Nitrogen-rich hypergolic ionic salts based on (2-methyltetrazol-5-yl)diazotates†
Qi Wanga,
Huijie Lua,
Fuqing Panga,
Jinglun Huangb,
Fude Nieb and
Fu-Xue Chen*a
aSchool of Chemical Engineering & the Environment, Beijing Institute of Technology, 5 South Zhongguancun street, Haidian district, Beijing 100081, China. E-mail: fuxue.chen@bit.edu.cn; Fax: +86-10-68912185
bInstitute of Chemical Materials, China Academy of Engineering Physics, Mianyang, 621050, Sichuan, China
First published on 9th June 2016
Abstract
A series of hypergolic (2-methyltetrazol-5-yl)diazotates have been synthesized by treatment of bases with the diazotatic acid, which were prepared by using isoamyl nitrite at low temperature. These hypergolic energetic salts showed remarkable short ignition delay times (IDs) by droplet test and a high specific impulse up to 291.2 s by calculation. All compounds were fully characterized using IR spectroscopy, 1H and 13C NMR spectroscopy and differential scanning calorimetry (DSC), and, in the case of aminoguanidinium (2-methyltetrazol-5-yl)diazotate (8) and diaminoguanidinium (2-methyltetrazol-5-yl)diazotate (9), with single crystal X-ray structuring.
Combustion of propellants in a closed chamber releases a significant volume of hot gases at high pressure, which provides propulsive force to propel rockets or missiles.1 In rockets, the most common bipropulsion system, composing of fuels and oxidizers, mainly uses hydrazine and its methylated derivatives as liquid hypergolic fuels because of their low ignition delay times (IDs) and high specific impulse (Isp).2 On the other hand, toxicity, carcinogenicity, and high handling cost of the hydrazine derivatives promote research into alternative propellant fuels, which are environmentally benign and concomitantly exhibit comparable performance to hydrazine.3 As promising future fuels, energetic salts and ionic liquids have attracted much attention in the past two decades.4 In the ignition process of energetic ionic salts, the anion is considered to have a dominant influence on ID time, compared with the cation.5 Accordingly, pursuing new hypergolic anions is an effective way to obtain good performance ionic salts with short IDs. At present, only few anions with hypergolicity have been reported, such as nitrate,6 azide,7 dicyanamide,8 nitrocy-anamide9 and B–H bond rich10 anions. As highlighted in a recent review by Zhang, increasing the oxygen balance of the anion in the high-nitrogen energetic ionic salts can improve the general performance,11 thus, we envisioned that the scarcely investigated tetrazole diazotic acid-based anion-derived energetic salts should exhibit good performance.
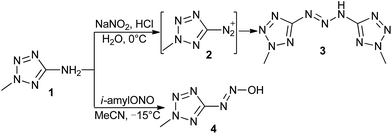
Diazotic acid has widely and long been accepted as the intermediate in diazotization of amine reacted with nitrous acid, which can be further transformed into diazonium salt at low pH.12 However, most of diazotic acids are rarely utilized due to instability, as well as the tautomer, nitrosamines. Only a few diazotic acids and diazotates have been isolated, e.g., in the synthesis of arylhydrazones13 and diazo14 compounds (Scheme 1a). Hantzsch synthesized alkanediazotates by decomposing N-nitrosourethans with a strong base (Scheme 1b).15 Much later, the decomposition mechanism of (Z)-2,2,2-trifluoro-1-arylethanediazoates in aqueous media was studied by Fishbein.16 In recent years, because of enthusiasm for nitrous oxide, a series of stable N-heterocyclic carbene (NHC)–N2O adducts, which can be described as imidazolium diazotates, were synthesized and investigated in chemical reactivity.17 Most recently, by reacting lithium dialkylamides with nitrous oxide, lithium diazotates were generated as intermediates to synthesize alkynyl and alkenyl substituted triazenes (Scheme 1c).18 In this communication, we report the syntheses and hypergolic properties of a series of (2-methyltetrazol-5-yl)diazotates (Scheme 1d).
Initially, in the presence of nitrite acid, 5-amino-2-methyltetrazole (1) was smoothly converted into 1,3-bis(2-methyltetrazol-5-yl)triazene (3)19 without observation of the known (2-methyltetrazol-5-yl)diazonium salt (2) intermediate (Scheme 2). Gratifyingly, by utilizing isoamyl nitrite in acetonitrile,20 the desired (2-methyltetrazol-5-yl)diazotic acid (4) was obtained in high yield. Furthermore, compound 4 did not react with weak acid (e.g. acetic acid) or dilute acid (e.g. diluted hydrochloric acid) but rapidly decomposed by treatment with concentrated strong acid (e.g. concentrated hydrochloric acid or methane sulfonic acid). The solution of diazotic acid 4 was stable below 0 °C and slowly decomposed at room temperature. In the solid form, 4 shows considerable stability at room temperature, while, interestingly, it spontaneously ignited upon exposing to the air in a few minutes. Subsequently, a series of stable (2-methyltetrazol-5-yl)diazotates were designed and synthesized, which displayed the expected hypergolic reactivity with white fuming nitric acid (WFNA).
Due to the acidity of the diazotic acid, compound 4 was readily converted to ammonium salt (5) and hydrazinium salt (6) by reacting with excessive aqueous ammonia and hydrazine hydrate, respectively. When the base was added to the reaction solution, the salt 5 or 6 was precipitated with high purity. To prepare guanidine based salts 7–10, barium (2-methyltetrazol-5-yl)diazotate was exploited as the precursor by treatment of 4 with barium hydroxide, followed by metathesis with guanidinium sulphates. All the salts were obtained in moderate to excellent yields (65–92%) (Scheme 3).
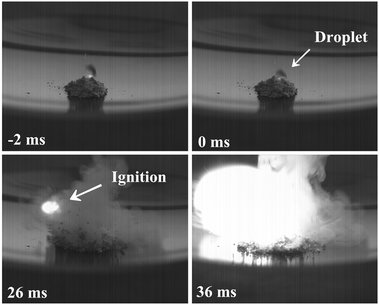
To observe and measure the actual ID, a droplet test was employed due to its flexibility, accuracy, and simple facility for the assessment of their potential for hypergolicity. An excess amount (50 μL) of WFNA was dropped onto a watch glass containing a small diazotate sample (ca. 20 mg). The ID time, from contact of the solid surface with the oxidizer until the first sign of a visible flame, was recorded by a high-speed camera recording 500 frames s−1.
The results from the droplet test are summarized in Table 1. Ignited with WFNA, all the salts revealed ID values ranging from 12 to 26 ms, except the aminoguanidinium salt (8) with a longer ID of 62 ms. Among them, the ammonium salt (5) exhibited the shortest ID of 12 ms, whereas guanidine based salts (7–10) displayed slight longer IDs (18–62 ms) (Fig. 1). In these (2-methyltetrazol-5-yl)diazotates, we supposed that the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O moiety was attributed as the crucial chemical bond to introduce hypergolicity with WFNA, analogous to the N–C
N–O moiety was attributed as the crucial chemical bond to introduce hypergolicity with WFNA, analogous to the N–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N moiety in dicyanamide or B–H bonds in dicyanoborohydride.21
N moiety in dicyanamide or B–H bonds in dicyanoborohydride.21
| Comp. | Tma [°C] | Tdb [°C] | ρc [g cm−3] | ΔHfd [kJ mol−1] | IDe [ms] | Ispf [s] |
|---|---|---|---|---|---|---|
| a Melting point (peak, DSC, 10 °C min−1).b Thermal decomposition temperature (onset) under nitrogen gas (DSC, 10 °C min−1).c Density (gas pycnometer, 25 °C).d Heat of formation (Gaussian 09W).e Ignition delay (using WFNA as the oxidizer).f Specific impulse (EXPLO5 V6.02). | ||||||
| 5 | — | 98 | 1.49 | 694.0 | 12 | 281.1 |
| 6 | 99 | 120 | 1.54 | 847.0 | 14 | 291.2 |
| 7 | 194 | 209 | 1.61 | 658.8 | 24 | 246.4 |
| 8 | 148 | 158 | 1.49 | 779.4 | 62 | 254.4 |
| 9 | 120 | 151 | 1.53 | 886.9 | 18 | 259.7 |
| 10 | 152 | 156 | 1.50 | 999.5 | 26 | 264.8 |
The structures of (2-methyltetrazol-5-yl)diazotates were confirmed by IR spectroscopy, 1H and 13C NMR spectroscopy, high resolution mass spectrometer as well as elemental analysis. In the IR spectra, two strong absorption bands at around 1400 cm−1 and 1680 cm−1 were attributed to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N
N and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of diazotates. The other intense absorption bands at 1260 cm−1 and 1480 cm−1 were assigned to the tetrazole ring.
O bonds of diazotates. The other intense absorption bands at 1260 cm−1 and 1480 cm−1 were assigned to the tetrazole ring.
The 1H NMR spectra of the salts 5–10 show a single resonance at 4.19–4.24 ppm, which belongs to the methyl groups of the anions. In the 13C NMR spectra, the carbon atom of the methyl groups could be found in the range of 39.0–39.2 ppm, and the one for the tetrazole rings is located in the range of 172.0–173.7 ppm.
Single crystals of 8 and 9 suitable for single-crystal X-ray diffraction were obtained by slow evaporation of an ethyl acetate–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) mixture at room temperature. Compound 8 crystallizes in the triclinic P
1 ratio) mixture at room temperature. Compound 8 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. 2), while 9 crystallizes in the monoclinic P2/c space group. All bond lengths, angles and torsion angles can be found in the ESI.† Longer than the bond length of a normal N
space group (Fig. 2), while 9 crystallizes in the monoclinic P2/c space group. All bond lengths, angles and torsion angles can be found in the ESI.† Longer than the bond length of a normal N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond (1.22 Å),22 the ones of N5–N6 in 8 and 9 are 1.285 (2) and 1.289 (16) Å, respectively. In contrast, the bond lengths of N6–O1 are only 1.289 (2) and 1.285 (16) Å, much shorter than the average of N–O single bond length (1.40 Å).22 In the N5–N6–O1, it is presumable that the electron delocalization, which results in the averaging of bond lengths, is caused by the resonance of the N
N double bond (1.22 Å),22 the ones of N5–N6 in 8 and 9 are 1.285 (2) and 1.289 (16) Å, respectively. In contrast, the bond lengths of N6–O1 are only 1.289 (2) and 1.285 (16) Å, much shorter than the average of N–O single bond length (1.40 Å).22 In the N5–N6–O1, it is presumable that the electron delocalization, which results in the averaging of bond lengths, is caused by the resonance of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond and N–O single bond. The moieties of the diazotate anion, both in 8 and 9, show nearly planar assemblies with torsion angles between the tetrazole ring and the N
N double bond and N–O single bond. The moieties of the diazotate anion, both in 8 and 9, show nearly planar assemblies with torsion angles between the tetrazole ring and the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O group of 1.2(3)° and 3.3(2)°, respectively.
N–O group of 1.2(3)° and 3.3(2)°, respectively.
Due to the presence of methyl and the absence of the π–π packing effect, the densities of the diazotates range from 1.49 to 1.54 g cm−3, except in the guanidinium salt 7 (1.61 g cm−3). The thermal stability of compound 5–10 was determined by differential scanning calorimetry (DSC). Guanidinium salt 7 exhibits the highest onset decomposition temperature at 209 °C among these salts. In contrast, ammonium salt 5 and hydrazinium salt 6 decompose at low onset temperatures (98 °C and 120 °C, respectively).
The calculation of the heat of formation was carried out using the program package Gaussian 09 (Revision E.01).23 By using B3LYP with the 6-31+G** basis set,24 the geometric optimization of the structures and frequency analyses were accomplished. Single-point energies were calculated at the MP2(full)/6-311++G** level. The heats of formation of the diazotates were calculated using the Born–Haber cycle.25 As a result of the high nitrogen content, all (2-methyltetrazol-5-yl)diazotate salts exhibit high positive heats of formation. And the specific impulse of these salts ranges from 246.4 s to 291.2 s, which are calculated by EXPLO5 V6.02. Salt 6 shows the highest specific impulse approaching 291.2 s but with poor thermal stability.
In summary, (2-methyltetrazol-5-yl)diazotatic acid was prepared by using isoamyl nitrite. A series of novel hypergolic energetic salts containing the (2-methyltetrazol-5-yl)diazotate anion were synthesized using a straightforward method. Most of these salts exhibit hypergolic reactivity with WFNA and attractive short IDs (12–26 ms). All salts show moderate to high specific impulse, and the highest is 291.2 s. All test and calculation results suggest that (2-methyltetrazol-5-yl)diazotates might be of interest for the additive in propellant formulations.
Acknowledgements
Financial support of this work from the Natural Science Foundation of China (NSFC21372027, 21172203) is gratefully acknowledged. The authors acknowledge collaborations with Professor Yanqiang Zhang (Institute of process engineering, Chinese Academy of Sciences, Beijing, China) in the calculations to predict the specific impulse of novel compounds.Notes and references
- J. P. Agrawal, High Energy Materials: Propellants, Explosives and Pyrotechnics, Wiley-VCH, Weinheim, 2010 Search PubMed.
- (a) S. Pichon, L. Catoire, N. Chaumeix and C. Paillard, J. Propul. Power, 2005, 21, 1057–1061 CrossRef CAS; (b) W. Daimon, Y. Gotoh and I. Kimura, J. Propul. Power, 1991, 7, 946–952 CrossRef CAS.
- (a) Y. Zhang and J. M. Shreeve, Angew. Chem., Int. Ed., 2011, 50, 935–937 CrossRef CAS PubMed; (b) S. Schneider, T. Hawkins, M. Rosander, G. Vaghjiani, S. Chambreau and G. Drake, Energy Fuels, 2008, 22, 2871–2872 CrossRef CAS; (c) H. Gao, Y.-H. Joo, B. Twamley, Z. Zhou and J. M. Shreeve, Angew. Chem., Int. Ed., 2009, 48, 2792–2795 CrossRef CAS PubMed.
- (a) Q. Zhang and J. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed; (b) R. P. Singh, R. D. Verma, D. T. Meshri and J. M. Shreeve, Angew. Chem., Int. Ed., 2006, 45, 3584–3601 CrossRef CAS PubMed; (c) H. Gao and J. M. Shreeve, Chem. Rev., 2011, 111, 7377–7436 CrossRef CAS PubMed.
- Q. Zhang and J. M. Shreeve, Chem.–Eur. J., 2013, 19, 15446–15451 CrossRef CAS PubMed.
- K. Wang, Y. Zhang, D. Chand, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2012, 18, 16931–16937 CrossRef CAS PubMed.
- Y.-H. Joo, H. Gao, Y. Zhang and J. M. Shreeve, Inorg. Chem., 2010, 49, 3282–3288 CrossRef CAS PubMed.
- Y. Zhang, H. Gao, Y. Guo, Y.-H. Joo and J. M. Shreeve, Chem.–Eur. J., 2010, 16, 3114–3120 CrossRef CAS PubMed.
- L. He, G.-H. Tao, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2010, 16, 5736–5743 CrossRef CAS PubMed.
- (a) S. Li, H. Gao and J. M. Shreeve, Angew. Chem., Int. Ed., 2014, 53, 2969–2972 CrossRef CAS PubMed; (b) Q. Zhang, P. Yin, J. Zhang and J. M. Shreeve, Chem.–Eur. J., 2014, 20, 6909–6914 CrossRef CAS PubMed.
- P. He, J.-G. Zhang, X. Yin, J.-T. Wu, L. Wu, Z.-N. Zhou and T.-L. Zhang, Chem.–Eur. J., 2016, 22, 7670–7685 CrossRef CAS PubMed.
- M. B. Smith and J. March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, Wiley-Interscience, New York, 5th edn, 2001 Search PubMed.
- (a) I. Fryšová, J. Slouka and T. Gucký, ARKIVOC, 2005, 15, 30–38 Search PubMed; (b) H. D. Porter and W. D. Peterson, Org. Synth., 1940, 20, 73 CrossRef CAS.
- (a) G. T. Morgan and W. R. Grist, J. Chem. Soc., Trans., 1918, 113, 688–694 RSC; (b) E. Müller and H. Hermann, Chem. Ber., 1963, 96, 570–583 CrossRef.
- (a) A. Hantzsch and M. Lehmann, Chem. Ber., 1902, 86, 897–905 CrossRef; (b) R. Huisgen and J. Reinertshofer, Justus Liebigs Ann. Chem., 1952, 575, 174–197 CrossRef CAS; (c) R. A. Moss, J. Org. Chem., 1965, 31, 1082–1087 CrossRef.
- J. I. Finneman and J. C. Fishbein, J. Am. Chem. Soc., 1996, 118, 7134–7138 CrossRef CAS.
- (a) A. G. Tskhovrebov, E. Solari, M. D. Wodrich, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2012, 51, 232–234 CrossRef CAS PubMed; (b) A. G. Tskhovrebov, E. Solari, M. D. Wodrich, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2012, 134, 1471–1473 CrossRef CAS PubMed; (c) A. G. Tskhovrebov, B. Vuichoud, E. Solari, R. Scopelliti and K. Severin, J. Am. Chem. Soc., 2013, 135, 9486–9492 CrossRef CAS PubMed; (d) E. Theuergarten, T. Bannenberg, M. D. Walter, D. Holschumacher, M. Freytag, C. G. Daniliuc, P. G. Jones and M. Tamm, Dalton Trans., 2014, 43, 1651–1662 RSC.
- G. Kiefer, T. Riedel, P. J. Dyson, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2015, 54, 302–305 CrossRef CAS PubMed.
- (a) T. M. Klapötke, N. K. Minar and J. Stierstorfer, Polyhedron, 2009, 28, 13–26 CrossRef; (b) T. V. Serebryanskaya, L. S. Ivashkevich, A. S. Lyakhov, P. N. Gaponik and O. A. Ivashkevich, Polyhedron, 2010, 29, 2844–2850 CrossRef CAS.
- U. Yasunobu and N. Takashi, JP Pat. 2009066935A, 2009.
- P. D. McCrary, G. Chatel, S. A. Alaniz, O. A. Cojocaru, P. A. Beasley, L. A. Flores, S. P. Kelley, P. S. Barber and R. D. Rogers, Energy Fuels, 2014, 28, 3460–3473 CrossRef CAS.
- F. H. Allen, O. Kennard and D. G. Watson, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision E.01, Gaussian, Inc, Wallingford CT, 2009 Search PubMed.
- R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989 Search PubMed.
- H. D. B. Jenkins, D. Tudeal and L. Glasser, Inorg. Chem., 2002, 41, 2364–2367 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental protocols and spectroscopic characterization data are provided. CCDC 1431004 and 1431005. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra11494f |
| This journal is © The Royal Society of Chemistry 2016 |