Oxybromination of phenol and aniline derivatives in H2O/scCO2 biphasic media
Benjamin
Ganchegui
and
Walter
Leitner
*
Institut für Technische und Makromolekulare Chemie, RWTH Aachen, Worringerweg 1, Aachen, 52074, Germany. E-mail: Leitner@itmc.rwth-aachen.de; Fax: +49-(0)241-8022177; Tel: +49-(0)241-8026480
First published on 3rd October 2006
Abstract
The oxybromination of phenols and anilines was achieved in the benign H2O/scCO2 biphasic system using NaBr–H2O2 as the bromine source without the need for metal catalysts or acidic additives. The reactivity of the system is associated with the intrinsic acidity of the medium and the in situ generation of percarbonic acid. High conversions of the starting material were achieved together with very good selectivities under optimized conditions.
Introduction
Brominated aromatic compounds are widely used as building blocks for fine chemicals.1 They are also present in the structure of many natural compounds of pharmacological interest.2 Most of the processes currently operating for the bromination of aryl compounds employ toxic, corrosive, and expensive molecular bromine, resulting in the formation of large amounts of HBr waste (eqn (1)). In view of the wide use of brominated aromatics, it is of interest to develop ecologically benign and economically attractive alternatives to these processes. In this context, oxybromination is safer and greener since it avoids the hazardous Br2, replacing it in most cases with a bromide salt in the presence of an oxidant agent under acidic conditions (eqn (2)).| Ar-H + Br2 → Ar-Br + HBr | (1) |
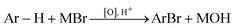 | (2) |
The systems reported for oxybromination so far require strongly acidic conditions, volatile organic solvents (VOCs) and metal or other catalysts (e.g. vanadium, copper, zeolites).3 The oxidizing equivalents are mostly provided by peroxides in the processes,4 although molecular oxygen could be activated in some instances.5–7 Kulkrani et al. reported a metal free system operating for the oxybromination of aniline and anisole derivatives in acetic acid as solvent.8 Liang et al. recently performed the oxybromination of aromatics in CH3CN–aqueous HBr mixtures, mediated by NaNO2.7
Increasing effort is currently devoted to the replacement of VOCs in modern organic synthesis and scCO2 is a promising alternative, since it is non-toxic and non-flammable.9 Moreover, it is cheap and readily available. In this article we describe an effective approach to the oxybromination of phenol and aniline derivatives performed without any metal catalyst or potential toxic/polluting acid addition in a biphasic system consisting of scCO2 and H2O.
Results and discussion
Background
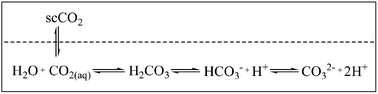
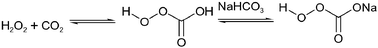
The use of an H2O/scCO2 biphasic system holds several potential advantages: in addition to the benign character of both scCO2 and water, the coexistence of water with scCO2 results in an intrinsic pH value around 3 of the water phase (Fig. 1). Moreover, the water phase recovers its neutral pH within a few minutes following the depressurization of CO2. Thus, this solvent system provides “switchable” acidic conditions without the need for external acid.In oxidation processes, the in situ generation of percarbonic acid from H2O2 and CO2 offers an additional possibility to exploit the chemical reactivity of the H2O/scCO2 system (eqn (3)). Eckert et al. invoked percarbonic acid to rationalise the rate enhancement observed in the metal free epoxidation of olefins with H2O2 in H2O/scCO2.10 Such an activating effect can also be achieved by the addition of NaHCO3 to an aqueous H2O2 solution.11 The formation of the sodium carboxylate of percarbonic acid then allows a higher concentration of the overall percarbonic species (eqn (4)). Such a bicarbonate activated hydrogen peroxide (BAP), in the absence of CO2, was used by Richardson et al. for the metal free epoxidation of olefins12 and sulfide oxidation13 in water. We therefore decided to investigate the reactivity of aromatic compounds with bromide salts in the presence of H2O2 using an H2O/scCO2 biphasic reaction medium in order to obtain the corresponding brominated products.
 | (3) |
 | (4) |
Screening of reaction conditions
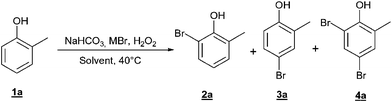
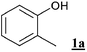
The so called “oxybromination” of an electron rich arene leads to a mixture of ortho- and para- brominated species, and polybromination is also possible. The use of an ortho-substituted substrate reduces the possible combinations to three products. Thus, in order to simplify the analytics, o-cresol1a was used as model substrate in a first screening for suitable reaction conditions (Table 1).|
|
||||||
|---|---|---|---|---|---|---|
| Entry | MBr : 1a | Equiv. NaHCO3 | Solvent system | Conv.a (%) | Selectivityb (%) | |
| 2a + 3ac | 4a | |||||
| (isolated yield) | ||||||
| a Standard conditions: o-cresol : MBr : H2O2 = 1 : 3 : 3, 40 °C, 4 h; GC conversion using octan-1-ol as standard. b Selectivity is expressed as the ratio of the GC peak areas; the only detected products are those 3 compounds. c Mono-brominated compounds were obtained as a 40 : 60 mixture of ortho- and para- isomers. d Reaction time = 2 h. e Reaction with a glass lined autoclave. | ||||||
| 1 | NaBr | — | H2O | 12 | 93 | 7 |
| 2 | NaBr | — | H2O/scCO2 | 54 | 78 | 22 |
| 3 | NaBr | 0.1 | H2O | 32 | 92 | 8 |
| 4 | NaBr | 0.1 | H2O/scCO2 | 91 | 89 (78) | 11 (8) |
| 5d | NaBr | 0.1 | H2O/scCO2 | 48 | 95 | 5 |
| 6e | NaBr | 0.1 | H2O/scCO2 | 93 | 86 | 14 |
| 7 | KBr | 0.1 | H2O/scCO2 | 86 | 91 | 9 |
| 8 | TBAB | 0.1 | H2O/scCO2 | 90 | 89 | 11 |
| 9 | NH4Br | 0.1 | H2O/scCO2 | 60 | 93 | 7 |
Submitting o-cresol1a to NaBr/H2O2 in water at 40 °C resulted in a poor conversion of 12% (entry 1). Using the biphasic H2O/scCO2 system under otherwise identical conditions, the conversion increased to 54% (entry 2 vs. 1). If the reaction was performed in absence of scCO2 but with a catalytic amount of NaHCO3, there was a noticeable enhancement compared to the CO2-free system, but conversion was still lower than in the H2O/scCO2 system (entry 3). This supports the involvement of percarbonic species in a BAP-type mechanism. The actual oxidant in this case would be percarbonic acid sodium salt (eqn (4)).11
The reason for less improvement with NaHCO3 than that observed in H2O/scCO2 is assumed to be the absence of acidic conditions necessary to achieve an efficient oxybromination.3 Indeed, by combining the effects of the biphasic H2O/scCO2 system with the addition of a catalytic amount of NaHCO3 the conversion could be raised to 91% within 4 hours, while the monobromination selectivity remained high (89%, entry 4). A shorter reaction time (entry 5) or a lower NaBr stoichiometry further improved the monobromo adduct selectivity albeit at the expense of a drop in conversion.
Since oxidation reactions in scCO2 can be initiated by the reactor walls14 and the conversion of bromide ions into bromine with hydrogen peroxide is known to be iron catalyzed,15 it was checked whether the iron-containing steel walls of the autoclave had an influence on the rate of the oxybromination. A test reaction was performed with a glass liner inside the autoclave. Conversion and selectivities were largely identical to those obtained in absence of the coating (entry 6 vs. 4). This indicates that either the reactor walls are not involved in the overall process or the bromide oxidation step is not rate limiting for this transformation.
Finally several bromide salts were tested (entries 7–9). Both KBr and [NBu4]Br exhibited comparable reactivities to NaBr whereas [NH4]Br was less effective. Therefore NaBr was kept as the standard bromine source for the rest of this work.
In summary, oxybromination of o-cresol could be achieved readily using H2O2/NaBr as the bromine source in an H2O/scCO2 biphasic system. More than 90% conversion and 90% selectivity for the monobrominated product was achieved. In a preparative scale experiment under the conditions of Table 1, entry 4, a mixture of the ortho- and para-brominated products (2a : 3a = 40 : 60) was isolated in 78% yield along with 8% of the disubstituted product 4a by flash chromatography after a classical work up.
Oxybromination of phenol derivatives
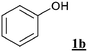
A series of phenol derivatives was submitted to the conditions described above. The results are shown in Table 2. After 3 hours, 98% conversion of phenol 1b with 88% monobromophenol selectivity was observed (entry 1a). It is assumed that the observed high monobromination selectivity results from two main factors: (i) for electronic reasons, an ortho- or para-bromination substitution has a deactivating effect toward a further electrophilic bromination of a phenol derivative , (ii) a brominated phenol is less water soluble than phenol itself.16 Indeed, the orange colour of the water phase indicates that the brominating species is mainly water soluble. Therefore, the partition of substrate, primary product and brominating agent in the biphasic mixture (Fig. 2) is expected to affect the selectivity control.| Entry | Substrate | Water solubilitya /g L–1 (at pH = 7) | NaBr (equiv.) | t/h | Conv.b (%) | Selectivityc (%) | ||
|---|---|---|---|---|---|---|---|---|
| Mono-Br | Di-Br | Tri-Br | ||||||
| a Conditions: substrate : NaHCO3 : NaBr : H2O2 = 1 : 0.1 : 3 : 3, 40 °C; solubilities from Acros Chemicals. b GC conversion with octan-1-ol as internal standard. c Selectivity is expressed as the ratio of the GC peak areas. | ||||||||
| 1a |
|
80 | 3 | 3 | 98 | 88 | 11 | 1 |
| 1b | 3 | 6 | 100 | 52 | 33 | 15 | ||
| 1c | 1 | 4 | 57 | 93 | 7 | 0 | ||
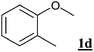
| 1d | 6 | 4 | 100 | 3 | 5 | 92 | ||
| 2a |
|
20 | 3 | 3 | 74 | 94 | 6 | — |
| 2b | 3 | 3.5 | 89 | 93 | 7 | — | ||
| 2c | 1 | 4 | 41 | 98 | 2 | — | ||
| 3a |

|
1 | 3 | 3 | 38 | 97 | 3 | — |
| 3b | 3 | 6 | 58 | 91 | 9 | — | ||
| 3c | 3 | 20 | 75 | 82 | 18 | — | ||
| 3d | 1 | 20 | 35 | 96 | 4 | — | ||
| 4 |
|
0.5 | 3 | 20 | 0 | — | — | — |
Increasing the reaction time resulted in a further reaction of the monobrominated adducts allowing the formation of di- and tribromophenol (entry 1b). Tuning the NaBr stoichiometry opens a further possibility to regulate the selectivity toward mono- or tribromophenol: a 1 : 1 ratio for 1b : NaBr led to a good monobromo selectivity (93%), albeit at conversion of only 57% (entry 1c). In the presence of 6 equivalents of NaBr, the reaction led to a full conversion along with 92% tribromophenol selectivity (entry 1d).
The influence of the water solubility of the substrates mentioned above is also evident from the relative reactivities of the substituted phenols 1a–d. o-Cresol1a proved less reactive than phenol 1b, even though it should be electronically more activated. Most significantly, the electronically almost identical substrate 1c proved far less reactive than 1a, in parallel to a largely reduced water solubility. Finally, the most hydrophobic substrate 1d proved unreactive. However, an additional deactivation effect by blocking the protic OH can not be excluded in this case.
Oxybromination of aniline derivatives
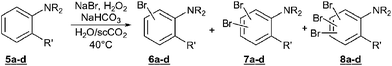
Given the successful and straightforward oxybromination of phenol derivatives in the H2O/scCO2 system, amino-substituted aromatics were tested under similar reaction conditions (Table 3). Aniline5a reacted smoothly and 98% conversion was achieved after 4 hours (entry 1). In contrast to the situation observed with phenol 1b, the disubstituted products 7a were formed with high preference from 5a. The o,o′- and o,p-isomers were typically observed in a ratio of 45 : 55. The monosubstituted product 6a (ortho : para = 70 : 30) can be obtained with good selectivity at reasonable conversion by reducing the amount of bromine source (entry 1b). Excellent yield for the monobrominated products (ortho : para = 35 : 65) were also achieved with substrate 5b (entry 2). The N-substituted derivatives 5c and 5d were far less reactive, which may again reflect their largely reduced water solubility (entries 3 and 4).|
|
|||||
|---|---|---|---|---|---|
| Entry | Substrate | NaBr (equiv.) | Time/h | Conv.a (%) | Sel.b (%) 6/7/(8) |
| a Conditions: substrate : NaHCO3 : NaBr : H2O2 = 1 : 0.1 : 3 : 3, 40 °C; GC conversion with octan-1-ol as internal standard. b Selectivity is expressed as the ratio of the GC peak areas. | |||||
| 1 |

|
3 | 4 | 98 | 29/70/(1) |
| 1 | 4 | 65 | 96/3/(1) | ||
| 2 |

|
3 | 4 | 94 | 91/9 |
| 1 | 4 | 43 | 98/2 | ||
| 3 |

|
3 | 20 | 22 | 99/1 |
| 4 |

|
3 | 20 | 0 | — |
Conclusion
This work provides a new method for the production of brominated phenol and aniline derivatives using NaBr–H2O2 as a bromine source in the benign H2O/scCO2 biphasic reaction medium. This system is suitable for the reaction of sufficiently water soluble substrates; mono-, di-, or tribrominated products could be obtained with high selectivities under suitable conditions. This new procedure represents an attractive green approach to brominated aromatics for several reasons: (i) it avoids the use and handling of Br2; (ii) no VOCs are used as solvents; (iii) the two components of the reaction medium, water and CO2, are non-toxic, non-flamable, cheap and readily available; (iv) the intrinsic reactivity of the biphasic system avoids the use of additional acids or metal catalysts.Experimental
Safety warning
Experiments using compressed gases must only be conducted in suitable equipment and under appropriate safety precautions.Reagents and analytics
Reactants were purchased from Acros and used without further purification. GC analyses were performed on a Hewlett Packard 5890 series II instrument, using octan-1-ol as standard. GC /MS were recorded on a Hewlett Packard 5973 quadrupole-spectrometer (EI, 70 eV). NMR spectra were recorded on a Brucker DPX-300 spectrometer (1H: 300 MHz, 13C: 75 MHz).Typical procedure
A 10 mL window-equipped stainless steel autoclave was charged with NaHCO3 (8.4 mg, 0.1 mmol), NaBr (309 mg, 3 mmol), o-cresol (108 mg, 1 mmol), 2 mL water and finally H2O2 (175 µL, 3 mmol). Then, a weighed amount of CO2 (8 g) was added using a compressor. The reactor was heated to 40 °C, whereby the pressure rose to 100–110 bar. After the given reaction time , the reactor was cooled down to 0 °C and CO2 was removed through a double cold trap within 20 minutes. The water phase in the reactor and the cold traps were extracted with ether and the combined extracts analysed by GC using octan-1-ol as standard. Products were identified by GC /MS and 1H NMR and comparison to authentic samples.2-bromo-6-methylphenol: 1H NMR (CDCl3, 300 MHz, 298 K): 2.28 (s, 3H, CH3); 5.35 (s, 1H, OH); 6.64–6.77 (m, 1H, ArH); 6.95–7.06 (m, 1H, ArH); 7.20–7.32 (m, 1H, ArH); MS (EI, 70 eV): 188, 186 (M+, 42); 107 (–Br, 100).
4-bromo-6-methylphenol: 1H NMR (CDCl3, 300 MHz, 298 K): 2.24 (s, 3H, CH3); 4.78 (s, 1H, OH); 6.51–6.63 (m, ArH); 7.10–7–19 (m, 1H, ArH); 7.19–7.26 (m, 1H, ArH); MS (EI, 70 eV): 188, 186 (M+, 72); 107 (–Br, 100).
2,4-dibromo-6-methylphenol: 1H NMR (CDCl3, 300 MHz, 298 K): 2.28 (s, 3H, CH3); 4.92 (s, 1H, OH); 7.02 (s, 1H, ArH); 7.24 (s, 1H, ArH); MS (EI, 70 eV): 268 (52), 266 (100), 264 (53); 187 (70); 185 (70).
Acknowledgements
Financial support of the German Ministry of Science and Education (bmbf; Lighthouse Project “Sustainable Aromatic Chemistry”) and the Fonds der Chemische Industrie is gratefully acknowledged.References
- I. P. Beletskaia and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS
.
- A. Butler and J. V. Walker, Chem. Rev., 1993, 93, 1937 CrossRef CAS
.
- G. Rothenberg and J. H. Clark, Green Chem., 2000, 2, 248–251 RSC
and references therein.
- M. Battacharjee, Polyhedron, 1992, 11, 2817 CrossRef CAS
; B. M. Choudary, Y. Udha and P. N. Reddy, Synlett, 1994, 450 CrossRef CAS
; K. Smith, P. He and A. Taylor, Green Chem., 1999, 1, 35 RSC
; U. Bora, G. Bose, M. K. Chaudhuri, S. S. Dhar, R. Gopinath, A. T. Khan and B. K. Patel, Org. Lett., 2000, 2, 247 CrossRef CAS
; G. Rothenberg and J. H. Clark, Org. Process Res. Dev., 2000, 4, 270 Search PubMed
; N. Narender, K. V. V. Krishna Mohan, R. Vinod Reddy, P. Srinivasu, S. J. Kulkrani and K. V. Ragavan, J. Mol. Catal. A: Chem., 2003, 192, 73 CrossRef CAS
; J. Salazar and R. Dorta, Synlett, 2004, 1318 CrossRef CAS
.
- R. Neumann and I. Asael, J. Chem. Soc., Chem. Commun., 1988, 1285 RSC
; E. J. Pressman, J. Y. Ofori, G. L. Soloveichik, J. L. Male and R. C. Mills, US Pat. 0143144 2004 Search PubMed
; G. L. Soloveichik, US Pat. 0049441, 2005 Search PubMed
; E. J. Pressman, J. L. Male, R. C. Mills and J. Y. Ofori, US Pat. 0059843, 2005 Search PubMed
; R. C. Mills and J. Y. Ofori, US Patent 0049440, 2005 Search PubMed
.
- R. Raja and P. Ratnasamy, J. Catal., 1997, 170, 244 CrossRef CAS
.
- G. Zhang, R. Liu, Q. Xu, L. Ma and X. Liang, Adv. Synth. Catal., 2006, 348, 862 CrossRef CAS
.
- K. V. V. Krishna Mohan, N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Rhagavan, Synth. Commun., 2004, 34, 2143 CrossRef
.
-
Chemical Synthesis Using Supercritical Fluids, ed. P. G. Jessop and W. Leitner, Wiley-VCH, Weinheim, 1999 Search PubMed
; A. Baiker, Chem. Rev., 1999, 99, 453 Search PubMed
; P. Licence, J. Ke, M. Sokolova, S. K. Ross and M. Poliakoff, Green Chem., 2003, 5, 99 CrossRef CAS
; W. Leitner, Acc. Chem. Res., 2002, 35, 746 RSC
.
- S. A. Nolen, J. Liu, J. S. Brown, P. Pollet, B. C. Eason, K. N. Griffith, R. Gläser, D. Bush, D. R. Lamb, C. L. Liotta and C. A. Eckert, Ind. Eng. Chem. Res., 2002, 41, 316 CrossRef CAS
.
- D. E. Richardson, H. Yao, K. M. Frank and D. A. Bennett, J. Am. Chem. Soc., 2000, 122, 1729 CrossRef CAS
.
- H. Yao and D. E. Richardson, J. Am. Chem. Soc., 2000, 122, 3220 CrossRef CAS
.
- D. A. Bennett, H. Yao and D. E. Richardson, Inorg. Chem., 2001, 40, 2996 CrossRef CAS
.
- N. Theyssen, Z. Hou and W. Leitner, Chem.–Eur. J., 2006, 12, 3401 CrossRef
and references therein.
-
I. V. Zakharov, Y. V. Geletii and V. A. Adamyan, USSR Pat., SU1623947, 1991 Search PubMed
.
- Solubility of phenol and p-bromophenol in water at 25 °C and pH = 7 are 80 g L–1 and 25 g L–1 respectively.
| This journal is © The Royal Society of Chemistry 2007 |