay -directed fractionation of extracts from the starfish Certonardoa semiregularis (Korea) led to the characterisation of the moderately cytotoxic saponin certonardoside P2 843, and inactive certonardoside I3 844.682 The isolation of the bioactive asterosaponins 845–850 from the cushion star Culcita novaeguineae (China) has been detailed in three separate reports.683–685 The triterpene nobilisidenols A851 and 852 were isolated from Holothuria nobilis (China).686
Triterpene glycosides, holothurins B2 853, B3 854 and B4 855, were reported from a collection of Holothuria polii (Bay of Naples).687 Six triterpene tetraosides, intercedensides D–I856–861, were isolated as cytotoxic constituents of the sea cucumber Mensamaria intercedens (China).688 This study also concluded that the sidechain stereochemistry of intercedenside A862689 should be corrected to (22Z). Bioassay -directed fractionation of an extract of the sea cucumber Pentacta quadrangularis (China) yielded the dual anti-angiogenic and anti-proliferative saponin, philinopside A863.690
However, no spectroscopic data were reported to support the structure shown. The sea cucumber Australostichopus mollis (New Zealand) was the source of the monosulfated triterpene glycosides mollisosides A864, B1 865 and B2 866,691 while Stichopus parvimensis (Baja California), yielded the oligoglycosides parvimosides A867 and B868.692 A smooth muscle-relaxing hexadecapeptide was characterised from the starfish Asterina pectinifera.693
13 Miscellaneous
Methoxylated polybrominated biphenyls, including the new analogue 869, have been identified as bioaccumulated natural products in a number of marine mammals.694,695 A further range of toxic peptides have been identified in skin secretions of the soapfish Grammistes sexlineatus.696 The hydroid Campanulariasp. (New Zealand) was the source of the guanidine 870,697 while a Chinese collection of the annelid Arenicola cristata led to the isolation of the cytotoxic sulfated sterol arenicolsterol A871.698 The modestly cytotoxic cymodienol872 and cymodiene873 were isolated from the sea grass Cymodocea nodosa (coastal areas of central Greece).699 In two separate studies, the mangrove plant Bruguiera gymnorrhiza (China) yielded phenols 874–878700 and diterpenes 879–881.701The phenol 876 was moderately active towards a range of microorganisms, while the diterpene 881 was mildly cytotoxic towards mouse fibroblast cells. A second species of Chinese mangrove plant, Bruguiera sexangula var. rhynchopetala, was the source of the diterpenes 882–884 and the disulfide quinone 885.702Bruguiera gymnorrhiza (China) yielded related diterpenes 886–889.703 Mild cytotoxicity was observed for 888 and 889.
14 Conclusions
In reviewing the literature for 2001, the observation was made that the rate of increase in publications of new compounds compared with 1965–2000 might have been easing.704 The publication rate for each of the years 2001–2005 has varied somewhat, but at the end of this period, using numbers extracted from MarinLit,68 the steady arithmetic increase observable since 1965 continues (see Fig. 1).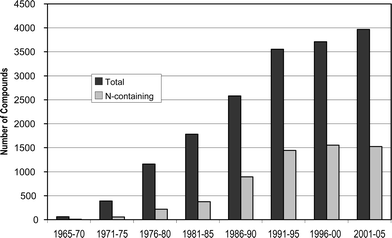 | ||
| Fig. 1 Numbers of marine natural products for the period 1965–2005. | ||
The geographic origins and the phyla sampled for 2001–2005 were examined to establish the differences, if any, compared with the sampling over the previous 36 years. These 2001–2005 data are shown in Fig. 2 and 3 as percentages of the totals for 1965–2005.
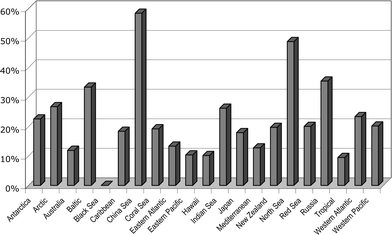 | ||
| Fig. 2 Citations for source regions for the period 2001–2005 as a percentage of the totals for 1965–2005. | ||
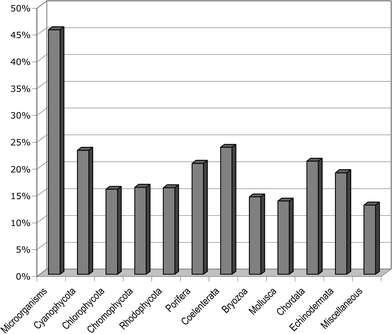 | ||
| Fig. 3 Citations for source phyla for the period 2001–2005 as a percentage of the totals for 1965–2005. | ||
Although the geographical descriptors used in MarinLit are not precise, the large increase from the China Sea as a source area is unmistakable. While the absolute numbers of samples are less, there has also been a notable increase in sampling from the North Sea, the Baltic and Russian territories.
Few people would be surprised by the observation that 45% of the accumulated literature on marine microorganisms has been published since 2000. Other phyla show (relatively) increased interest also. These “traditional” phyla, such as Porifera, Chordata and Coelenterata, are significant as the absolute numbers for 1965–2000 reported for these phyla are much larger than those for microorganisms. It is pertinent to note that some 40% of the work for 2001–2005 on coelenterates has been sourced from the China Sea.
Analysis of the data for 2001–2005 period against the accumulated records from 1965 onwards suggests that the study of marine natural products is thriving, attracting new research groupings and making rapid progress in exploring alternative source phyla and geographical areas.
15 References
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2005, 22, 15 RSC.
- C.-Y. Wang, M.-Y. Geng and H.-S. Guan, Chin. J. New Drugs, 2005, 14, 278 Search PubMed.
- A. M. S. Mayer and M. T. Hamann, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2005, 140, 265 Search PubMed.
- M. S. Butler, Nat. Prod. Rep., 2005, 22, 162 RSC.
- G. M. Cragg and D. J. Newman, Pure Appl. Chem., 2005, 77, 1923 CrossRef CAS.
- G. M. Cragg and D. J. Newman, Pure Appl. Chem., 2005, 77, 7 CrossRef CAS.
- X.-H. Yan and Y.-W. Guo, Chin. J. Nat. Med., 2005, 3, 65 Search PubMed.
- S. Singh, B. N. Kate and U. C. Banerjee, Crit. Rev. Biotechnol., 2005, 25, 73 CrossRef CAS.
- T. Okino, Kaiyo Seibutsu Seibun no Riyo, 2005, 47 Search PubMed.
- S. Tsukamoto, Kaiyo Seibutsu Seibun no Riyo, 2005, 60 Search PubMed.
- D. Sipkema, M. C. R. Franssen, R. Osinga, J. Tramper and R. H. Wijffels, Mar. Biotechnol., 2005, 7, 142 CrossRef CAS.
- K. Iguchi, Kaiyo Seibutsu Seibun no Riyo, 2005, 97 Search PubMed.
- S. Fukuzawa, Kaiyo Seibutsu Seibun no Riyo, 2005, 132 Search PubMed.
- T. Miyamoto, Kaiyo Seibutsu Seibun no Riyo, 2005, 145 Search PubMed.
- M. S. Kelly, Prog. Mol. Subcell. Biol., 2005, 39, 139 Search PubMed.
- Y. Yokota, Prog. Mol. Subcell. Biol., 2005, 39, 251 Search PubMed.
- M. Ishibashi, Kaiyo Seibutsu Seibun no Riyo, 2005, 159 Search PubMed.
- T. J. Heckrodt and J. Mulzer, Top. Curr. Chem., 2005, 244, 1 CAS.
- E. Zubia, M. J. Ortega and J. Salva, Mini–Rev. Org. Chem., 2005, 2, 389 Search PubMed.
- J. Sun, H. Lin, S. Li, G. Liu, C. Zhang and H. Zhang, Zhongcaoyao, 2005, 36, 609 Search PubMed.
- H. N. Kamel and M. Slattery, Pharm. Biol., 2005, 43, 253 CrossRef CAS.
- T. L. Simmons, E. Andrianasolo, K. McPhail, P. Flatt and W. H. Gerwick, Mol. Cancer Ther., 2005, 4, 333 Search PubMed.
- M.-L. Bourguet-Kondracki and J. M. Kornprobst, Adv. Biochem. Eng. Biotechnol., 2005, 97, 105 Search PubMed.
- Y. Nogata, Kaiyo Seibutsu Seibun no Riyo, 2005, 290 Search PubMed.
- D. Alonso, A. Castro and A. Martinez, Expert Opin. Ther. Pat., 2005, 15, 1377 Search PubMed.
- L. Gomez-Paloma, M. C. Monti, S. Terracciano, A. Casapullo and R. Riccio, Curr. Org. Chem., 2005, 9, 1419 CrossRef CAS.
- R. A. Keyzers and M. T. Davies-Coleman, Chem. Soc. Rev., 2005, 34, 355 RSC.
- I. P. Singh, S. B. Bharate and K. K. Bhutani, Curr. Sci., 2005, 89, 269 CAS.
- N. Dias, H. Vezin, A. Lansiaux and C. Bailly, Top. Curr. Chem., 2005, 253, 89 CAS.
- L. Zhang, R. An, J. Wang, N. Sun, S. Zhang, J. Hu and J. Kuai, Curr. Opin. Microbiol., 2005, 8, 276 CrossRef CAS.
- A. T. Bull, J. E. M. Stach, A. C. Ward and M. Goodfellow, Antonie van Leeuwenhoek, 2005, 87, 65 CrossRef.
- C. Imada, Antonie van Leeuwenhoek, 2005, 87, 59 CrossRef CAS.
- B. S. Moore, J. A. Kalaitzis and L. Xiang, Antonie van Leeuwenhoek, 2005, 87, 49 CrossRef CAS.
- P. R. Jensen, T. J. Mincer, P. G. Williams and W. Fenical, Antonie van Leeuwenhoek, 2005, 87, 43 CrossRef CAS.
- H. P. Fiedler, C. Bruntner, A. T. Bull, A. C. Ward, M. Goodfellow, O. Potterat, C. Puder and G. Mihm, Antonie van Leeuwenhoek, 2005, 87, 37 CrossRef CAS.
- J. Clayden, B. Read and K. R. Hebditch, Tetrahedron, 2005, 61, 5713 CrossRef CAS.
- T. Yasumoto, Proc. Jpn. Acad., Ser. B, 2005, 81, 43 Search PubMed.
- K. Yasumoto, Kagaku to Seibutsu, 2005, 43, 153 Search PubMed.
- C. Wiegand and S. Pflugmacher, Toxicol. Appl. Pharmacol., 2005, 203, 201 CrossRef CAS.
- M. A. Friedman and B. E. Levin, J. Int. Neuropsychol. Soc., 2005, 11, 331 CAS.
- L. E. Fleming, L. C. Backer and D. G. Baden, Environ. Health Perspect., 2005, 113, 618 CAS.
- G. P. Rossini, Toxicology, 2005, 207, 451 CrossRef CAS.
- M. C. C. Batoréu, E. Dias, P. Pereira and S. Franca, Environ. Toxicol. Pharmacol., 2005, 19, 401 CrossRef CAS.
- G. A. Codd, L. F. Morrison and J. S. Metcalf, Toxicol. Appl. Pharmacol., 2005, 203, 264 CrossRef CAS.
- Y. Harayama and Y. Kita, Curr. Org. Chem., 2005, 9, 1567 CrossRef CAS.
- E. M. Antunes, B. R. Copp, M. T. Davies-Coleman and T. Samaai, Nat. Prod. Rep., 2005, 22, 62 RSC.
- L. N. Rogosa, N. F. Salakhutdinov and G. A. Tolstikov, Russ. J. Bioorg. Chem., 2005, 31, 507 Search PubMed.
- J. P. Bergé and G. Barnathan, Adv. Biochem. Eng. Biotechnol., 2005, 96, 49 Search PubMed.
- W. Gul and M. T. Hamann, Life Sci., 2005, 78, 442 CrossRef CAS.
- M. Somei and F. Yamada, Nat. Prod. Rep., 2005, 22, 73 RSC.
- R. G. S. Berlinck and M. H. Kossuga, Nat. Prod. Rep., 2005, 22, 516 RSC.
- J.-F. Liu, S.-P. Guo and B. Jiang, Chin. J. Org. Chem., 2005, 25, 788 CAS.
- T. Ogamino and S. Nishiyama, J. Synth. Org. Chem., Jpn., 2005, 63, 583 CAS.
- J. D. Connolly and R. A. Hill, Nat. Prod. Rep., 2005, 22, 230 RSC.
- Z. Jin, Nat. Prod. Rep., 2005, 22, 196 RSC.
- V. M. Dembitsky, T. A. Gloriozova and V. V. Poroikov, Mini–Rev. Med. Chem., 2005, 5, 319 Search PubMed.
- M. Kita and D. Uemura, Chem. Lett., 2005, 34, 454 CrossRef CAS.
- M. Ohba, Recent Res. Dev. Org. Chem., 2005, 9, 71 Search PubMed.
- N. S. Sarma, M. S. R. Krishna and S. R. Rao, Mar. Drugs, 2005, 3, 84 Search PubMed.
- P.-J. Sung, P.-C. Chang, L.-S. Fang, J.-H. Sheu, W.-C. Chen, Y.-P. Chen and M.-R. Lin, Heterocycles, 2005, 65, 195 CrossRef CAS.
- B. S. Moore, Nat. Prod. Rep., 2005, 22, 580 RSC.
- A. Fontana, A. Cutignano and G. d'Ippolito, in Biocatalysis: Chemistry and Biology, ed. A. Trincone, Research Signpost, Trivandrum, India, 2005, p. 167 Search PubMed.
- J. L. Fortman and D. H. Sherman, ChemBioChem, 2005, 6, 960 CrossRef CAS.
- J. Piel, D. Butzke, N. Fusetani, D. Hui, M. Platzer, G. Wen and S. Matsunaga, J. Nat. Prod., 2005, 68, 472 CrossRef CAS.
- J. Piel, Naturwiss. Rundsch., 2005, 58, 5 Search PubMed.
- G. M. Nicholas and A. J. Phillips, Nat. Prod. Rep., 2005, 22, 144 RSC.
- W. E. Houssen and M. Jaspars, Methods Biotechnol., 2005, 20, 353 Search PubMed.
- MarinLit database, Department of Chemistry, University of Canterbury: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml.
- R. H. Feling, G. O. Buchanan, T. J. Mincer, C. A. Kauffman, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2003, 42, 355 CrossRef CAS.
- P. G. Williams, G. O. Buchanan, R. H. Feling, C. A. Kauffman, P. R. Jensen and W. Fenical, J. Org. Chem., 2005, 70, 6196 CrossRef CAS.
- G. O. Buchanan, P. G. Williams, R. H. Feling, C. A. Kauffman, P. R. Jensen and W. Fenical, Org. Lett., 2005, 7, 2731 CrossRef CAS.
- D. S. Fukuda, J. S. Mynderse, P. J. Baker, D. M. Berry, L. D. Boeck, R. C. Yao, F. P. Mertz, W. M. Nakatsukasa, J. Mabe and J. Ott, J. Antibiot., 1990, 43, 623 CAS.
- D. S. Fukuda, J. S. Mynderse and R. C. Yao, U.S. Patent 4904590, 1990.
- I. E. Soria-Mercado, P. R. Jensen, W. Fenical, S. Kassel and J. Golen, Acta Crystallogr., Sect. E, 2004, 60, 1627.
- I. E. Soria-Mercado, A. Prieto-Davo, P. R. Jensen and W. Fenical, J. Nat. Prod., 2005, 68, 904 CrossRef CAS.
- Y. Matsuo, M. Suzuki, H. Kasai, Y. Shizuri and S. Harayama, Environ. Microbiol., 2003, 5, 25 CrossRef CAS.
- Y. Matsuo, H. Imagawa, M. Nishizawa and Y. Shizuri, Science, 2005, 307, 1598 CrossRef CAS.
- K. Kanoh, Y. Matsuo, K. Adachi, H. Imagawa, M. Nishizawa and Y. Shizuri, J. Antibiot., 2005, 58, 289 CrossRef CAS.
- P. Romero, L. Malet, L. M. Canedo, C. Cuevas and J. Fernando Reyes, PCT Int. Appl., WO 2005000880 A2 20050106, 2005.
- A. J. Blackman and C. Li, Aust. J. Chem., 1994, 47, 1625 CAS.
- N. Lindquist and W. Fenical, Experientia, 1991, 47, 504 CAS.
- B. Carte and D. J. Faulkner, J. Org. Chem., 1983, 48, 2314 CrossRef CAS.
- C. Holmstrom, S. James, B. A. Neilan, D. C. White and S. Kjelleberg, Int. J. Syst. Bacteriol., 1998, 48, 1205 Search PubMed.
- A. Franks, P. Haywood, C. Holmström, S. Egan, S. Kjelleberg and N. Kumar, Molecules, 2005, 10, 1286 Search PubMed.
- J. Vicar, M. Budesinsky and K. Blaha, Collect. Czech. Chem. Commun., 1973, 38, 1940 CAS.
- J. Vicar, J. Smolikova and K. Blaha, Collect. Czech. Chem. Commun., 1973, 38, 1957 CAS.
- M. Mitova, M. L. Tutino, G. Infusini, G. Marino and S. De Rosa, Mar. Biotechnol., 2005, 7, 523 CrossRef CAS.
- R. R. Manam, S. Teisan, D. J. White, B. Nicholson, J. Grodberg, S. T. C. Neuteboom, K. S. Lam, D. A. Mosca, G. K. Lloyd and B. C. M. Potts, J. Nat. Prod., 2005, 68, 240 CrossRef CAS.
- F. Li, R. P. Maskey, S. Qin, I. Sattler, H. H. Fiebig, A. Maier, A. Zeeck and H. Laatsch, J. Nat. Prod., 2005, 68, 349 CrossRef CAS.
- H.-S. Lee, H. J. Shin, K. H. Jang, T. S. Kim, K.-B. Oh and J. Shin, J. Nat. Prod., 2005, 68, 623 CrossRef CAS.
- V. R. Macherla, J. Liu, C. Bellows, S. Teisan, B. Nicholson, K. S. Lam and B. C. M. Potts, J. Nat. Prod., 2005, 68, 780 CrossRef.
- I. Kock, R. P. Maskey, M. A. F. Biabani, E. Helmke and H. Laatsch, J. Antibiot., 2005, 58, 530 CrossRef CAS.
- A. Gorajana, B. V. V. S. N. Kurada, S. Peela, P. Jangam, S. Vinjamuri, E. Poluri and A. Zeeck, J. Antibiot., 2005, 58, 526 CrossRef CAS.
- Y.-Q. Tang, I. Sattler, R. Thiericke, S. Grabley and X.-Z. Feng, J. Antibiot., 2000, 53, 934 CAS.
- M. P. Sobolevskaya, S. Fotso, U. Havash, V. A. Denisenko, E. Helmke, N. G. Prokofeva, T. A. Kuznetsova, H. Laatsch and G. B. Elyakov, Chem. Nat. Compd., 2004, 40, 282 CrossRef CAS.
- W. H. Kim, J. H. Jung and E. Lee, J. Org. Chem., 2005, 70, 8190 CrossRef CAS.
- H. Mertens, O. Schmidt and K. Adam, Patents DE 1083011, 1960, and GB 875720.
- J. F. McGhie, W. A. Ross, D. Evans and J. E. Tomlin, J. Chem. Soc., 1962, 350 RSC.
- J. S. Dickschat, T. Martens, T. Brinkhoff, M. Simon and S. Schulz, Chem. Biodiversity, 2005, 2, 837 Search PubMed.
- R. K. Phipps, J. W. Blunt, A. L. J. Cole and M. H. G. Munro, ARKIVOC, 2004, 94 CAS.
- X.-X. Han, C.-B. Cui, Q.-Q. Gu, W.-M. Zhu, H.-B. Liu, J.-Y. Gu and H. Osada, Tetrahedron Lett., 2005, 46, 6137 CrossRef CAS.
- T. Mülhaupt, H. Kaspar, S. Otto, M. Reichert, G. Bringmann and T. Lindel, Eur. J. Org. Chem., 2005, 2, 334 CrossRef.
- M. Winter, F. Gautschi, I. Flament, M. Stoll and I. M. Goldman, U.S. Patent, 3924015, 1975.
- J. S. Dickschat, H. Reichenbach, I. Wagner-Döbler and S. Schulz, Eur. J. Org. Chem., 2005, 4141 CrossRef CAS.
- S. Tsukamoto, H. Hirota, M. Imachi, M. Fujimuro, H. Onuki, T. Ohta and H. Yokosawa, Bioorg. Med. Chem. Lett., 2005, 15, 191 CrossRef CAS.
- S. A. Waksman, E. S. Horning and E. L. Spencer, Science, 1942, 96, 202 CrossRef CAS.
- K. Tatsuta, T. Tsuchiya, N. Mikami, S. Umezawa, H. Umezawa and H. Naganawa, J. Antibiot., 1974, 27, 579 CAS.
- O. F. Smetanina, A. I. Kalinovskii, Y. V. Khudyakov, O. P. Moiseenko, M. V. Pivkin, N. I. Menzorova, Y. T. Sibirtsev and T. A. Kuznetsova, Chem. Nat. Compd., 2005, 41, 243 CrossRef CAS.
- M.-A. Ouyang, R. Liu and Y.-H. Kuo, J. Asian Nat. Prod. Res., 2005, 7, 761 Search PubMed.
- M. Tsuda, M. Sasaki, T. Mugishima, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2005, 68, 273 CrossRef CAS.
- M. Sasaki, M. Tsuda, M. Sekiguchi, Y. Mikami and J. Kobayashi, Org. Lett., 2005, 7, 4261 CrossRef CAS.
- M. Tsuda, Y. Kasai, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, Org. Lett., 2004, 6, 3087 CrossRef CAS.
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao and J. Kobayashi, J. Org. Chem., 2005, 70, 9430 CrossRef CAS.
- G. Bringmann, G. Lang, T. A. M. Gulder, H. Tsuruta, J. Mühlbacher, K. Maksimenka, S. Steffens, K. Schaumann, R. Stöhr, J. Wiese, J. F. Imhoff, S. Perovic-Ottstadt, O. Boreiko and W. E. G. Müller, Tetrahedron, 2005, 61, 7252 CrossRef CAS.
- W. Liu, Q. Gu, W. Zhu, C. Cui, G. Fan, T. Zhu, H. Liu and Y. Fang, Tetrahedron Lett., 2005, 46, 4993 CrossRef CAS.
- W. Liu, Q. Gu, W. Zhu, C. Cui and G. Fan, J. Antibiot., 2005, 58, 441 CrossRef CAS.
- W. Liu, Q. Gu, W. Zhu, C. Cui and G. Fan, J. Antibiot., 2005, 58, 621 CrossRef CAS.
- R. Andrarde, W. A. Ayer and P. P. Mebe, Can. J. Chem., 1992, 70, 2526 CAS.
- N. Abe, T. Murata and A. Hirota, Biosci., Biotechnol., Biochem., 1998, 62, 661 CAS.
- G. A. Warr, J. A. Veitch, A. W. Walsh, G. A. Hesler, D. M. Pirnit, J. E. Leet, P.-F. M. Lin, I. A. Medina, K. D. McBrien, S. Forenza, J. M. Clark and K. S. Lam, J. Antibiot., 1996, 49, 234 CAS.
- J. He, U. Lion, I. Sattler, F. A. Gollmick, S. Grabley, J. Cai, M. Meiners, H. Schünke, K. Schaumann, U. Dechert and M. Krohn, J. Nat. Prod., 2005, 68, 1397 CrossRef CAS.
- Z. H. Xin, W. M. Zhu, Q. Q. Gu, Y. C. Fang, L. Duan and C. B. Cui, Chin. Chem. Lett., 2005, 16, 1227 CAS.
- A. Takatsuki, S. Suzuki, K. Ando, G. Tamura and K. Arima, J. Antibiot., 1968, 21, 671 CAS.
- R. Sakai, T. Nakamura, T. Nishino, M. Yamamoto, A. Miyamura, H. Miyamoto, N. Ishiwata, N. Komatsu, H. Kamiya and N. Tsuruzoe, Bioorg. Med. Chem., 2005, 13, 6388 CrossRef CAS.
- K. Adachi, K. Kanoh, P. Wisespongp, M. Nishijima and Y. Shizuri, J. Antibiot., 2005, 58, 145 CrossRef CAS.
- K. Krohn, J. Dai, U. Flörke, H.-J. Aust, S. Dräger and B. Schulz, J. Nat. Prod., 2005, 68, 400 CrossRef.
- X. Li, M. K. Kim, U. Lee, S.-K. Kim, J. S. Kang, H. D. Choi and B. W. Son, Chem. Pharm. Bull., 2005, 53, 453 CrossRef CAS.
- Y. Kasai, K. Komatsu, H. Shigemori, M. Tsuda, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2005, 68, 777 CrossRef CAS.
- H. Wei, T. Itoh, M. Kinoshita, N. Kotoku, S. Aoki and M. Kobayashi, Tetrahedron, 2005, 61, 8054 CrossRef CAS.
- T. Yamada, M. Iritani, K. Minoura, K. Kawai and A. Numata, Org. Biomol. Chem., 2004, 2, 2131 RSC.
- T. Yamada, M. Doi, A. Miura, W. Harada, M. Hiramura, K. Minoura, R. Tanaka and A. Numata, J. Antibiot., 2005, 58, 185 CrossRef CAS.
- M. Cueto, P. R. Jensen, C. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Nat. Prod., 2001, 64, 1444 CrossRef CAS.
- D.-C. Oh, P. R. Jensen, C. A. Kauffman and W. Fenical, Bioorg. Med. Chem., 2005, 13, 5267 CrossRef CAS.
- C. J. Smith, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, J. Jompa, A. Tahir and C. M. Ireland, J. Nat. Prod., 2000, 63, 142 CrossRef CAS.
- R. Garcia, J. M. Seco, S. A. Vazquez, E. Quinoa and R. Riguera, J. Org. Chem., 2002, 67, 4579 CrossRef CAS.
- X. Franck, M. E. Vaz Araujo, J.-C. Jullian, R. Hocquemiller and B. Figadere, Tetrahedron Lett., 2001, 42, 2801 CrossRef CAS.
- S. Gesner, N. Cohen, M. Ilan, O. Yarden and S. Carmeli, J. Nat. Prod., 2005, 68, 1350 CrossRef CAS.
- A. M. J. Senderowicz, G. Kaur, E. Sainz, C. Laing, W. D. Inman, J. Rodriguez, P. Crews, L. Malspeis, M. R. Grever, E. A. Sausville and K. L. K. Duncan, J. Natl. Cancer Inst., 1995, 87, 46 CrossRef CAS.
- P. Crews, L. V. Manes and M. Boehler, Tetrahedron Lett., 1986, 27, 2797 CrossRef CAS.
- T. M. Zabriskie, J. A. Klocke, C. M. Ireland, A. H. Marcus, T. F. Molinski, D. J. Faulkner, C. Xu and J. Clardy, J. Am. Chem. Soc., 1986, 108, 3123 CrossRef.
- O. E. Christian, J. Compton, K. R. Christian, S. L. Mooberry, F. A. Valeriote and P. Crews, J. Nat. Prod., 2005, 68, 1592 CrossRef CAS.
- X. Wu, X. Liu, G. Jiang, Y. Lin, W. Chan and L. L. P. Vrijmoed, Chem. Nat. Compd., 2005, 41, 27 CrossRef CAS.
- Y. Lin, X. Wu, S. Feng, G. Jiang, J. Luo, S. Zhou, L. L. Vrijmoed, E. B. Jones, K. Krohn, K. Steingrover and F. Zsila, J. Org. Chem., 2001, 66, 6252 CrossRef CAS.
- X. Y. Wu, X. H. Liu, Y. C. Lin, J. H. Luo, Z. G. She, L. Houjin, W. L. Chan, S. Antus, T. Kurtan, B. Elsässer and K. Krohn, Eur. J. Org. Chem., 2005, 4061 CrossRef CAS.
- H. Raistrick and D. J. Ross, Biochem. J., 1952, 50, 635 CAS.
- P. Cai, A. T. McPhail, E. Krainer, B. Katz, C. Pearce, C. Boros, B. Caceres, D. Smith and D. R. Houck, Tetrahedron Lett., 1999, 40, 1479 CrossRef CAS.
- X. Lin, Y. Huang, M. Fang, J. Wang, Z. Zheng and W. Su, FEMS Microbiol. Lett., 2005, 251, 53 CrossRef CAS.
- B. Han, K. L. McPhail, H. Gross, D. E. Goeger, S. L. Mooberry and W. H. Gerwick, Tetrahedron, 2005, 61, 11723 CrossRef CAS.
- B. Han, D. Goeger, C. S. Maier and W. H. Gerwick, J. Org. Chem., 2005, 70, 3133 CrossRef CAS.
- S. Carmely and Y. Kashman, Tetrahedron Lett., 1985, 26, 511 CrossRef CAS.
- E. H. Andrianasolo, H. Gross, D. Goeger, M. Musafija-Girt, K. McPhail, R. M. Leal, S. L. Mooberry and W. H. Gerwick, Org. Lett., 2005, 7, 1375 CrossRef CAS.
- T. Teruya, K. Kobayashi, K. Suenaga and H. Kigoshi, Tetrahedron Lett., 2005, 46, 4001 CrossRef CAS.
- J. B. MacMillan and T. F. Molinski, J. Nat. Prod., 2005, 68, 604 CrossRef CAS.
- Y. Ito and A. Butler, Limnol. Oceanogr., 2005, 50, 1918 CAS.
- N. Morsy, S. Matsuoka, T. Houdai, N. Matsumori, S. Adachi, M. Murata, T. Iwashita and T. Fujita, Tetrahedron, 2005, 61, 8606 CrossRef CAS.
- R. Echigoya, L. Rhodes, Y. Oshima and M. Satake, Harmful Algae, 2005, 4, 383 Search PubMed.
- T. Kubota, A. Takahashi, M. Tsuda and J. Kobayashi, Mar. Drugs, 2005, 3, 113 Search PubMed.
- M. Tsuda, Y. Kariya, R. Iwamoto, E. Fukushi, J. Kawabata and J. Kobayashi, Mar. Drugs, 2005, 3, 1 Search PubMed.
- J. Wu, L. J. Long, Y. Song, S. Zhang, Q. X. Li, H. S. Huang and Z. H. Xiao, Chem. Pharm. Bull., 2005, 53, 330 CrossRef CAS.
- M. L. Souto, J. J. Fernández, J. M. Franco, B. Paz, L. V. Gil and M. Norte, J. Nat. Prod., 2005, 68, 420 CrossRef CAS.
- C. O. Miles, A. L. Wilkins, A. D. Hawkes, A. Selwood, D. J. Jensen, J. Aasen, R. Munday, I. A. Samdal, L. R. Briggs, V. Beuzenberg and A. L. MacKenzie, Toxicon, 2004, 44, 325 CrossRef CAS.
- C. O. Miles, A. L. Wilkins, A. D. Hawkes, A. I. Selwood, D. J. Jensen, R. Munday, J. M. Cooney and V. Beuzenberg, Toxicon, 2005, 45, 61 CrossRef CAS.
- J. Aasen, S. L. MacKinnon, P. LeBlanc, J. A. Walter, P. Hovgaard, T. Aune and M. A. Quilliam, Chem. Res. Toxicol., 2005, 18, 509 CrossRef CAS.
- M. Satake, Y. Tanaka, Y. Ishikura, Y. Oshima, H. Naoki and T. Yasumoto, Tetrahedron Lett., 2005, 46, 3537 CrossRef CAS.
- K. Tanaka, Y. Itagaki, M. Satake, H. Naoki, T. Yasumoto, K. Nakanishi and N. Berova, J. Am. Chem. Soc., 2005, 127, 9561 CrossRef.
- A. J. Bourdelais, H. M. Jacocks, J. L. C. Wright, P. M. Bigwarfe, Jr. and D. G. Baden, J. Nat. Prod., 2005, 68, 2 CrossRef CAS.
- B. Suárez-Gómez, M. L. Souto, P. G. Cruz, J. J. Fernández and M. Norte, J. Nat. Prod., 2005, 68, 596 CrossRef CAS.
- C.-K. Lu, H.-N. Chou, C.-K. Lee and T.-H. Lee, Org. Lett., 2005, 7, 3893 CrossRef CAS.
- M. Kita, M. Kondo, T. Koyama, K. Yamada, T. Matsumoto, K.-H. Lee, J.-T. Woo and D. Uemura, J. Am. Chem. Soc., 2004, 126, 4794 CrossRef CAS.
- M. Kita, N. Ohishi, K. Washida, M. Kondo, T. Koyama, K. Yamada and D. Uemura, Bioorg. Med. Chem., 2005, 13, 5253 CrossRef CAS.
- A. A. Carlos, B. K. Baillie, M. Kawachi and T. Maruyama, J. Phycol., 1999, 35, 1054 CrossRef CAS.
- K. Onodera, H. Nakamura, Y. Oba and M. Ojika, Tetrahedron, 2003, 59, 1067 CrossRef CAS.
- K. Onodera, H. Nakamura, Y. Oba, Y. Ohizumi and M. Ojika, J. Am. Chem. Soc., 2005, 127, 10406 CrossRef CAS.
- G. d'Ippolito, A. Cutignano, R. Briante, F. Febbraio, G. Cimino and A. Fontana, Org. Biomol. Chem., 2005, 3, 4065 RSC.
- A. P. Rauter, M. M. Filipe, C. Prata, J. P. Noronha, M. A. M. Sampayo, J. Justino and J. Bermejo, Fitoterapia, 2005, 76, 433 CrossRef CAS.
- J. Hiort, K. Maksimenka, M. Reichert, S. Perovic-Ottstadt, W. H. Lin, V. Wray, K. Steube, K. Schaumann, H. Weber, P. Proksch, R. Ebel, W. E. G. Mueller and G. Bringmann, J. Nat. Prod., 2004, 67, 1532 CrossRef CAS.
- J. Hiort, K. Maksimenka, M. Reichert, S. Perovic-Ottstadt, W. H. Lin, V. Wray, K. Steube, K. Schaumann, A. Weber, P. Proksch, R. Ebel, W. E. G. Müller and G. Bringmann, J. Nat. Prod., 2005, 68, 1821 CrossRef CAS.
- Y. H. Ye, H. L. Zhu, Y. C. Song, J. Y. Liu and R. X. Tan, J. Nat. Prod., 2005, 68, 1106 CrossRef CAS.
- J. I. Jimenez and P. J. Scheuer, J. Nat. Prod., 2001, 64, 200 CrossRef CAS.
- H. Chen, Y. Feng, Z. Xu and T. Ye, Tetrahedron, 2005, 61, 11132 CrossRef CAS.
- D. B. Stierle and A. A. Stierle, Experientia, 1992, 48, 1165 CAS.
- X. Gao and D. G. Hall, J. Am. Chem. Soc., 2005, 127, 1628 CrossRef CAS.
- T. S. Bugni, D. Abbanat, V. S. Bernan, W. M. Maiese, M. Greenstein, R. M. Van Wagoner and C. M. Ireland, J. Org. Chem., 2000, 65, 7195 CrossRef CAS.
- G. Mehta and S. C. Pan, Tetrahedron Lett., 2005, 46, 5219 CrossRef CAS.
- B. Bister, D. Bischoff, M. Ströbele, J. Riedlinger, A. Reicke, F. Wolter, A. T. Bull, H. Zähner, H.-P. Fiedler and R. D. Süssmuth, Angew. Chem., Int. Ed., 2004, 43, 2574 CrossRef CAS.
- C. W. Zapf, B. A. Harrison, C. Drahl and E. J. Sorensen, Angew. Chem., Int. Ed., 2005, 44, 6533 CrossRef CAS.
- M. K. Renner, Y. C. Shen, X. C. Cheng, P. R. Jensen, W. Frankmolle, C. A. Kauffman, W. Fenical, E. Lobkovsky and J. Clardy, J. Am. Chem. Soc., 1999, 121, 11273 CrossRef CAS.
- S. J. Wen, T. S. Hu and Z. J. Yao, Tetrahedron, 2005, 61, 4931 CrossRef CAS.
- B. Han, K. L. McPhail, A. Ligresti, V. Di Marzo and W. H. Gerwick, J. Nat. Prod., 2003, 66, 1364 CrossRef CAS.
- I. R. Davies, M. Cheeseman, D. Gangani Niyadurupola and S. D. Bull, Tetrahedron Lett., 2005, 46, 5547 CrossRef CAS.
- H. Luesch, R. Pangilinan, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2001, 64, 304 CrossRef CAS.
- Y. Peng, H. Pang, Z. Xu and T. Ye, Lett. Org. Chem., 2005, 2, 703 Search PubMed.
- M. Yamazaki, H. Fujimoto and E. Okuyama, Chem. Pharm. Bull., 1978, 26, 111 CAS.
- S. S. Afiyatullov, A. I. Kalinovskii, M. V. Pivkin, P. S. Dmitrenok and T. A. Kuznetsova, Chem. Nat. Compd., 2005, 41, 236 CrossRef CAS.
- H. H. Wasserman, G. C. Rodgers and D. D. Keith, J. Am. Chem. Soc., 1969, 91, 1263 CrossRef CAS.
- R. Liu, C.-B. Cui, L. Duan, Q.-Q. Gu and W.-M. Zhu, Arch. Pharm. Res., 2005, 28, 1341 Search PubMed.
- F. Peypoux, F. Besson, G. Michel, C. Lenzen, L. Dierickx and L. Delcambe, J. Antibiot., 1980, 33, 1146 CAS.
- G. K. Oleinikova, A. S. Dmitrenok, V. G. Voinov, E. L. Chaikina, L. S. Shevchenko and T. A. Kuznetsova, Chem. Nat. Compd., 2005, 41, 461 CrossRef CAS.
- G. K. Oleinikova, A. S. Dmitrenok, V. G. Voinov, E. L. Chaikina, L. S. Shevchenko and T. A. Kuznetsova, Chem. Nat. Compd., 2005, 41, 240 CrossRef CAS.
- A. Closse, R. Mauli, H. P. Sigg and A. G. Sandoz, Helv. Chim. Acta, 1965, 49, 204 CAS.
- G. Assante, L. Camarda, G. Nasini and L. Merlini, Phytopathol. Mediterr., 1980, 19, 163 Search PubMed.
- M. Sequin-Frey and C. Tamm, Helv. Chim. Acta, 1971, 54, 851 CrossRef CAS.
- A. R. Burnett and R. H. Thomson, J. Chem. Soc. C, 1968, 857 RSC.
- Y. Li, X. Li and B. W. Son, Nat. Prod. Sci., 2005, 11, 136 CAS.
- T. Seki, M. Satake, L. Mackenzie, H. F. Kaspar and T. Yasumoto, Tetrahedron Lett., 1995, 36, 7093 CrossRef.
- M. Dragunow, M. Trzoss, M. A. Brimble, R. Cameron, V. Beuzenberg, P. Holland and D. Mountfort, Environ. Toxicol. Pharmacol., 2005, 20, 305 CrossRef CAS.
- F. M. Lovel, J. Am. Chem. Soc., 1966, 88, 4510 CrossRef.
- P. R. Burkholder, R. M. Pfister and F. H. Leitz, Appl. Microbiol., 1966, 14, 649 Search PubMed.
- J. D. Peschke, U. Hanefeld and H. Laatsch, Biosci., Biotechnol., Biochem., 2005, 69, 628 CrossRef CAS.
- A. D. Rodriguez, J.-G. Shi and S. D. Huang, J. Nat. Prod., 1999, 62, 1228 CrossRef CAS.
- J. M. Boehnlein, L. Z. Santiago-Vázquez and R. G. Kerr, Mar. Ecol.: Prog. Ser., 2005, 303, 105 CrossRef CAS.
- J. S. Sandler, W. Fenical, B. M. Gulledge, A. R. Chamberlin and J. J. La Clair, J. Am. Chem. Soc., 2005, 127, 9320 CrossRef CAS.
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2006, 23, 26 RSC.
- H. Kogen, K. Tago, S. Kaneko, K. Hamano, K. Onodera, H. Haruyama, K. Minagawa, T. Kinoshita, T. Ishikawa, T. Tanimoto and Y. Tsujita, J. Antibiot., 1996, 49, 624 CAS.
- J. T. Handley and A. J. Blackman, Aust. J. Chem., 2005, 58, 39 CrossRef CAS.
- R. J. Andersen and O. Taglialatela-Scafati, J. Nat. Prod., 2005, 68, 1428 CrossRef CAS.
- D. Y. Shi, L. J. Han, J. Sun, S. Li, S. J. Wang, Y. C. Yang, X. Fan and J. G. Shi, Chin. Chem. Lett., 2005, 16, 777 CAS.
- S.-W. Yin, C.-Y. Wang, X.-M. Li and B.-G. Wang, Biochem. Syst. Ecol., 2005, 33, 1288 CrossRef CAS.
- M. T. Hamann, C. S. Otto, P. J. Scheuer and D. C. Dunbar, J. Org. Chem., 1996, 61, 6594 CrossRef CAS.
- M. T. Hamann, C. S. Otto, P. J. Scheuer and D. C. Dunbar, J. Org. Chem., 1998, 63, 4856 CrossRef.
- L. Bourel-Bonnet, K. V. Rao, M. T. Hamann and A. Ganesan, J. Med. Chem., 2005, 48, 1330 CrossRef CAS.
- T. Kajiwara, A. Hatanaka, T. Kawai, M. Ishihara and T. Tuneya, Nippon Suisan Gakkaishi, 1987, 53, 1901 Search PubMed.
- Y. Akakabe, K. Washizu, K. Matsui and T. Kajiwara, Biosci., Biotechnol., Biochem., 2005, 69, 1348 CrossRef CAS.
- K. H. Jang, B. H. Lee, B. W. Choi, H.-S. Lee and J. Shin, J. Nat. Prod., 2005, 68, 716 CrossRef CAS.
- K. Inaoka, Y. Nishizawa, H. Tone and T. Kamiya, Jpn. Kokai Tokkyo Koho, JP H04-49259, 1992.
- M. Iwashima, J. Mori, X. Ting, T. Matsunaga, K. Hayashi, D. Shinoda, H. Saito, U. Sankawa and T. Hayashi, Biol. Pharm. Bull., 2005, 28, 374 CrossRef CAS.
- J. Mori, M. Iwashima, H. Wakasugi, H. Saito, T. Matsunaga, M. Ogasawara, S. Takahashi, H. Suzuki and T. Hayashi, Chem. Pharm. Bull., 2005, 53, 1159 CrossRef CAS.
- D. Abatis, C. Vagias, D. Galanakis, J. N. Norris, D. Moreau, C. Roussakis and V. Roussis, Tetrahedron Lett., 2005, 46, 8525 CrossRef CAS.
- M. Ishitsuka, T. Kusumi, Y. Nomura, T. Konno and H. Kakisawa, Chem. Lett., 1979, 1269 CrossRef CAS.
- L.-A. Tziveleka, D. Abatis, K. Paulus, R. Bauer, C. Vagias and V. Roussis, Chem. Biodiversity, 2005, 2, 901 Search PubMed.
- W. H. Gerwick, W. Fenical, N. Fritsch and J. Clardy, Tetrahedron Lett., 1979, 145 CrossRef CAS.
- O. M. M. Sabry, S. Andrews, K. L. McPhail, D. E. Goeger, A. Yokochi, K. T. LePage, T. F. Murray and W. H. Gerwick, J. Nat. Prod., 2005, 68, 1022 CrossRef CAS.
- F. Song, X. Xu, S. Li, S. Wang, J. Zhao, P. Cao, Y. Yang, X. Fan, J. Shi, L. He and Y. Lü, J. Nat. Prod., 2005, 68, 1309 CrossRef CAS.
- A. Ortalo-Magné, G. Culioli, R. Valls, B. Pucci and L. Piovetti, Phytochemistry, 2005, 66, 2316 CrossRef CAS.
- J. F. Biard, J. F. Verbist, R. Floch and Y. Letourneux, Tetrahedron Lett., 1980, 21, 1849 CrossRef CAS.
- T. Kusumi, Y. Shibata, M. Ishizuka, T. Kinoshita and H. Kakizawa, Chem. Lett., 1979, 277 CrossRef CAS.
- C. K. Tsang, A. Ina, T. Goto and Y. Kamei, Neuroscience, 2005, 132, 633 CrossRef CAS.
- C. F. Cross, E. J. Bevan and J. F. Briggs, Chem.–Ztg., 1907, 31, 725 Search PubMed.
- H. S. Kang, H. Y. Chung, J. H. Jung, B. W. Son and J. S. Choi, Chem. Pharm. Bull., 2003, 51, 1012 CrossRef CAS.
- Y. Fukuyama, M. Kodama, I. Miura, Z. Kinzyo, M. Kido, H. Mori, Y. Nakayama and M. Takahashi, Chem. Pharm. Bull., 1989, 37, 349 CAS.
- Y. Fukuyama, M. Kodama, I. Miura, Z. Kinzyo, H. Mori, Y. Nakayama and M. Takahashi, Chem. Pharm. Bull., 1990, 38, 133 CAS.
- Y. Fukuyama, I. Miura, Z. Kinzyo, Y. Nakayama, M. Takahashi and M. Kido, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1983, 26, 126 Search PubMed.
- Y. C. Kim, R. B. An, N. Y. Yoon, T. J. Nam and J. S. Choi, Arch. Pharm. Res., 2005, 28, 1376 Search PubMed.
- M. Ochi, H. Kotsuki, K. Muraoka and T. Tokoroyama, Bull. Chem. Soc. Jpn., 1979, 52, 629 CAS.
- K. Kurata, K. Taniguchi and M. Suzuki, Phytochemistry, 1996, 41, 749 CrossRef CAS.
- W. Fenical, J. J. Sims, D. Squatrito, R. M. Wing and P. Radlick, J. Org. Chem., 1973, 38, 2383 CrossRef CAS.
- T. Laube, W. Beil and K. Seifert, Tetrahedron, 2005, 61, 1141 CrossRef CAS.
- A. A. El-Gamal, W.-L. Wang and C.-Y. Duh, J. Nat. Prod., 2005, 68, 815 CrossRef CAS.
- N. K. Kubota, H. Iwamoto, Y. Fukazawa and Y. Uchio, Heterocycles, 2005, 65, 2675 CrossRef CAS.
- J. Sun, D. Shi, M. Ma, S. Li, S. Wang, L. Han, Y. Yang, X. Fan, J. Shi and L. He, J. Nat. Prod., 2005, 68, 915 CrossRef CAS.
- K. Yamada, H. Yazawa, D. Uemura, M. Toda and Y. Hirata, Tetrahedron, 1969, 25, 3509 CrossRef CAS.
- H. Nemoto, M. Nagamochi, H. Ishibashi and K. Fukumoto, J. Org. Chem., 1994, 59, 74 CrossRef CAS.
- J. Sun, L. J. Han, D. Y. Shi, X. Fan, S. J. Wang, S. Li, Y. C. Yang and J. G. Shi, Chin. Chem. Lett., 2005, 16, 1611 CAS.
- D. J. Goldsmith, T. K. John, C. D. Kwong and G. R. Painter, III, J. Org. Chem., 1980, 45, 3989 CrossRef CAS.
- Y. Shizuri and K. Yamada, Phytochemistry, 1985, 24, 1385 CrossRef CAS.
- M. Kladi, C. Vagias, G. Furnari, D. Moreau, C. Roussakis and V. Roussis, Tetrahedron Lett., 2005, 46, 5723 CrossRef CAS.
- S. Mao and Y. Guo, Helv. Chim. Acta, 2005, 88, 1034 CrossRef CAS.
- J. J. Fernández, M. L. Souto, L. V. Gil and M. Norte, Tetrahedron, 2005, 61, 8910 CrossRef CAS.
- M. Kuniyoshi, P. G. Wahome, T. Miono, T. Hashimoto, M. Yokoyama, K. L. Shrestha and T. Higa, J. Nat. Prod., 2005, 68, 1314 CrossRef.
- M. Suzuki, T. Kawamoto, C. S. Vairappan, T. Ishii, T. Abe and M. Masuda, Phytochemistry, 2005, 66, 2787 CrossRef CAS.
- O. J. McConnell and W. Fenical, J. Org. Chem., 1978, 43, 4238 CrossRef CAS.
- V. J. Paul, O. J. McConnell and W. Fenical, J. Org. Chem., 1980, 45, 3401 CrossRef CAS.
- J. Kimura, Y. Tobita, T. Motoyama, Y. Ataka and Y. Takada, J. Nat. Prod., 2005, 68, 585 CrossRef CAS.
- S. Mataka, H. Eguchi, K. Takahashi, T. Hatta and M. Tashiro, Bull. Chem. Soc. Jpn., 1989, 62, 3127.
- K. Nishizawa and J. Y. Satoh, Chem. Soc. Jpn., 1975, 48, 1875 Search PubMed.
- J. E. Lightowler and H. J. Rylance, Br. J. Pharmacol., 1964, 22, 221 CAS.
- V. L. Afanas'eva, A. V. Lyubeshkin, S. S. Kovaleva, M. R. Bagreeva, O. S. Anisimova and R. G. Glushkov, Khim.-Farm. Zh., 1987, 21, 1367 CAS.
- W. Wang, Y. Okada, H. Shi, Y. Wang and T. Okuyama, J. Nat. Prod., 2005, 68, 620 CrossRef CAS.
- J. Zhao, M. Ma, S. Wang, S. Li, P. Cao, Y. Yang, Y. Lü, J. Shi, N. Xu, X. Fan and L. He, J. Nat. Prod., 2005, 68, 691 CrossRef CAS.
- D. B. Stierle, R. M. Wing and J. J. Sims, Tetrahedron, 1979, 35, 2855 CrossRef CAS.
- M. D. Higgs, D. J. Vanderah and D. J. Faulkner, Tetrahedron, 1977, 33, 2775 CrossRef CAS.
- C. Ireland, M. O. Stallard, D. J. Faulkner, J. Finer and J. Clardy, J. Org. Chem., 1976, 41, 2461 CrossRef CAS.
- M. G. Knott, H. Mkwananzi, C. E. Arendse, D. T. Hendricks, J. J. Bolton and D. R. Beukes, Phytochemistry, 2005, 66, 1108 CrossRef CAS.
- W.-H. Zhang, H.-M. Zhong and C.-T. Che, J. Asian Nat. Prod. Res., 2005, 7, 59 Search PubMed.
- M. Lorenzo, M. Cueto, A. San-Martín, V. Fajardo and J. Darias, Tetrahedron, 2005, 61, 9550 CrossRef CAS.
- M. Kitamura, T. Koyama, Y. Nakano and D. Uemura, Chem. Lett., 2005, 34, 1272 CrossRef CAS.
- J. Kubanek, A. C. Prusak, T. W. Snell, R. A. Giese, K. I. Hardcastle, C. R. Fairchild, W. Aalbersberg, C. Raventos-Suarez and M. E. Hay, Org. Lett., 2005, 7, 5261 CrossRef CAS.
- M. A. Graber, W. H. Gerwick and D. P. Cheney, Tetrahedron Lett., 1996, 37, 4635 CrossRef CAS.
- H. Miyaoka, Y. Hara, I. Shinohara, T. Kurokawa and Y. Yamada, Tetrahedron Lett., 2005, 46, 7945 CrossRef CAS.
- A. Lopez and W. H. Gerwick, Lipids, 1987, 22, 190 CrossRef CAS.
- T. Tsuzuki, K. Tanaka, S. Kuwahara and T. Miyazawa, Lipids, 2005, 40, 147 CrossRef CAS.
- E. Kurosawa, A. Fukuzawa and T. Irie, Tetrahedron Lett., 1973, 42, 4135 CrossRef.
- H. Lee, H. Kim, T. Yoon, B. Kim, S. Kim, H.-D. Kim and D. Kim, J. Org. Chem., 2005, 70, 8723 CrossRef CAS.
- M. Maeda, T. Kodama, T. Tanaka, H. Yoshizumi, T. Takemoto, K. Nomoto and T. Fujita, Chem. Pharm. Bull., 1986, 34, 4892 CAS.
- J. Clayden, F. E. Knowles and I. R. Baldwin, J. Am. Chem. Soc., 2005, 127, 2412 CrossRef CAS.
- J. Y. Su, Y. L. Zhong, L. M. Zeng, H. M. Wu and K. Ma, Phytochemistry, 1995, 40, 195 CrossRef CAS.
- J. Justicia, J. L. Oller-López, A. G. Campãna, J. E. Oltra, J. M. Cuerva, E. Buñuel and D. J. Cárdenas, J. Am. Chem. Soc., 2005, 127, 14911 CrossRef CAS.
- H. J. Park, Park, H. Y. Chung, J. Kim and J. S. Choi, J. Fish. Sci. Technol., 1999, 2, 1 Search PubMed.
- H. Park, M. Kurokawa, K. Shiraki, N. Nakamura, J. Choi and M. Hattori, Biol. Pharm. Bull., 2005, 28, 2258 CrossRef CAS.
- S. Tilvi, M. Majik and C. G. Naik, Eur. J. Mass Spectrom., 2005, 11, 345 CrossRef CAS.
- G. W. Zhang, X. Q. Ma, C. X. Zhang, J. Y. Su, W. C. Ye, X. Q. Zhang, X. S. Yao and L. M. Zeng, Helv. Chim. Acta, 2005, 88, 885 CrossRef CAS.
- S. N. Ayyad, Mansoura Sci. Bull., A: Chem., 2004, 31, 115 Search PubMed.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, Eur. J. Org. Chem., 2005, 2, 368 CrossRef.
- C. Emura, R. Higuchi, T. Miyamoto and R. W. M. Van Soest, J. Org. Chem., 2005, 70, 3031 CrossRef CAS.
- V. Costantino, M. D'Esposito, E. Fattorusso, A. Mangoni, N. Basilico, S. Parapini and D. Taramelli, J. Med. Chem., 2005, 48, 7411 CrossRef CAS.
- T. N. Makarieva, A. G. Guzii, V. A. Denisenko, P. S. Dmitrenok, E. A. Santalova, E. V. Pokanevich, T. F. Molinski and V. A. Stonik, J. Nat. Prod., 2005, 68, 255 CrossRef CAS.
- L. Barbieri, V. Costantino, E. Fattorusso and A. Mangoni, J. Nat. Prod., 2005, 68, 1527 CrossRef CAS.
- T. N. Makarieva, V. A. Denisenko, P. S. Dmitrenok, A. G. Guzii, E. A. Santalova, V. A. Stonik, J. B. MacMillan and T. F. Molinski, Org. Lett., 2005, 7, 2897 CrossRef CAS.
- N. L. Segraves and P. Crews, J. Nat. Prod., 2005, 68, 118 CrossRef CAS.
- K. Warabi, T. Hamada, Y. Nakao, S. Matsunaga, H. Hirota, R. W. M. van Soest and N. Fusetani, J. Am. Chem. Soc., 2005, 127, 13262 CrossRef CAS.
- T. A. Mansoor, B. H. Bae, J. Hong, C. O. Lee, K. S. Im and J. H. Jung, Lipids, 2005, 40, 981 CrossRef CAS.
- M. Ishibashi, S. Takeuchi and J. Kobayashi, Tetrahedron Lett., 1993, 34, 3749 CrossRef CAS.
- M. Tsuda, T. Endo, M. Perpelescu, S. Yoshida, K. Watanabe, J. Fromont, Y. Mikami and J. Kobayashi, Tetrahedron, 2003, 59, 1137 CrossRef CAS.
- H. Mizutani, M. Watanabe and T. Honda, Synlett, 2005, 793 CAS.
- S. C. Jain, R. Kumar, R. Goswami, M. K. Pandey, S. Khurana, L. Rohatgi and K. Gyanda, Pure Appl. Chem., 2005, 77, 185 CrossRef CAS.
- S. Tsukamoto, M. Takahashi, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 682 CrossRef CAS.
- A. P. Dobbs, A. Venturelli, L. A. Butler and R. J. Parker, Synlett, 2005, 4, 652 CrossRef.
- A. Kawakami, T. Miyamoto, R. Higuchi, M. Kuwano and R. W. M. van Soest, Tetrahedron Lett., 2001, 42, 3335 CrossRef CAS.
- N. Sudhakar, A. Ravi Kumar, A. Prabhakar, B. Jagadeesh and B. Venkateswara Rao, Tetrahedron Lett., 2005, 46, 325 CrossRef CAS.
- P. Bhaket, K. Morris, C. S. Stauffer and A. Datta, Org. Lett., 2005, 7, 875 CrossRef CAS.
- I. Kuroda, M. Musman, I. I. Ohtani, T. Ichiba, J. Tanaka, D. G. Gravalos and T. Higa, J. Nat. Prod., 2002, 65, 1505 CrossRef CAS.
- V. Ledroit, C. Debitus, C. Lavaud and G. Massiot, Tetrahedron Lett., 2003, 44, 225 CrossRef CAS.
- R. G. Linington, M. Robertson, A. Gauthier, B. B. Findlay, R. van Soest and R. J. Andersen, Org. Lett., 2002, 4, 4089 CrossRef CAS.
- J. Sun, X. Han and B. Yu, Synlett, 2005, 437.
- K. Watanabe, Y. Tsuda, M. Hamada, M. Omori, G. Mori, K. Iguchi, H. Naoki, T. Fujita and R. W. M. Van Soest, J. Nat. Prod., 2005, 68, 1001 CrossRef CAS.
- M. L. Lerch, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 2003, 66, 667 CrossRef CAS.
- B. W. Gung, C. Gibeau and A. Jones, Tetrahedron: Asymmetry, 2005, 16, 3107 CrossRef CAS.
- H. Tada and F. Yasuda, Chem. Lett., 1984, 779 CrossRef CAS.
- N. Fusetani, M. Sugano, S. Matsunaga and K. Hashimoto, Tetrahedron Lett., 1987, 28, 4311 CrossRef CAS.
- S. López, F. Fernández-Trillo, P. Midón, L. Castedo and C. Saá, J. Org. Chem., 2005, 70, 6346 CrossRef CAS.
- T. Yosief, A. Rudi, Y. Wolde-ab and Y. Kashman, J. Nat. Prod., 1998, 61, 491 CrossRef CAS.
- A. Fontana, G. d'Ippolito, L. D'Souza, E. Mollo, P. S. Parameswaram and G. Cimino, J. Nat. Prod., 2001, 64, 131 CrossRef CAS.
- C. Xu, J. M. Raible and P. H. Dussault, Org. Lett., 2005, 7, 2509 CrossRef CAS.
- N. Sata, H. Abinsay, W. Y. Yoshida, F. D. Horgen, N. Sitachitta, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2005, 68, 1400 CrossRef CAS.
- M. Holzwarth, J. M. Trendel, P. Albrecht, A. Maier and W. Michaelis, J. Nat. Prod., 2005, 68, 759 CrossRef CAS.
- R. A. Epifanio, L. S. Pinheiro and N. C. Alves, J. Braz. Chem. Soc., 2005, 16, 1367 CAS.
- R. J. Capon, S. Singh, S. Ali and S. Sotheeswaran, Aust. J. Chem., 2005, 58, 18 CrossRef CAS.
- F. Berrué, O. P. Thomas, R. Fernández and P. Amade, J. Nat. Prod., 2005, 68, 547 CrossRef CAS.
- F. Berrué, O. P. Thomas, C. F. L. Bon, F. Reyes and P. Amade, Tetrahedron, 2005, 61, 11843 CrossRef CAS.
- C. Campagnuolo, E. Fattorusso, A. Romano, O. Taglialatela-Scafati, N. Basilico, S. Parapini and D. Taramelli, Eur. J. Org. Chem., 2005, 23, 5077 CrossRef.
- D. B. Stierle and D. J. Faulkner, J. Org. Chem., 1980, 45, 3396 CrossRef CAS.
- M. Akiyama, Y. Isoda, M. Nishimoto, A. Kobayashi, D. Togawa, N. Hirao, A. Kuboki and S. Ohira, Tetrahedron Lett., 2005, 46, 7483 CrossRef CAS.
- A. S. Ratnayake, R. A. Davis, M. K. Harper, C. A. Veltri, C. D. Andjelic, L. R. Barrows and C. M. Ireland, J. Nat. Prod., 2005, 68, 104 CrossRef CAS.
- A. C. Granato, J. H. H. L. de Oliveira, M. H. R. Seleghim, R. G. S. Berlinck, M. L. Macedo, A. G. Ferreira, R. M. da Rocha, E. Hajdu, S. Peixinho, C. O. Pessoa, M. O. Moraes and B. C. Cavalcanti, Quim. Nova, 2005, 28, 192 CAS.
- S. Cao, C. Foster, M. Brisson, J. S. Lazo and D. G. I. Kingston, Bioorg. Med. Chem., 2005, 13, 999 CrossRef CAS.
- N. Sata, M. A. Galario, N. Sitachitta, P. J. Scheuer and M. Kelly, J. Nat. Prod., 2005, 68, 262 CrossRef CAS.
- R. H. Cichewicz, F. A. Valeriote and P. Crews, Org. Lett., 2004, 6, 1951 CrossRef CAS.
- G. R. Pettit, J. Xu, J. Chapuis, R. K. Pettit, L. P. Tackett, D. L. Doubek, J. N. A. Hooper and J. M. Shmidt, J. Med. Chem., 2004, 47, 1149 CrossRef CAS.
- X. Jiang, J. García-Fortanet and J. K. De Brabander, J. Am. Chem. Soc., 2005, 127, 11254 CrossRef CAS.
- M. E. Green, J. C. Rech and P. E. Floreancig, Org. Lett., 2005, 7, 4117 CrossRef CAS.
- S. Kanazawa, N. Fusetani and S. Matsunaga, Tetrahedron Lett., 1993, 34, 1065 CrossRef CAS.
- N. Cramer, S. Laschat, A. Baro, H. Schwalbe and C. Richter, Angew. Chem., Int. Ed., 2005, 44, 820 CrossRef CAS.
- N. Fusetani, T. Sugawara, S. Matsunaga and H. Hirota, J. Org. Chem., 1991, 56, 4971 CrossRef CAS.
- S. Nishimura, S. Matsunaga, M. Yoshida, H. Hirota, S. Yokoyama and N. Fusetani, Bioorg. Med. Chem., 2005, 13, 449 CrossRef CAS.
- P. T. Northcote, J. W. Blunt and M. H. G. Munro, Tetrahedron Lett., 1991, 32, 6411 CrossRef CAS.
- M. E. Bordeleau, J. Mathews, J. Wojnar, L. Linquist, O. Novac, E. Janowsky, N. Sonenburg, P. Northcote, P. Teesdale-Spittle and J. Pelletier, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10460 CrossRef CAS.
- W. K. Low, Y. J. Dang, T. Schneider-Poetsch, Z. G. Shi, N. S. Choi, W. C. Merrick, D. Romo and J. O. Liu, Mol. Cell, 2005, 20, 709 CrossRef CAS.
- T. Araki, S. Matsunaga and N. Fusetani, Biosci., Biotechnol., Biochem., 2005, 69, 1318 CrossRef CAS.
- N. Kotoku, L. Cao, S. Aoki and M. Kobayashi, Heterocycles, 2005, 65, 563 CrossRef CAS.
- T. Hamada, T. Sugawara, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1994, 35, 719 CrossRef CAS.
- T. Hamada, T. Sugawara, S. Matsunaga and N. Fusetani, Tetrahedron Lett., 1994, 35, 609 CrossRef CAS.
- T. Hamada, S. Matsunaga, G. Yano and N. Fusetani, J. Am. Chem. Soc., 2005, 127, 110 CrossRef CAS.
- K. C. Nicolaou, D. Schlawe, D. W. Kim, D. A. Longbottom, R. G. de Noronha, D. E. Lizos, R. R. Manam and D. J. Faulkner, Chem.–Eur. J., 2005, 11, 6197 CrossRef CAS.
- A. Randazzo, G. Bifulco, C. Giannini, M. Bucci, C. Debitus, G. Cirino and L. Gomez-Paloma, J. Am. Chem. Soc., 2001, 123, 10870 CrossRef CAS.
- K. C. Nicolaou, D. W. Kim, D. Schlawe, D. E. Lizos, R. G. de Noronha and D. A. Longbottom, Angew. Chem., Int. Ed., 2005, 44, 4925 CrossRef CAS.
- G. R. Pettit and R. Tan, J. Nat. Prod., 2005, 68, 60 CrossRef CAS.
- D. E. Williams, B. O. Patrick, H. W. Behrisch, R. Van Soest, M. Roberge and R. J. Andersen, J. Nat. Prod., 2005, 68, 327 CrossRef CAS.
- G. R. Pettit, J. Xu, A. Dorsaz and M. D. Williams, Bioorg. Med. Chem. Lett., 1995, 5, 1339 CrossRef CAS.
- A. Napolitano, I. Bruno, R. Riccio and L. Gomez-Paloma, Tetrahedron, 2005, 61, 6808 CrossRef CAS.
- A. Zampella, M. V. D'Auria, L. Gomez-Paloma, A. Casapullo, L. Minale, C. Debitus and Y. Henin, J. Am. Chem. Soc., 1996, 118, 6202 CrossRef CAS.
- A. Zampella, R. D'Orsi, V. Sepe, A. Casapullo, M. C. Monti and M. V. D'Auria, Org. Lett., 2005, 7, 3585 CrossRef CAS.
- N. Oku, A. G. Benson Krishnamoorthy, R. L. Ferguson, M. Z. Lipton, L. R. Phillips, K. R. Gustafson and J. B. McMahon, J. Org. Chem., 2005, 70, 6842 CrossRef CAS.
- P. W. Ford, K. R. Gustafson, T. C. McKee, N. Shigematsu, L. K. Maurizi, L. K. Pannell, D. E. Williams, E. D. de Silva and P. Lassota, J. Am. Chem. Soc., 1999, 121, 5899 CrossRef CAS.
- K. Makino, E. Nagata and Y. Hamada, Tetrahedron Lett., 2005, 46, 6827 CrossRef CAS.
- N. Oku, K. R. Gustafson, L. K. Cartner, J. A. Wilson, N. Shigamatsu, S. Hess, L. K. Pannell, M. R. Boyd and J. B. McMahon, J. Nat. Prod., 2004, 67, 1407 CrossRef CAS.
- D. E. Williams, P. Austin, A. R. Diaz-Marrero, R. Van Soest, T. Matainaho, C. D. Roskelley, M. Roberge and R. J. Andersen, Org. Lett., 2005, 7, 4173 CrossRef CAS.
- P. Phuwapraisirisan, S. Matsunaga and N. Fusetani, Org. Lett., 2005, 7, 2233 CrossRef CAS.
- S. Tsukamoto, K. Koimaru and K. Ohta, Mar. Drugs, 2005, 3, 29 Search PubMed.
- G. R. Pettit, Z. A. Chicaz, F. Gao, C. L. Herald, M. R. Boyd, J. M. Schmidt and J. N. A. Hooper, J. Org. Chem., 1993, 58, 1302 CrossRef CAS.
- R. K. Pettit, T. Woyke, S. Pon, Z. A. Cichacz, G. R. Pettit and C. L. Herald, Med. Mycol., 2005, 43, 453 Search PubMed.
- V. A. Klenchin, R. King, J. Tanaka, G. Marriott and I. Rayment, Chem. Biol., 2005, 12, 287 CrossRef CAS.
- N. Takada, H. Sato, K. Suenaga, H. Arimoto, K. Yamada, K. Ueda and D. Uemura, Tetrahedron Lett., 1999, 40, 6309 CrossRef CAS.
- T. R. Hoye and J. Wang, J. Am. Chem. Soc., 2005, 127, 6950 CrossRef CAS.
- M. R. Rao and D. J. Faulkner, J. Nat. Prod., 2002, 65, 386 CrossRef CAS.
- C. S. Barry, N. Bushby, J. P. H. Charmant, J. D. Elsworth, J. R. Harding and C. L. Willis, Chem. Commun., 2005, 5097 RSC.
- M. S. Agrawal and B. F. Bowden, Mar. Drugs, 2005, 3, 9 Search PubMed.
- B. Carte and D. J. Faulkner, Tetrahedron, 1981, 37, 2335 CrossRef CAS.
- Y. M. Xu, R. K. Johnson and S. M. Hecht, Bioorg. Med. Chem., 2005, 13, 657 CrossRef CAS.
- R. Kazlauskas, R. O. Lidgard, P. T. Murphy and R. J. Wells, Tetrahedron Lett., 1980, 21, 2277 CrossRef CAS.
- R. Kazlauskas, R. O. Lidgard, P. T. Murphy, R. J. Wells and J. F. Blount, Aust. J. Chem., 1981, 34, 765 CAS.
- E. O. Pordesimo and F. J. Schmitz, J. Org. Chem., 1990, 55, 4704 CrossRef CAS.
- M. S. Butler, T. K. Lim, R. J. Capon and L. S. Hammond, Aust. J. Chem., 1991, 44, 287 CAS.
- E. A. Couladouros, E. N. Pitsinos, V. I. Moutsos and G. Sarakinos, Chem.–Eur. J., 2005, 11, 406 CrossRef.
- P. Sauleau, P. Retailleau, J. Vacelet and M.-L. Bourguet-Kondracki, Tetrahedron, 2005, 61, 955 CrossRef CAS.
- A. Ardá, J. Rodríguez, R. M. Nieto, C. Bassarello, L. Gomez-Paloma, G. Bifulco and C. Jiménez, Tetrahedron, 2005, 61, 10093 CrossRef CAS.
- R. J. Capon, D. Vuong, E. Lacey and J. H. Gill, J. Nat. Prod., 2005, 68, 179 CrossRef CAS.
- R. J. Capon, D. Vuong, M. McNally, T. Peterle, N. Trotter, E. Lacey and J. H. Gill, Org. Biomol. Chem., 2005, 3, 118 RSC.
- H. Suzuki, K. Shindo, A. Ueno, T. Miura, M. Takei, M. Sakakibara, H. Fukamachi, J. Tanaka and T. Higa, Bioorg. Med. Chem. Lett., 1999, 9, 1361 CrossRef CAS.
- R. A. Fairhurst, D. Janus and A. Lawrence, Org. Lett., 2005, 7, 4697 CrossRef CAS.
- J. G. Ondeyka, K. B. Herath, H. Jayasuriya, J. D. Polishook, G. F. Bills, A. W. Dombrowski, M. Mojena, G. Koch, J. DiSalvo, J. DeMartino, Z. Guan, W. Nanakorn and C. Morenberg, Mol. Diversity, 2005, 9, 123 Search PubMed.
- M. Tsuda, K. Hirano and J. Kobayashi, Tetrahedron Lett., 1999, 40, 4819 CrossRef CAS.
- S. P. Romeril, V. Lee, J. E. Baldwin and T. D. W. Claridge, Tetrahedron, 2005, 61, 1127 CrossRef CAS.
- H. Ishiyama, M. Tsuda, T. Endo and J. Kobayashi, Molecules, 2005, 10, 312 Search PubMed.
- R. A. Keyzers, C. E. Arendse, D. T. Hendricks, T. Samaai and M. T. Davies-Coleman, J. Nat. Prod., 2005, 68, 506 CrossRef CAS.
- G. Lang, A. Pinkert, J. W. Blunt and M. H. G. Munro, J. Nat. Prod., 2005, 68, 1796 CrossRef CAS.
- N. K. Utkina, A. E. Makarchenko and V. A. Denisenko, J. Nat. Prod., 2005, 68, 1424 CrossRef CAS.
- N. L. Segraves and P. Crews, J. Nat. Prod., 2005, 68, 1484 CrossRef CAS.
- S. Rubnov, C. Chevallier, O. Thoison, C. Debitus, O. Laprevote, D. Grénard and T. Sévenet, Nat. Prod. Res., 2005, 19, 75 CrossRef CAS.
- M. Tsuda, Y. Takahashi, J. Fromont, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2005, 68, 1277 CrossRef CAS.
- B. Q. Bao, Q. S. Sun, X. S. Yao, J. K. Hong, C. O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 711 CrossRef CAS.
- K. B. Oh, W. Mar, S. Kim, J. Y. Kim, M. N. Oh, J. G. Kim, D. Shin, C. J. Sim and J. Shin, Bioorg. Med. Chem. Lett., 2005, 15, 4927 CrossRef CAS.
- D. T. A. Youssef, J. Nat. Prod., 2005, 68, 1416 CrossRef CAS.
- Y. Iinuma, S. Kozawa, H. Ishiyama, M. Tsuda, E. Fukushi, J. Kawabata, J. Fromont and J. Kobayashi, J. Nat. Prod., 2005, 68, 1109 CrossRef CAS.
- V. K. Rao, N. Kasanah, S. Wahyuono, B. L. Tekwani, R. F. Schinazi and M. T. Hamann, J. Nat. Prod., 2004, 67, 1314 CrossRef CAS.
- A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS.
- T. Kouko, K. Matsumura and T. Kawasaki, Tetrahedron, 2005, 61, 2309 CrossRef CAS.
- A. Aiello, M. D'Esposito, E. Fattorusso, M. Menna, W. E. G. Müller, S. Perovic-Ottstadt, H. Tsuruta, T. A. M. Gulder and G. Bringmann, Tetrahedron, 2005, 61, 7266 CrossRef CAS.
- A. R. Carroll, A. Ngo, R. J. Quinn, J. Redburn and J. N. A. Hooper, J. Nat. Prod., 2005, 68, 804 CrossRef CAS.
- N. B. Perry, L. Ettouati, M. Litaudon, J. W. Blunt and M. H. G. Munro, Tetrahedron, 1994, 50, 3987 CrossRef CAS.
- M. Simone, E. Erba, G. Damia, F. Vikhanskaya, A. M. Di Francesco, R. Riccardi, C. Bailly, C. Cuevas, J. M. F. Sousa-Faro and M. D'Incalci, Eur. J. Cancer, 2005, 41, 2366 CrossRef CAS.
- G. R. Pettit, J. C. Knight, J. C. Collins, D. L. Herald, R. K. Pettit, M. R. Boyd and V. G. Young, J. Nat. Prod., 2000, 63, 793 CrossRef CAS.
- C. Chan, R. Heid, S. Zheng, J. Guo, B. Zhou, T. Furuuchi and S. J. Danishefsky, J. Am. Chem. Soc., 2005, 127, 4596 CrossRef CAS.
- B. S. Davidson, Tetrahedron Lett., 1992, 33, 3721 CrossRef CAS.
- P. Magnus and K. S. Matthews, J. Am. Chem. Soc., 2005, 127, 12476 CrossRef CAS.
- J. W. Lane, Y. Chen and R. M. Williams, J. Am. Chem. Soc., 2005, 127, 12684 CrossRef CAS.
- K. Warabi, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Org. Chem., 2003, 68, 2765 CrossRef CAS.
- A. Fürstner, M. M. Domostoj and B. Scheiper, J. Am. Chem. Soc., 2005, 127, 11620 CrossRef.
- J. F. Hu, J. Peng, A. B. Kazi, M. Kelly and M. T. Hamann, J. Chem. Res., 2005, 7, 427 Search PubMed.
- E. Fattorusso and O. Taglialatela-Scafati, Tetrahedron Lett., 2000, 41, 9917 CrossRef CAS.
- G. Papeo, M. A. G. Z. Frau, D. Borghi and M. Varasi, Tetrahedron Lett., 2005, 46, 8635 CrossRef CAS.
- J. Patel, N. Pelloux-Léon, F. Minassian and Y. Vallée, J. Org. Chem., 2005, 70, 9081 CrossRef CAS.
- W. A. Gallimore, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2005, 68, 1420 CrossRef CAS.
- J. C. Braekman, D. Daloze, H. Tavares, E. Hajdu and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 193 CrossRef CAS.
- Z. D. Aron and L. E. Overman, J. Am. Chem. Soc., 2005, 127, 3380 CrossRef CAS.
- R. G. S. Berlinck, J. C. Braekman, D. Daloze, I. Bruno, R. Riccio, S. Ferri, S. Spampinato and E. Speroni, J. Nat. Prod., 1993, 56, 1007 CrossRef CAS.
- L. E. Overman and Y. H. Rhee, J. Am. Chem. Soc., 2005, 127, 15652 CrossRef CAS.
- K. Ravinder, A. V. Reddy, T. V. Raju and Y. Venkateswarlu, ARKIVOC, 2005, 51 CAS.
- S. Matsunaga, H. Kobayashi, R. W. M. van Soest and N. Fusetani, J. Org. Chem., 2005, 70, 1893 CrossRef CAS.
- F. C. Schroeder, T. R. Kau, P. A. Silver and J. Clardy, J. Nat. Prod., 2005, 68, 574 CrossRef CAS.
- S. N. Georgiades and J. Clardy, Org. Lett., 2005, 7, 4091 CrossRef CAS.
- A. Kijjoa, J. Bessa, R. Wattanadilok, P. Sawangwong, M. S. J. Nascimento, M. Pedro, A. M. S. Silva, G. Eaton, R. van Soest and W. Herz, Z. Naturforsch., B: Chem. Sci., 2005, 60, 904 CAS.
- Y. Gopichand and F. J. Schmitz, Tetrahedron Lett., 1979, 3921 CrossRef CAS.
- G. M. König and A. D. Wright, Heterocycles, 1993, 36, 1351 Search PubMed.
- E. W. Rogers, M. F. de Oliveira, R. G. S. Berlinck, G. M. König and T. F. Molinski, J. Nat. Prod., 2005, 68, 891 CrossRef CAS.
- R. D. Encarnacion, E. Sandoval, J. Malmstrom and C. Christophersen, J. Nat. Prod., 2000, 63, 874 CrossRef.
- T. Ogamino and S. Nishiyama, Tetrahedron Lett., 2005, 46, 1083 CrossRef CAS.
- R. J. Capon and N. S. Trotter, J. Nat. Prod., 2005, 68, 1689 CrossRef CAS.
- H. Wu, H. Nakamura, J. Kobayashi, Y. Ohizumi and Y. Hirata, Tetrahedron Lett., 1984, 25, 3719 CrossRef CAS.
- A. K. Bakkestuen, L. L. Gundersen, D. Petersen, B. T. Utenova and A. Vik, Org. Biomol. Chem., 2005, 3, 1025 RSC.
- T. Hattori, K. Adachi and Y. Shizuri, J. Nat. Prod., 1997, 60, 411 CrossRef CAS.
- H. Nakamura, H. Wu, Y. Ohizumi and Y. Hirata, Tetrahedron Lett., 1984, 25, 2989 CrossRef CAS.
- I. S. Marcos, N. García, A. J. Sexmero, P. Basabe, D. Díez and J. G. Urones, Tetrahedron, 2005, 61, 11672 CrossRef CAS.
- E. Pérez-García, E. Zubía, M. J. Ortega and J. L. Carballo, J. Nat. Prod., 2005, 68, 653 CrossRef CAS.
- C. E. McNamara, L. Larsen, N. B. Perry, J. L. Harper, M. V. Berridge, E. W. Chia, M. Kelly and V. L. Webb, J. Nat. Prod., 2005, 68, 1431 CrossRef CAS.
- K. Ueda, T. Ueta, E. R. O. Siwu, M. Kita and D. Uemura, Chem. Lett., 2005, 34, 1530 CrossRef CAS.
- Y. Venkateswarlu, D. J. Faulkner, J. L. R. Steiner, E. Corcoran and J. Clardy, J. Org. Chem., 1991, 56, 6271 CrossRef CAS.
- A. Rudi, Y. Benayahu and Y. Kashman, Org. Lett., 2004, 6, 4013 CrossRef CAS.
- B. S. Olson and D. Trauner, Synlett, 2005, 4, 700.
- H. Liu, M. Namikoshi, K. Akano, H. Kobayashi, H. Nagai and X. Yao, J. Asian Nat. Prod. Res., 2005, 7, 661 Search PubMed.
- E. Goclik, G. M. König, A. D. Wright and R. Kaminsky, J. Nat. Prod., 2000, 63, 1150 CrossRef CAS.
- J. H. Kwak, F. J. Schmitz and M. Kelly, J. Nat. Prod., 2000, 63, 1153 CrossRef CAS.
- L. Yang, D. E. Williams, A. Mui, C. Ong, G. Krystal, R. van Soest and R. J. Andersen, Org. Lett., 2005, 7, 1073 CrossRef CAS.
- S. S. Nasu, B. K. S. Yeung, M. T. Hamann, P. J. Scheuer, M. Kelly-Borges and K. Goin, J. Org. Chem., 1995, 60, 7290 CrossRef CAS.
- E. J. Alvarez-Manzaneda, R. Chahboun, I. B. Pérez, E. Cabrera, E. Alvarez and R. Alvarez-Manzaneda, Org. Lett., 2005, 7, 1477 CrossRef CAS.
- G. Cimino, S. De Stefano and L. Minale, Tetrahedron, 1973, 29, 2565 CrossRef CAS.
- C. J. Davis, T. E. Hurst, A. M. Jacob and C. J. Moody, J. Org. Chem., 2005, 70, 4414 CrossRef CAS.
- Y. Letourneux, J. M. Brunel, R. Fernandez, M. Dherbomez and C. Debitus, Heterocycl. Commun., 2005, 11, 291 CAS.
- M. J. Martín, F. Berrué, P. Amade, R. Fernández, A. Francesch, F. Reyes and C. Cuevas, J. Nat. Prod., 2005, 68, 1554 CrossRef CAS.
- A. M. Piggott and P. Karuso, Molecules, 2005, 10, 1292 Search PubMed.
- H. Ishiyama, A. Hashimoto, J. Fromont, Y. Hoshino, Y. Mikami and J. Kobayashi, Tetrahedron, 2005, 61, 1101 CrossRef CAS.
- R. H. Cichewicz, L. J. Clifford, P. R. Lassen, X. L. Cao, T. B. Freedman, L. A. Nafie, J. D. Deschamps, V. A. Kenyon, J. R. Flanary, T. R. Holman and P. Crews, Bioorg. Med. Chem., 2005, 13, 5600 CrossRef CAS.
- V. J. Paul, Y. Seo, K. W. Cho, J. R. Rho, J. Shin and P. R. Bergquist, J. Nat. Prod., 1997, 60, 1115 CrossRef CAS.
- A. Rudi, Y. Benayahu and Y. Kashman, J. Nat. Prod., 2005, 68, 280 CrossRef CAS.
- H. Shimogawa, T. Teruya, K. Suenaga and H. Kigoshi, Bull. Chem. Soc. Jpn., 2005, 78, 1345 CrossRef CAS.
- A. Rudi, Y. Erez, Y. Benayahu and Y. Kashman, Tetrahedron Lett., 2005, 46, 8613 CrossRef CAS.
- M. L. Ciavatta, A. Fontana, S. Puliti, G. Scognamiglio and G. Cimino, Tetrahedron, 1999, 55, 12629 CrossRef CAS.
- M. L. Ciavatta, M. Gavagnin, E. Manzo, R. Puliti, C. A. Mattia, L. Mazzarella, G. Cimino, J. S. Simpson and M. J. Garson, Tetrahedron, 2005, 61, 8049 CrossRef CAS.
- G. Cimino, S. De Stefano, A. Guerriero and L. Minale, Tetrahedron Lett., 1975, 3723 CrossRef CAS.
- H. Gaspar, M. Gavagnin, G. Calado, F. Castelluccio, E. Mollo and G. Cimino, Tetrahedron, 2005, 61, 11032 CrossRef CAS.
- M. Tischler, R. J. Andersen, M. I. Choudhary and J. Clardy, J. Org. Chem., 1991, 56, 42 CrossRef CAS.
- M. Kolympadi, M. Liapis and V. Ragoussis, Tetrahedron, 2005, 61, 2003 CrossRef CAS.
- S. Cao, C. Foster, J. S. Lazo and D. G. I. Kingston, Bioorg. Med. Chem., 2005, 13, 5094 CrossRef CAS.
- M. Li, L. Li, Z. Deng, Y. Qiu and W. Lin, Bopuxue Zazhi, 2004, 21, 323 Search PubMed.
- Z. G. Yu, K. S. Bi and Y. W. Guo, Helv. Chim. Acta, 2005, 88, 1004 CrossRef CAS.
- G. R. Pettit, R. Tan and Z. A. Cichacz, J. Nat. Prod., 2005, 68, 1253 CrossRef CAS.
- D. T. A. Youssef, L. A. Shaala and S. Emara, J. Nat. Prod., 2005, 68, 1782 CrossRef CAS.
- G. Cimino, S. De Stefano, L. Minale and E. Fattorusso, Tetrahedron, 1972, 28, 333 CrossRef CAS.
- H. J. Choi, Y. H. Choi, S. B. Yee, E. Im, J. H. Jung and N. D. Kim, Mol. Carcinog., 2005, 44, 162 CrossRef CAS.
- M. R. Kernan, D. J. Faulkner, L. Parkanyi, J. Clardy, M. S. de Carvalho and R. S. Jacobs, Experientia, 1989, 45, 388 CAS.
- M. S. Buchanan, A. Edser, G. King, J. Whitmore and R. J. Quinn, J. Nat. Prod., 2001, 64, 300 CrossRef CAS.
- P. Basabe, S. Delgado, I. S. Marcos, D. Diez, A. Diego, M. De Román and J. G. Urones, J. Org. Chem., 2005, 70, 9480 CrossRef CAS.
- T. A. Mansoor, J. Hong, C. O. Lee, S. J. Bae, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 331 CrossRef CAS.
- L. Li, Z. Deng, H. Fu, J. Li, W. Lin and P. Proksch, J. Asian Nat. Prod. Res., 2005, 7, 115 Search PubMed.
- P. Sauleau and M.-L. Bourguet-Kondracki, Steroids, 2005, 70, 954 CrossRef CAS.
- X. C. Huang, Y. W. Guo, E. Mollo and G. Cimino, Helv. Chim. Acta, 2005, 88, 281 CrossRef CAS.
- A. Mandeau, C. Debitus, M. F. Ariès and B. David, Steroids, 2005, 70, 873 CrossRef CAS.
- H. Mitome, N. Shirato, A. Hoshino, H. Miyaoka, Y. Yamada and R. W. M. Van Soest, Steroids, 2005, 70, 63 CrossRef CAS.
- A. Zampella, R. D'Orsi, V. Sepe, S. De Marino, N. Borbone, A. Valentin, C. Debitus, F. Zollo and M. V. D'Auria, Eur. J. Org. Chem., 2005, 20, 4359 CrossRef.
- D. Xiao, X. Peng, S. Deng, W. Ma and H. Wu, Chin. J. Org. Chem., 2005, 25, 1606 CAS.
- J. S. Sandler, S. L. Forsburg and D. J. Faulkner, Tetrahedron, 2005, 61, 1199 CrossRef CAS.
- S. A. Tang, Z. W. Deng, J. Li, H. Z. Fu, Y. H. Pei, S. Zhang and W. H. Lin, Chin. Chem. Lett., 2005, 16, 353 CAS.
- C. F.-L. Bon, F. Berrué, O. P. Thomas, F. Reyes and P. Amade, J. Nat. Prod., 2005, 68, 1284 CrossRef.
- H. F. Dai, R. A. Edrada, R. Ebel, M. Nimtz, V. Wray and P. Proksch, J. Nat. Prod., 2005, 68, 1231 CrossRef CAS.
- E. W. Rogers and T. F. Molinski, J. Nat. Prod., 2005, 68, 450 CrossRef CAS.
- G. Q. Li, X. Li, Z. W. Deng, H. S. Guan and W. H. Lin, Chin. Chem. Lett., 2005, 16, 494 CAS.
- N. Fusetani, T. Toyoda, N. Asai, S. Matsunaga and T. Maruyama, J. Nat. Prod., 1996, 59, 796 CrossRef CAS.
- N. Alam, B. H. Bae, J. Hong, C. O. Lee, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 1059 CrossRef CAS.
- M. Kita, M. Kitamura, T. Koyama, T. Teruya, H. Matsumoto, Y. Nakano and D. Uemura, Tetrahedron Lett., 2005, 46, 8583 CrossRef CAS.
- L. Quijano, F. Cruz, I. Navarrete, P. Gomez and T. Rios, Lipids, 1994, 29, 731 CrossRef CAS.
- A. Han, J. Song, D. S. Jang, H. Min, S. K. Lee and E. Seo, Arch. Pharm. Res., 2005, 28, 290 Search PubMed.
- P. Muralidhar, M. M. Kumar, N. Krishna, C. B. Rao and D. V. Rao, Chem. Pharm. Bull., 2005, 53, 168 CrossRef CAS.
- X. He, S. Yan, L. Zeng and J. Su, Zhongshan Daxue Xuebao, Ziran Kexueban, 2005, 44, 113 Search PubMed.
- C. Subrahmanyam, S. Ratnar Kumar and G. Damodar Reddy, Indian J. Chem., Sect. B, 2005, 44, 2186.
- J.-Y. Su, Y.-Y. Kuang, L.-M. Zeng and H. Li, J. Asian Nat. Prod. Res., 2005, 7, 107 Search PubMed.
- V. Anjaneyulu and P. Radhika, Indian J. Chem., Sect. B, 1999, 38, 457.
- P. Radhika, P. R. Rao, J. Archana and N. K. Rao, Biol. Pharm. Bull., 2005, 28, 1311 CrossRef CAS.
- C. A. Ospina, A. D. Rodríguez, J. A. Sánchez, E. Ortega-Barria, T. L. Capson and A. M. S. Mayer, J. Nat. Prod., 2005, 68, 1519 CrossRef CAS.
- J.-H. Su, A. F. Ahmed, P.-J. Sung, Y.-C. Wu and J.-H. Sheu, J. Nat. Prod., 2005, 68, 1651 CrossRef CAS.
- A. F. Ahmed, Y.-H. Kuo, C.-F. Dai and J.-H. Sheu, J. Nat. Prod., 2005, 68, 1208 CrossRef CAS.
- H.-C. Huang, C.-H. Chao, J.-H. Liao, M. Y. Chiang, C.-F. Dai, Y.-C. Wu and J.-H. Sheu, Tetrahedron Lett., 2005, 46, 7711 CrossRef.
- S. P. Garzón, A. D. Rodríguez, J. A. Sánchez and E. Ortega-Barria, J. Nat. Prod., 2005, 68, 1354 CrossRef CAS.
- A. A. H. El-Gamal, E.-P. Chiu, C.-H. Li, S.-Y. Cheng, C.-F. Dai and C.-Y. Duh, J. Nat. Prod., 2005, 68, 1749 CrossRef CAS.
- S.-H. Qi, S. Zhang, X. Li and Q.-X. Li, J. Nat. Prod., 2005, 68, 1288 CrossRef CAS.
- N. S. Reddy, J. K. Reed, R. E. Longley and A. E. Wright, J. Nat. Prod., 2005, 68, 248 CrossRef CAS.
- A. Ata, H. Y. Win, D. Holt, P. Holloway, E. P. Segstro and G. S. Jayatilake, Helv. Chim. Acta, 2004, 87, 1090 CrossRef CAS.
- C. Duque, M. Puyana, G. Narváez, O. Osorno, N. Hara and Y. Fujimoto, Tetrahedron, 2004, 60, 10627 CrossRef CAS.
- I. I. Rodríguez, Y.-P. Shi, O. J. Garciá, A. D. Rodríguez, A. M. S. Mayer, J. A. Sánchez, E. Ortega-Barria and J. González, J. Nat. Prod., 2004, 67, 1672 CrossRef CAS.
- A. D. Rodríguez, personal communication, 2006.
- T. Ferns and R. G. Kerr, Tetrahedron, 2005, 61, 12358 CrossRef CAS.
- A. A. H. El-Gamal, S.-K. Wang, C.-F. Dai, I.-G. Chen and C.-Y. Duh, J. Nat. Prod., 2005, 68, 74 CrossRef CAS.
- J. Y. Su, R. L. Yang, Y. Y. Kuang and L. M. Zeng, J. Nat. Prod., 2000, 63, 1543 CrossRef CAS.
- C.-X. Zhang, S.-J. Yan, G.-W. Zhang, W.-G. Lu, J.-Y. Su, L.-M. Zeng, L.-Q. Gu, X.-P. Yang and Y.-J. Lian, J. Nat. Prod., 2005, 68, 1087 CrossRef CAS.
- R. Jia, Y.-W. Guo, E. Mollo and G. Cimino, Helv. Chim. Acta, 2005, 88, 1028 CrossRef CAS.
- M. Gutiérrez, T. L. Capson, H. M. Guzmán, J. González, E. Ortega-Barria, E. Quiñoa and R. Riguera, J. Nat. Prod., 2005, 68, 614 CrossRef CAS.
- G. Li, Y. Zhang, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, J. Nat. Prod., 2005, 68, 649 CrossRef CAS.
- S. W. Yin, Y. P. Shi, X. M. Li and B. G. Wang, Chin. Chem. Lett., 2005, 16, 1489 CAS.
- A. F. Ahmed, R.-T. Shiue, G.-H. Wang, C.-F. Dai, Y.-H. Kuo and J.-H. Sheu, Tetrahedron, 2003, 59, 7337 CrossRef CAS.
- Y.-J. Tseng, A. F. Ahmed, C.-F. Dai, M. Y. Chiang and J.-H. Sheu, Org. Lett., 2005, 7, 3813 CrossRef CAS.
- M. Kobayashi and T. Hamaguchi, Chem. Pharm. Bull., 1988, 36, 3780 CAS.
- Y.-L. Zhang, X.-L. Tang, G.-Q. Li and W.-H. Lin, Qingdao Keji Daxue Xuebao, Ziran Kexueban, 2005, 26, 215 Search PubMed.
- A. A. H. El-Gamal, S.-K. Wang and C.-Y. Duh, Org. Lett., 2005, 7, 2023 CrossRef CAS.
- A. A. H. El-Gamal, S.-K. Wang and C.-Y. Duh, Tetrahedron Lett., 2005, 46, 6095 CrossRef CAS.
- J. Marrero, A. D. Rodríguez and C. L. Barnes, Org. Lett., 2005, 7, 1877 CrossRef CAS.
- T. Iwagawa, K. Babazono, M. Nakatani, M. Doe, Y. Morimoto and K. Takemura, Heterocycles, 2005, 65, 607 CrossRef CAS.
- T. Iwagawa, N. Nishitani, M. Nakatani, M. Doe, Y. Morimoto and K. Takemura, J. Nat. Prod., 2005, 68, 31 CrossRef CAS.
- T. Iwagawa, N. Nishitani, M. Nakatani, M. Doe, Y. Morimoto and K. Takemura, J. Nat. Prod., 2005, 68, 818 CrossRef CAS.
- T. Iwagawa, K. Babazono, H. Okamura, M. Nakatani, M. Doe, Y. Morimoto, M. Shiro and K. Takemura, Heterocycles, 2005, 65, 2083 CrossRef CAS.
- T. Iwagawa, K. Babazono, H. Okamura, M. Nakatani, M. Doe, Y. Morimoto, M. Shiro and K. Takemura, Heterocycles, 2005, 65, 3093 CAS.
- P.-J. Sung, W.-P. Hu, L.-S. Fang, T.-Y. Fan and J.-J. Wang, Nat. Prod. Res., 2005, 19, 689 CrossRef CAS.
- A. Hoshino, H. Mitome, S. Tamai, H. Takiyama and H. Miyaoka, J. Nat. Prod., 2005, 68, 1328 CrossRef CAS.
- T. Barsby and J. Kubanek, J. Nat. Prod., 2005, 68, 511 CrossRef CAS.
- Y.-C. Lin, Y.-L. Huang, A. T. Khalil, M.-H. Chen and Y.-C. Shen, Chem. Pharm. Bull., 2005, 53, 128 CrossRef CAS.
- S.-H. Qi, S. Zhang, Y.-M. Wen, Z.-H. Xiao and Q.-X. Li, Helv. Chim. Acta, 2005, 88, 2349 CrossRef CAS.
- J. J. Morales, D. Lorenzo and A. D. Rodríguez, J. Nat. Prod., 1991, 54, 1368 CrossRef CAS.
- M. T. Crimmins and J. M. Ellis, J. Am. Chem. Soc., 2005, 127, 17200 CrossRef CAS.
- L. Chill, N. Berrer, Y. Benayahu and Y. Kashman, J. Nat. Prod., 2005, 68, 19 CrossRef CAS.
- P. Bernardelli, O. M. Moradei, D. Friedrich, J. Yang, F. Gallou, B. P. Dyck, R. W. Doskotch, T. Lange and L. A. Paquette, J. Am. Chem. Soc., 2001, 123, 9021 CrossRef CAS.
- J.-H. Su, H.-C. Huang, C.-H. Chao, L.-Y. Yan, Y.-C. Wu, C.-C. Wu and J.-H. Shen, Bull. Chem. Soc. Jpn., 2005, 78, 877 CrossRef CAS.
- A. F. Ahmed, M.-H. Wu, G.-H. Wang, Y.-C. Wu and J.-H. Sheu, J. Nat. Prod., 2005, 68, 1051 CrossRef CAS.
- A. A. H. El-Gamal, C.-Y. Chiang, S.-H. Huang, S.-K. Wang and C.-Y. Duh, J. Nat. Prod., 2005, 68, 1336 CrossRef CAS.
- Y.-C. Shen, Y.-C. Lin, A. F. Ahmed and Y.-H. Kuo, Tetrahedron Lett., 2005, 46, 4793 CrossRef CAS.
- A. A. H. El-Gamal, S.-K. Wang and C.-Y. Duh, Tetrahedron Lett., 2005, 46, 4499 CrossRef CAS.
- G. G. Mellado, E. Zubía, M. J. Ortega and P. J. López-González, J. Nat. Prod., 2005, 68, 1111 CrossRef CAS.
- X. H. Yan and Y. W. Guo, Chin. Chem. Lett., 2005, 16, 356 CAS.
- R. Jia, Y.-W. Guo, E. Mollo and G. Cimino, Nat. Prod. Res., 2005, 19, 789 CrossRef CAS.
- C.-H. Chao, L.-F. Huang, S.-L. Wu, J.-H. Su, H.-C. Huang and J.-H. Sheu, J. Nat. Prod., 2005, 68, 1366 CrossRef CAS.
- P. Jin, Z. Deng, Y. Pei, H. Fu, J. Li, L. van Ofwegen, P. Proksch and W. Lin, Steroids, 2005, 70, 487 CrossRef CAS.
- G. Li, Z. Deng, H. Guan, L. van Ofwegen, P. Proksch and W. Lin, Steroids, 2005, 70, 13 CrossRef CAS.
- G.-W. Zhang, X.-Q. Ma, H. Kurihara, C.-X. Zhang, X.-S. Yao, J.-Y. Su and L.-M. Zeng, Org. Lett., 2005, 7, 991 CrossRef CAS.
- W. Zhang, Y.-W. Guo, M. Gavagnin, E. Mollo and G. Cimino, Helv. Chim. Acta, 2005, 88, 87 CrossRef CAS.
- W. Zhang, G.-M. Yao and Y.-W. Guo, Acta Crystallogr., Sect. E, 2005, 61, o2884 CrossRef.
- C. Subrahmanyam and S. R. Kumar, J. Chem. Res., Synop., 2000, 182 Search PubMed.
- J. Yang, S.-H. Qi, S. Zhang, J. Wu and Z.-H. Xiao, Chin. J. Chem., 2005, 23, 1218 CrossRef CAS.
- C.-H. Chao, L.-F. Huang, Y.-L. Yang, J.-H. Su, G.-H. Wang, M. Y. Chiang, Y.-C. Wu, C.-F. Dai and J.-H. Sheu, J. Nat. Prod., 2005, 68, 880 CrossRef CAS.
- C. B. Rao, K. V. Ramana, D. V. Rao, E. Fahy and D. J. Faulkner, J. Nat. Prod., 1988, 51, 954 CrossRef CAS.
- G.-W. Zhang, X.-Q. Ma, S.-J. Yan, L.-M. Zeng and J.-Y. Su, Gaodeng Xuexiao Huaxue Xuebao, 2005, 26, 81 CAS.
- S. Qi, S. Zhang, J. Huang, Z. Xiao, J. Wu and Q. Li, Magn. Reson. Chem., 2005, 43, 266 CrossRef CAS.
- H. T. D'Armas, B. S. Mootoo and W. F. Reynolds, J. Nat. Prod., 2000, 63, 1669 CrossRef CAS.
- A. Rueda, E. Zubia, M. J. Ortega and J. Salva, Steroids, 2001, 66, 897 CrossRef CAS.
- H. Jayasuriya, K. B. Herath, J. G. Ondeyka, Z. Guan, R. P. Borris, S. Tiwari, W. de Jong, F. Chavez, J. Moss, D. W. Stevenson, H. T. Beck, M. Slattery, N. Zamora, M. Schulman, A. Ali, N. Sharma, K. MacNaul, N. Hayes, J. G. Menke and S. B. Singh, J. Nat. Prod., 2005, 68, 1247 CrossRef CAS.
- S. Cao, C. Foster, J. S. Lazo and D. G. I. Kingston, Bioorg. Med. Chem., 2005, 13, 5830 CrossRef CAS.
- H. Nagai, Kaiyo Seibutsu Seibun no Riyo, 2005, 106 Search PubMed.
- T. Honma, Y. Hasegawa, M. Ishida, H. Nagai, Y. Nagashima and K. Shiomi, Toxicon, 2005, 45, 33 CrossRef CAS.
- T. Honma, S. Minagawa, H. Nagai, M. Ishida, Y. Nagashima and K. Shiomi, Toxicon, 2005, 46, 768 CrossRef CAS.
- A. P. Il'ina, M. M. Monastyrnaya, I. N. Sokotun, T. A. Egorov, Y. A. Nazarenko, G. N. Likhatskaya and E. P. Kozlovskaya, Russ. J. Bioorg. Chem., 2005, 31, 34 Search PubMed.
- R. B. Cunha, A. N. C. Santana, P. C. Amaral, M. D. F. Carvalho, D. M. F. Carvalho, E. A. Cavalheiro, B. Maigret, C. A. O. Ricart, B. A. Cardi, M. V. Sousa and K. M. Carvalho, Toxicon, 2005, 45, 207 CrossRef CAS.
- A. J. Blackman and D. J. Matthews, Heterocycles, 1985, 23, 2829 CrossRef CAS.
- M. Ramirez-Osuna, D. Chavez, L. Hernandez, E. Molins, R. Somanathan and G. Aguirre, Molecules, 2005, 10, 295 Search PubMed.
- H. Kawashima, Lipids, 2005, 40, 627 CrossRef CAS.
- G. R. Pettit, Y. Tang and J. C. Knight, J. Nat. Prod., 2005, 68, 974 CrossRef CAS.
- R. J. Andersen, D. J. Faulkner, H. Cun-heng, G. D. Van Duyne and J. Clardy, J. Am. Chem. Soc., 1985, 107, 5492 CrossRef CAS.
- M. Vanhuyse, J. Kluza, C. Tardy, G. Otero, C. Cuevas, C. Bailly and A. Lansiaux, Cancer Lett., 2005, 221, 165 CrossRef CAS.
- C. M. Ireland and P. J. Scheuer, Science, 1979, 205, 922 CrossRef CAS.
- E. Manzo, M. L. Ciavatta, M. Gavagnin, E. Mollo, S. Wahidulla and G. Cimino, Tetrahedron Lett., 2005, 46, 465 CrossRef CAS.
- M. Cueto, L. D'Croz, J. L. Maté, A. San-Martín and J. Darias, Org. Lett., 2005, 7, 415 CrossRef CAS.
- J. E. Barbarow, A. K. Miller and D. Trauner, Org. Lett., 2005, 7, 2901 CrossRef CAS.
- J. E. Moses, R. M. Adlington, R. Rodriguez, S. J. Eade and J. E. Baldwin, Chem. Commun., 2005, 1687 RSC.
- D. R. Zuidema, A. K. Miller, D. Trauner and P. B. Jones, Org. Lett., 2005, 7, 4959 CrossRef CAS.
- V. Di Marzo, R. R. Vardaro, L. De Petrocellis, G. Villani, R. Minei and G. Cimino, Experientia, 1991, 47, 1221 CAS.
- D. R. Zuidema and P. B. Jones, J. Nat. Prod., 2005, 68, 481 CrossRef CAS.
- M. Gavagnin, E. Mollo, G. Cimino and J. Ortea, Tetrahedron Lett., 1996, 37, 4259 CrossRef CAS.
- D. W. Jeffery, M. V. Perkins and J. M. White, Org. Lett., 2005, 7, 1581 CrossRef CAS.
- D. C. Manker and D. J. Faulkner, Tetrahedron, 1987, 43, 3677 CrossRef CAS.
- A. Pathak, J. Aslaoui and C. Morin, J. Org. Chem., 2005, 70, 4184 CrossRef CAS.
- T. Maoka, Y. Fujiwara, K. Hashimoto and N. Akimoto, Chem. Pharm. Bull., 2005, 53, 1207 CrossRef CAS.
- J.-K. Seo, J. M. Crawford, K. L. Stone and E. J. Noga, Biochem. Biophys. Res. Commun., 2005, 338, 1998 CrossRef CAS.
- G. Bulaj, P. J. West, J. E. Garrett, M. Marsh, M.-M. Zhang, R. S. Norton, B. J. Smith, D. Yoshikami and B. M. Olivera, Biochemistry, 2005, 44, 7259 CrossRef CAS.
- M. B. Aguilar, E. López-Vera, J. S. Imperial, A. Falcón, B. M. Olivera and E. P. Heimer dela Cotera, Peptides, 2005, 26, 23 CrossRef CAS.
- M. C. V. Braga, K. Konno, F. C. V. Portaro, J. C. de Freitas, T. Yamane, B. M. Olivera and D. C. Pimenta, Toxicon, 2005, 45, 113 CrossRef.
- S. Sudarslal, S. Majumdar, P. Ramasamy, R. Dhawan, P. P. Pal, M. Ramaswami, A. K. Lala, S. K. Sikdar, S. P. Sarma, K. S. Krishnan and P. Balaram, FEBS Lett., 2003, 553, 209 CrossRef CAS.
- S. P. Sarma, G. S. Kumar, S. Sudarslal, P. Iengar, P. Ramasamy, S. K. Sikdar, K. S. Krishnan and P. Balaram, Chem. Biodiversity, 2005, 2, 535 Search PubMed.
- T. Grkovic, D. R. Appleton and B. R. Copp, Chem. N. Z., 2005, 69, 12 Search PubMed.
- C. E. Kicklighter, S. Shabani, P. M. Johnson and C. D. Derby, Curr. Biol., 2005, 15, 549 CrossRef CAS.
- T. Dias, I. Brito, L. Moujir, N. Paiz, J. Darias and M. Cueto, J. Nat. Prod., 2005, 68, 1677 CrossRef.
- E. Manzo, M. L. Ciavatta, M. Gavagnin, R. Puliti, E. Mollo, Y.-W. Guo, C. A. Mattia, L. Mazzarella and G. Cimino, Tetrahedron, 2005, 61, 7456 CrossRef CAS.
- M. Norte, A. G. González, F. Cataldo, M. L. Rodríguez and I. Brito, Tetrahedron, 1991, 47, 9411 CrossRef CAS.
- D. Awakura, K. Fujiwara and A. Murai, Chem. Lett., 1999, 461 CrossRef CAS.
- D. Iliopoulou, C. Vagias, C. Harvala and V. Roussis, Phytochemistry, 2002, 59, 111 CrossRef CAS.
- K. L. McPhail and M. T. Davies-Coleman, Nat. Prod. Res., 2005, 19, 449 CrossRef CAS.
- S. Tsukamoto, Y. Yamashita and T. Ohta, Mar. Drugs, 2005, 3, 22 Search PubMed.
- S. Suntornchashwej, N. Chaichit, M. Isobe and K. Suwanborirux, J. Nat. Prod., 2005, 68, 951 CrossRef CAS.
- M. Gavagnin, M. Carbone, M. Nappo, E. Mollo, V. Roussis and G. Cimino, Tetrahedron, 2005, 61, 617 CrossRef CAS.
- K. Suenaga, T. Shibata, N. Takada, H. Kigoshi and K. Yamada, J. Nat. Prod., 1998, 61, 515 CrossRef CAS.
- Y. Morimoto, Y. Nishikawa and M. Takaishi, J. Am. Chem. Soc., 2005, 127, 5806 CrossRef CAS.
- G. R. Pettit, Y. Kamano, H. Kizu, C. Dufresne, C. L. Herald, R. J. Bontems, J. M. Schmidt, F. E. Boettner and R. A. Nieman, Heterocycles, 1989, 28, 553 CrossRef CAS.
- M. A. Ali, R. B. Bates, Z. D. Crane, C. W. Dicus, M. R. Gramme, E. Hamel, J. Marcischak, D. S. Martinez, K. J. McClure, P. Nakkiew, G. R. Pettit, C. C. Stessman, B. A. Sufi and G. V. Yarick, Bioorg. Med. Chem., 2005, 13, 4138 CrossRef CAS.
- M. Gavagnin, M. Carbone, E. Mollo and G. Cimino, Tetrahedron Lett., 2003, 44, 1495 CrossRef CAS.
- E. J. Alvarez-Manzaneda, R. Chahboun, I. Barranco, E. C. Torres, E. Alvarez and R. Alvarez-Manzaneda, Tetrahedron Lett., 2005, 46, 5321 CrossRef CAS.
- V. Kulciţki, N. Ungur, M. Gavagnin, M. Carbone and G. Cimino, Eur. J. Org. Chem., 2005, 1816 CrossRef CAS.
- K. L. McPhail, M. T. Davies-Coleman and J. Starmer, J. Nat. Prod., 2001, 64, 1183 CrossRef CAS.
- C.-H. Li, S.-J. Da, X.-S. Chen, G.-L. Zhou, Z.-X. Xie and Y. Li, Huaxue Xuebao, 2005, 63, 2158 CAS.
- T. P. Brady, S. H. Kim, K. Wen, C. Kim and E. A. Theodorakis, Chem.–Eur. J., 2005, 11, 7175 CrossRef CAS.
- J. E. Hochlowski, D. J. Faulkner, G. K. Matsumoto and J. Clardy, J. Org. Chem., 1983, 48, 1142 CrossRef CAS.
- A. Abad, C. Agulló, A. C. Cuñat and A. B. García, Tetrahedron, 2005, 61, 1961 CrossRef CAS.
- T. Miyamoto, K. Sakamoto, K. Arao, T. Komori, R. Higuchi and T. Sasaki, Tetrahedron, 1996, 52, 8187 CrossRef CAS.
- A. Fontana, P. Cavaliere, S. Wahidulla, C. G. Naik and G. Cimino, Tetrahedron, 2000, 56, 7305 CrossRef CAS.
- T. Teruya, K. Suenaga, S. Maruyama, M. Kurotaki and H. Kigoshi, Tetrahedron, 2005, 61, 6561 CrossRef CAS.
- J.-F. Biard, C. Roussakis, J.-M. Kornprobst, D. Gouiffes-Barbin, J.-F. Verbist, P. Cotelle, M. P. Foster, C. M. Ireland and C. Debitus, J. Nat. Prod., 1994, 57, 1336 CrossRef CAS.
- G. Zuber, M.-R. Goldsmith, T. D. Hopkins, D. N. Beratan and P. Wipf, Org. Lett., 2005, 7, 5269 CrossRef CAS.
- P. Wipf and T. D. Hopkins, Chem. Commun., 2005, 3421 RSC.
- S. C. Lievens and T. F. Molinski, Org. Lett., 2005, 7, 2281 CrossRef CAS.
- A. Simon-Levert, A. Arrault, N. Bontemps-Subielos, C. Canal and B. Banaigs, J. Nat. Prod., 2005, 68, 1412 CrossRef CAS.
- L. K. Shubina, S. N. Fedorov, O. S. Radchenko, N. N. Balaneva, S. A. Kolesnikova, P. S. Dmitrenok, A. Bode, Z. Dong and V. A. Stonik, Tetrahedron Lett., 2005, 46, 559 CrossRef CAS.
- G. Guella, I. Mancini and F. Pietra, Helv. Chim. Acta, 1987, 70, 621 CrossRef CAS.
- A. Aiello, E. Fattorusso, P. Luciano, A. Mangoni and M. Menna, Eur. J. Org. Chem., 2005, 5024 CrossRef CAS.
- A. Aiello, E. Fattorusso, P. Luciano, A. Macho, M. Menna and E. Muñoz, J. Med. Chem., 2005, 48, 3410 CrossRef CAS.
- M. J. McKay, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 2005, 68, 1776 CrossRef CAS.
- K. Ravinder, A. V. Reddy, P. Krishnaiah, P. Ramesh, S. Ramakrishna, H. Laatsch and Y. Venkateswarlu, Tetrahedron Lett., 2005, 46, 5475 CrossRef CAS.
- H. Liu, S. B. Pratasik, T. Nishikawa, T. Shida, K. Tachibana, T. Fujiwara, H. Nagai, H. Kobayashi and M. Namikoshi, Tetrahedron Lett., 2004, 45, 7015 CrossRef CAS.
- H. Liu, T. Fujiwara, T. Nishikawa, Y. Mishima, H. Nagai, T. Shida, K. Tachibana, H. Kobayashi, R. E. P. Mangindaan and M. Namikoshi, Tetrahedron, 2005, 61, 8611 CrossRef CAS.
- S. Malla Reddy, M. Srinivasulu, N. Satyanarayana, A. K. Kondapi and Y. Venkateswarlu, Tetrahedron, 2005, 61, 9242.
- A. J. Blackman, C. P. Li, D. C. R. Hockless, B. W. Skelton and A. H. White, Tetrahedron, 1993, 49, 8645 CrossRef CAS.
- J. F. Baird, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691 CrossRef CAS.
- H. H. Issa, J. Tanaka, R. Rachmat, A. Setiawan, A. Trianto and T. Higa, Mar. Drugs, 2005, 3, 78 Search PubMed.
- E. W. Schmidt, J. T. Nelson, D. A. Rasko, S. Sudek, J. A. Eisen, M. G. Haygood and J. Ravel, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7315 CrossRef CAS.
- P. F. Long, W. C. Dunlap, C. N. Battershill and M. Jaspars, ChemBioChem, 2005, 6, 1760 CrossRef CAS.
- L. J. Perez and D. J. Faulkner, J. Nat. Prod., 2003, 66, 247 CrossRef CAS.
- S.-L. You and J. W. Kelly, Tetrahedron, 2005, 61, 241 CrossRef CAS.
- Y. Yonezawa, N. Tani and C.-G. Shin, Bull. Chem. Soc. Jpn., 2005, 78, 1492 CrossRef CAS.
- A. Rudi, L. Chill, M. Aknin and Y. Kashman, J. Nat. Prod., 2003, 66, 575 CrossRef CAS.
- S.-L. You and J. W. Kelly, Tetrahedron Lett., 2005, 46, 2567 CrossRef CAS.
- S. Kumar-Roiné, M. Frostin, A. Al-Mourabit, A.-G. Duigou, J. Pusset, S. Ali, M. Sauvain, S. Sotheeswaran and D. Laurent, ACGC Chem. Res. Commun., 2005, 18, 25 Search PubMed.
- A. J. Freyer, A. D. Patil, L. Killmer, N. Troupe, M. Mentzer, B. Carte, L. Faucette and R. K. Johnson, J. Nat. Prod., 1997, 60, 986 CrossRef CAS.
- B. M. Trost and D. R. Fandrick, Org. Lett., 2005, 7, 823 CrossRef CAS.
- A. D. Patil, A. J. Freyer, R. Reichwein, B. Carte, L. B. Killmer, L. Faucette, R. K. Johnson and D. J. Faulkner, Tetrahedron Lett., 1997, 38, 363 CrossRef CAS.
- H. Abe, S. Aoyagi and C. Kibayashi, J. Am. Chem. Soc., 2005, 127, 1473 CrossRef CAS.
- S. Dutta, H. Abe, S. Aoyagi, C. Kibayashi and K. S. Gates, J. Am. Chem. Soc., 2005, 127, 15004 CrossRef CAS.
- F. Kong and D. J. Faulkner, Tetrahedron Lett., 1991, 32, 3667 CrossRef CAS.
- J. Kubanek, D. E. Williams, E. D. de Silva, T. Allen and R. J. Andersen, Tetrahedron Lett., 1995, 36, 6189 CrossRef CAS.
- H. Tsuneki, Y. You, N. Toyooka, T. Sasaoka, H. Nemoto, J. A. Dani and I. Kimura, Biol. Pharm. Bull., 2005, 28, 611 CrossRef CAS.
- H. Kang and W. Fenical, J. Org. Chem., 1997, 62, 3254 CrossRef CAS.
- A. Hamasaki, J. M. Zimpleman, I. Hwang and D. L. Boger, J. Am. Chem. Soc., 2005, 127, 10767 CrossRef CAS.
- S. Faivre, S. Chièze, C. Delbaldo, N. Ady-Vago, C. Guzman, L. Lopez-Lazaro, S. Lozahic, J. Jimeno, F. Pico, J. P. Armand, J. A. L. Martin and E. Raymond, J. Clin. Oncol., 2005, 23, 7871 CrossRef CAS.
- E. F. A. Brandon, R. D. van Ooijen, R. W. Sparidans, L. L. Lázaro, A. J. R. Heck, J. H. Beijnen and J. H. M. Schellens, J. Mass Spectrom., 2005, 40, 821 CrossRef CAS.
- J. Fayette, I. R. Coquard, L. Alberti, D. Ranchère, H. Boyle and J.-Y. Blay, Oncologist, 2005, 10, 827 Search PubMed.
- P. Allavena, M. Signorelli, M. Chieppa, E. Erba, G. Bianchi, F. Marchesi, C. O. Olimpio, C. Bonardi, A. Garbi, A. Lissoni, F. de Braud, J. Jimeno and M. D'Incalci, Cancer Res., 2005, 65, 2964 CrossRef CAS.
- T. Hamada, M. Asanuma, T. Ueki, F. Hayashi, N. Kobayashi, S. Yokoyama, H. Michibata and H. Hirota, J. Am. Chem. Soc., 2005, 127, 4216 CrossRef CAS.
- N. P. Mischenko, S. A. Fedoreyev, N. D. Pokhilo, V. P. Anufriev, V. A. Denisenko and V. P. Glazunov, J. Nat. Prod., 2005, 68, 1390 CrossRef CAS.
- J. R. Rho and Y. H. Kim, Bull. Korean Chem. Soc., 2005, 26, 1457 CAS.
- F. Kisa, K. Yamada, M. Kaneko, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 382 CrossRef CAS.
- T. Maruta, T. Saito, M. Inagaki, O. Shibata and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 1255 CrossRef CAS.
- K. Yamada, R. Matsubara, M. Kaneko, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2001, 49, 447 CrossRef CAS.
- K. Yamada, N. Wada, H. Onaka, R. Matsubara, R. Isobe, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 788 CrossRef CAS.
- K. Yamada, H. Onaka, M. Tanaka, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 1333 CrossRef CAS.
- M. Inagaki, T. Miyamoto, R. Isobe and R. Higuchi, Chem. Pharm. Bull., 2005, 53, 1551 CrossRef CAS.
- L.-X. Zhang, X. Fan and J.-G. Shi, J. Asian Nat. Prod. Res., 2005, 7, 25 Search PubMed.
- A. A. Kicha, A. I. Kalinovsky, N. V. Ivanchina, Y. N. El'kin and V. A. Stonik, Russ. Chem. Bull., 1994, 43, 1726 CrossRef.
- A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, Russ. Chem. Bull., 2005, 54, 1266 CrossRef CAS.
- E. V. Levina, A. I. Kalinovsky, P. V. Andriyashenko, P. S. Dmitrenok, D. L. Aminin and V. A. Stonik, J. Nat. Prod., 2005, 68, 1541 CrossRef CAS.
- H. D. Chludil and M. S. Maier, J. Nat. Prod., 2005, 68, 1279 CrossRef CAS.
- E. V. Levina, A. I. Kalinovsky, V. A. Stonik, P. S. Dmitrenok and P. V. Andriyashchenko, Russ. J. Bioorg. Chem., 2005, 31, 467 Search PubMed.
- W. Wang, H. Jang, J. Hong, C.-O. Lee, S.-J. Bae, S. Shin and J. H. Jung, Arch. Pharm. Res., 2005, 28, 285 Search PubMed.
- H. F. Tang, Y. H. Yi, L. Li, P. Sun, D. Z. Zhou and B. S. Liu, Chin. Chem. Lett., 2005, 16, 619 CAS.
- H.-F. Tang, Y.-H. Yi, L. Li, P. Sun, S.-Q. Zhang and Y.-P. Zhao, Planta Med., 2005, 71, 458 CrossRef CAS.
- H.-F. Tang, Y.-H. Yi, L. Li, P. Sun, S.-Q. Zhang and Y.-P. Zhao, J. Nat. Prod., 2005, 68, 337 CrossRef CAS.
- J. Wu, Y. Yi, H. Wu, Q. He, Z. Zou and S. Zhang, Zhongguo Tianran Yaowu, 2005, 3, 276 Search PubMed.
- A. S. Silchenko, V. A. Stonik, S. A. Avilov, V. I. Kalinin, A. I. Kalinovsky, A. M. Zaharenko, A. V. Smirnov, E. Mollo and G. Cimino, J. Nat. Prod., 2005, 68, 564 CrossRef CAS.
- Z. Zou, Y. Yi, H. Wu, X. Yao, L. Du, W. Jiuhong, C.-C. Liaw and K.-H. Lee, J. Nat. Prod., 2005, 68, 540 CrossRef CAS.
- Z.-R. Zou, Y.-H. Yi, H.-M. Wu, J.-H. Wu, C.-C. Liaw and K.-H. Lee, J. Nat. Prod., 2003, 66, 1055 CrossRef CAS.
- Y. Tong, X. Zhang, F. Tian, Y. Yi, Q. Xu, L. Li, L. Tong, L. Lin and J. Ding, Int. J. Cancer, 2005, 114, 843 CrossRef CAS.
- G. Moraes, P. T. Northcote, A. S. Silchenko, A. S. Antonov, A. I. Kalinovsky, P. S. Dmitrenok, S. A. Avilov, V. I. Kalinin and V. A. Stonik, J. Nat. Prod., 2005, 68, 842 CrossRef CAS.
- A. M. de Moncerrat Iñiguez-Martinez, G. Guerra-Rivas, T. Rios and L. Quijano, J. Nat. Prod., 2005, 68, 1669 CrossRef.
- E. J. Kim, C.-H. Kim, H.-J. Go, I. H. Kim, S. H. An, H.-Y. Sohn, H. Y. Park, H. D. Yoon, Y.-C. Chang, Y.-K. Hong and N. G. Park, J. Korean Fish. Soc., 2005, 38, 148 Search PubMed.
- E. L. Teuten, L. Xu and C. M. Reddy, Science, 2005, 307, 917 CrossRef CAS.
- G. Marsh, M. Athanasiadou, I. Athanassiadis, A. Bergman, T. Endo and K. Haraguchi, Environ. Sci. Technol., 2005, 39, 8684 CrossRef CAS.
- N. Sugiyama, M. Araki, M. Ishida, Y. Nagashima and K. Shiomi, Toxicon, 2005, 45, 595 CrossRef CAS.
- W. E. Houssen and M. Jaspars, J. Nat. Prod., 2005, 68, 453 CrossRef CAS.
- B. Chen, X. R. Shen and J. L. Kong, Chin. J. Chem., 2005, 23, 599 CrossRef CAS.
- I. Kontiza, C. Vagias, J. Jakupovic, D. Moreau, C. Roussakis and V. Roussis, Tetrahedron Lett., 2005, 46, 2845 CrossRef CAS.
- L. Han, X. Huang, I. Sattler, U. Moellmann, H. Fu, W. Lin and S. Grabley, Planta Med., 2005, 71, 160 CrossRef CAS.
- L. Han, X. Huang, I. Sattler, H.-M. Dahse, H. Fu, S. Grabley and W. Lin, Pharmazie, 2005, 60, 705 CAS.
- S. Bao, Z. Deng, H. Fu, P. Proksch and W. Lin, Helv. Chim. Acta, 2005, 88, 2757 CrossRef CAS.
- L. Han, X. Huang, I. Sattler, H.-M. Dahse, H. Fu, W. Lin and S. Grabley, J. Nat. Prod., 2004, 67, 1620 CrossRef CAS.
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2003, 20, 1 RSC.
| This journal is © The Royal Society of Chemistry 2007 |