Asymmetric transfer hydrogenation of ketones and imines with novel water-soluble chiral diamine as ligand in neat water
Li
Li
ab,
Jiashou
Wu
a,
Fei
Wang
a,
Jian
Liao
a,
Hua
Zhang
a,
Chunxia
Lian
a,
Jin
Zhu
*a and
Jingen
Deng
*a
aKey Laboratory of Asymmetric Synthesis and Chirotechnology of Sichuan Province and Union Laboratory of Asymmetric Synthesis, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, 610041, China. E-mail: jgdeng@cioc.ac.cn; Fax: +86 8522 3978
bGraduate School of Chinese Academy of Sciences, Beijing, China
First published on 16th November 2006
Abstract
A novel water-soluble rhodium(III)catalyst prepared from o,o′-aminated N-tosyl-1,2-diphenylethylenediamine(S,S)-3 and [Cp*RhCl2]2, which was efficient for the asymmetric transfer hydrogenation of ketones and imines in neat water with high reactivity and excellent enantioselectivity , has been developed.
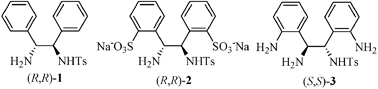
Asymmetric transfer hydrogenation (ATH) of prochiral ketones and imines is a pivotal reaction for the synthesis of chiral alcohols and amines owing to its ease of handling, lower cost and safety.1 Among the various chiral catalysts reported, the most notable is the ruthenium catalyst Ru-TsDPEN [TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine, (R,R)-1] developed by Noyori and Ikariya.2 This catalyst has been applied to the asymmetric transfer hydrogenation of both ketone and imine, leading to good to excellent ees with 2-propanol2 and HCOOH-NEt3,3 respectively, as hydrogen donor as well as solvent.
As a consequence of the increasing demand for efficient and environmentally friendly methods, there has been considerable interest in the development of water-soluble catalytic systems which allow catalytic reactions to occur in water.4 We have reported a water-soluble catalyst based on the Noyori–Ikariya system, which has been successfully used in the asymmetric transfer hydrogenation of ketones and imines in aqueous media.5 Although the ligand (R,R)-2 in the catalyst can be easily synthesized, its purification was rather difficult. Herein, we report a novel water-soluble catalytic system with chiral vicinal diamine (S,S)-3 as ligand for the ATH of ketones and imines in neat (i.e. organic solvent- and surfactant -free) water, which is easily prepared and purified from the nitro-precursor via catalytic hydrogenation.6
Results and discussion
We started with the ATH of acetophenone by using transition metal complexes of the ligand, (S,S)-3 as catalysts with HCO2Na7 as hydrogen source in neat water. The precatalyst was generated by in situ reacting (S,S)-3 with [Cp*RhCl2]2, [Cp*IrCl2]2, and [RuCl2(p-cymene)]2, respectively, in degassed water at 40 °C for 1 h. To our delight, with the water-soluble Rh-(S,S)-3 complex,8(S)-phenethyl alcohol was obtained after 0.5 h at 28 °C with high conversion and enantioselectivity (97% and 97% ee, Table 1, entry 1). Other types of metal complexes were tested as catalyst precursors and were much less reactive (entries 2 and 3). The Rh-(R,R)-2 exhibited low conversion and the enantioselectivity was inferior (entry 4).| Entry | Ligand | Metal complexes | Conv. (%)b | ee (%)b |
|---|---|---|---|---|
| a Unless otherwise indicated, the reaction was carried out in 1 mL of water under argon atmosphere at 28 °C for 0.5 h using 0.4 mmol ketone, [M-(S,S)-3] : [acetophenone] : [HCO2Na] = 1 : 100 : 500. The configuration of phenethyl alcohol was determined by comparison of the sign of optical rotation with literature data.2 b Determined by GC analysis. | ||||
| 1 | (S,S)-3 | [Cp*RhCl2]2 | 97 | 97 |
| 2 | (S,S)-3 | [Cp*IrCl2]2 | 29 | 94 |
| 3 | (S,S)-3 | [RuCl2(p-cymene)]2 | 33 | 95 |
| 4 | (R,R)-2 | [Cp*RhCl2]2 | 9 | 10 |
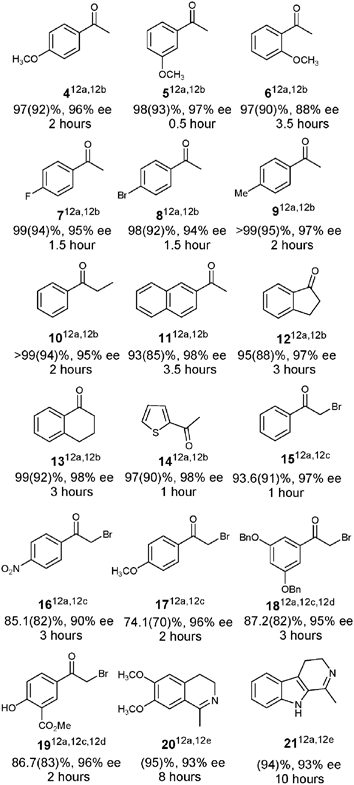
Encouraged by the result obtained with Rh-(S,S)-3, we then extended this system to a wide range of substituted acetophenones4–9, the related ketones 10–13 and the heteroaryl ketone 14. All the ketones can be transformed to the corresponding secondary alcohols at fast rates in an organic solvent free system under optimization conditions as shown in Fig. 1. A substituent on the ortho-site of the acetophenone decreases the enantioselectivity (88% ee for 6). When the Rh-(S,S)-3 was applied to propiophenone 10, 2′-acetonaphthone 11, and cyclic substrates 12 and 13, 95% to 98% ees were achieved. In the reaction of heteroatom ketone 14, chiral alcohol was obtained in excellent enantioselectivity (98% ee) with 97% conversion in 1 h.
Optical pure 2-bromo-1-arylethanols were the key intermediates of β-adrenergic receptor agonists, which were usually obtained from the corresponding α-bromomethylaromatic ketones by reduction employing CBS9oxazaborolidine or biocatalysts .10 In aqueous media, α-bromomethylaromatic ketones 15–19 can also be converted to corresponding chiral 2-bromo alcohols quickly with high enantioselectivity (90% to 97% ee) by using Rh-(S,S)-3 as catalyst , in which the reduced products of 18 and 19 are the key medicinal intermediates of anti-asthma drugs, terbutaline and salbutamol, respectively.11 Moreover, high activity and enantioselectivity were observed for the transfer hydrogenation of imines 20 and 21 (93% ee and 95% yield for 20, 93% ee and 94% yield for 21).
In conclusion, we have developed a novel water-soluble catalyst prepared from the chiral vicinal diamine, (S,S)-3 and [Cp*RhCl2]2, which have been shown to be efficient for the catalytic asymmetric transfer hydrogenation of ketones and imines with sodium formate as hydrogen donor in neat water. It is also notable that this catalytic system can catalyze the ATH of α-bromomethylaromatic ketones and imines besides simple ketones and give high yields and enantioselectivities within a few hours at 28 °C.5,7
Experimental
1H NMR spectra was recorded on Bruker (300 MHz) equipment in CDCl3 with TMS (δ 0.00) as internal standard . Enantiomeric excess was determined by GC on chiral CP-cyclodex B-236 M/CP-Chirasil-DEX columns or HPLC on Chiral OD/OJ columns. The concentration of rhodium in both organic and aqueous layers was determined by ICP-MS analysis. M-(S,S)-3 complexes were synthesized following Noyori et al.'s procedure.13 The α-bromomethylaromatic ketones14 and imines15 were prepared as reported in the literature. All other reagents were purchased from commercial sources and were used without further purification.Typical procedure for asymmetric transfer hydrogenation of ketones and imines
The mixture of (S,S)-3 (1.7 mg, 0.0044 mmol) and [RhCl2(Cp*)]2 (1.3 mg, 0.002 mmol) was degassed three times. 1 mL of degassed water was added and the mixture was stirred at 40 °C for 1 h. Ketone/imine (0.4 mmol) and HCO2Na·2H2O (208 mg, 2.0 mmol) were added to the solution. The mixture was degassed three times and was stirred under argon at 28 °C. When the reaction was completed (determined by TLC), the reaction mixture was extracted with CH2Cl2 (5 mL) three times. For ketones, the conversion and enantioselectivity were determined by GC . For α-bromomethylaromatic ketones, the yield was determined by 1H-NMR using 1,3,5-trimethylbenzene as an internal standard . Isolated yield was obtained by flash chromatography .Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 20572108 and 203900507).Notes and references
- For a recent review, see: S. Gladiali and E. Alberico, Chem. Soc. Rev., 2006, 35, 226–236 Search PubMed.
- S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 7562–7563 CrossRef CAS.
- A. Fujii, S. Hashiguchi, N. Uematsu, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1996, 118, 2521–2522 CrossRef CAS; N. Uematsu, A. Fujii, S. Hashiguchi, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1996, 118, 4916–4917 CrossRef CAS.
- For a comprehensive review, see: D. Sinou, Adv. Synth. Catal., 2002, 344, 221–237 Search PubMed.
- Y. Ma, H. Liu, L. Chen, X. Cui, J. Zhu and J. Deng, Org. Lett., 2003, 5, 2103–2106 CrossRef CAS; J. Wu, F. Wang, Y. Ma, X. Cui, L. Cun, J. Zhu, J. Deng and B. Yu, Chem. Commun., 2006, 1766–1768 RSC.
- D. Xue, Y. C. Chen, X. Cui, Q. W. Wang, J. Zhu and J. G. Deng, J. Org. Chem., 2005, 70, 3584–3591 CrossRef CAS.
- H. Y. Rhyoo, H. J. Park and Y. K. Chung, Chem. Commun., 2001, 2064–2065 RSC; S. Ogo, T. Abura and Y. Watanabe, Organometallics, 2002, 21, 2964–2969 CrossRef CAS; X. Wu, X. Li, W. Hems, F. King and J. Xiao, Org. Biomol. Chem., 2004, 2, 1818–1821 RSC; X. Wu, D. Vinci, T. Ikariya and J. Xiao, Chem. Commun., 2005, 4447–4449 RSC.
- After biphasic separation with Et2O/AcOEt (1 : 1, v/v), a little catalyst was extracted into the organic layer. For Rh-(S,S)-3, the concentration of rhodium in both organic and aqueous layers was 0.85 µg mL–1 and 55.1 µg mL–1, respectively.
- E. J. Corey and C. J. Helal, Angew. Chem., Int. Ed., 1998, 37, 1986–2012 CrossRef CAS.
- A. Goswami, R. L. Bezbaruah, J. Goswami, N. Borthakur, D. Dey and A. K. Hazarika, Tetrahedron: Asymmetry, 2000, 11, 3701–3709 CrossRef CAS.
- F. Wang, H. Liu, L. Cun, J. Zhu, J. Deng and Y. Jiang, J. Org. Chem., 2005, 70, 9424–9429 CrossRef CAS.
- (a) Reactions were carried out in 1 mL of water under an argon atmosphere at 28 °C by using 0.4 mmol ketone, [Rh-(S,S)-3] : [ketone] : [HCO2Na] = 1 : 100 : 500; data in parentheses were the isolated yields. (b) The conversion and ee were determined by GC, and the configuration of alcohols was S-form. (c) Yield was determined by 1H NMR, ee was determined by GC or HPLC, and the configuration of alcohols was R-form. (d) 0.5 Equivalent of Triton X-100 was used.11 (e) Ee was determined by HPLC, and the configuration of amines was R-form.
- K. J. Haack, S. Hashiguchi, A. Fujii, T. Ikariya and R. Noyori, Angew. Chem., Int. Ed. Engl., 1997, 36, 285–288 CrossRef CAS.
- L. C. King and G. K. Ostrum, J. Org. Chem., 1964, 29, 3459–3461 CrossRef CAS.
- P. N. Craig, F. P. Nabenhauer, P. M. Williams, E. Macko and J. Joner, J. Am. Chem. Soc., 1952, 74, 1316–1317 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2007 |