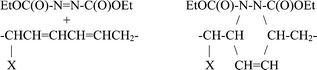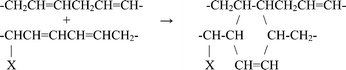Novel modified soybean oil containing hydrazino-ester: synthesis and characterization
Atanu
Biswas
*a,
Brajendra K.
Sharma
bc,
J. L.
Willet
a,
K.
Vermillion
d,
Sevim Z.
Erhan
b and
H. N.
Cheng
*e
aPlant Polymer Research Unit, National Center for Agricultural Utilization Research, USDA/Agricultural Research Services, 1815 N. University Street, Peoria, IL 61604, USA
bFood and Industrial Oil Research Unit, National Center for Agricultural Utilization Research, USDA/Agricultural Research Services, 1815 N. University Street, Peoria, IL 61604, USA
cDepartment of Chemical Engineering, Pennsylvania State University, University Park, PA 16802, USA
dNew Crops and Processing Technology Research Unit, National Center for Agricultural Utilization Research, USDA/Agricultural Research Services, 1815 N. University Street, Peoria, IL 61604, USA
eHercules Incorporated Research Center, 500 Hercules Road, Wilmington, DE 19808-1599, USA
First published on 31st October 2006
Abstract
A novel synthetic approach for chemical modification of vegetable oils is presented. The structural modification is carried out using diethyl azodicarboxylate (DEAD) in the absence of catalyst and solvent. In a microwave oven the reaction can be achieved in 5–15 minutes. The reaction can also proceed using conventional heat, albeit for a longer time. The products are characterized by 1H, 13C, and two-dimensional NMR.
Introduction
There has been a lot of interest in using vegetable oils (particularly soybean oil) as renewable raw materials for new industrial products. It is important to develop a range of relatively facile reactions on vegetable oils in order to facilitate their use. This article represents a step in this direction. The reaction reported is novel and fast, does not require catalyst or solvent, has high yields and is achievable even with a microwave oven. It appears to be a useful reaction that has many potential applications for these oils.There has been a constant demand for environmentally friendly lubricants. The interest intensified during the last decade due to strict government and environmental regulations.1 Most of the current lubricants originate from petroleum stock, which is toxic to the environment and difficult to dispose of. Vegetable oils with high oleic content are considered to be potential candidates to substitute conventional mineral oil-based lubricating oils and synthetic esters.2,3 Vegetable oils are preferred to synthetic fluids because they are renewable resources and potentially cheaper.
Vegetable oils as lubricants are preferred because they are biodegradable and non-toxic, unlike conventional mineral-based oils.2,4 Apart from these, they have advantages like low volatility, high viscosity index, good boundary lubrication properties, and high solubilizing power for polar contaminants and additive molecules. On the other hand, vegetable oils have poor oxidative stability,5,6 primarily due to the presence of bis-allylic protons, and are highly susceptible to radical attack and subsequently undergo oxidative degradation to form polar oxy compounds. This phenomenon may result in insoluble deposits and increases in oil acidity and viscosity, but can be partly mitigated through the use of antioxidants. Vegetable oils can also show poor corrosion protection.7 Low temperature studies have also shown that most vegetable oils undergo cloudiness, precipitation, poor flow, and solidification at −10 °C upon long-term exposure to cold temperature8,9 in sharp contrast to mineral oil-based fluids.
In a previous account, Biswas et al.10 reported a method to prepare amino derivatives of soybean oil. Our objective in this work is to explore new pathways to attach nitrogen to vegetable oil. The structural modification is carried out using diethyl azodicarboxylate (DEAD) in the absence of any catalyst and solvent. It has been demonstrated that this reaction is very versatile and can be conducted under different reaction conditions. For example, we can prepare the reaction product using a microwave oven in about 10 minutes, thereby saving a lot of time and energy.
Experimental
Microwave-assisted reaction of soybean oil with DEAD
Microwave reactions were carried out using an Ethos™ MicroSYNTH 1600 Microwave Labstation from Milestone Inc. Reactions were performed in a 50 mL quartz pressure vessel, QRS1550 from Milestone. A mixture of soybean oil 5.32 g (6.2 mmole), 3.1 g of DEAD (18.6 mmole, 3 molar equivalents) were taken into a cross-linked fluoropolymer reactor with a Weflon™ stir bar and placed in a Milestone Ethos™ Labstation. Using the Easywave™ operating software and the fiber-optic temperature probe, the reaction mixture was irradiated with simultaneous magnetic stirring. It took 3 min to reach 120 °C and was held at that temperature for another 10 min. The soybean oil/DEAD adduct was obtained as a viscous, honey-colored oil.Conventional thermal synthesis
The same reaction was carried out for 4 hours in a 25 ml round bottom flask with overhead stirrer and a heating mantle as the heat source at 60–120 °C.Room temperature synthesis in water
A mixture of 5.32 g of soybean oil (6.2 mmole), 3.1 g of DEAD (18.6 mmole, 3 molar equivalents) and 15 ml of water were stirred at room temperature for 3–24 hours. The yellow color of the reaction mixture slowly disappeared and became colorless. Along with the soybean/DEAD adduct, we also obtained white crystals of reduced DEAD (DEADH2). The white solid was readily removed to give the soybean/DEAD adduct. If needed, DEADH2 could be separately crystallized from ethyl acetate–hexane (m.p. 131–133 °C).NMR spectroscopy
All 13C NMR spectra were recorded quantitatively with a Bruker ARX-500 spectrometer (Bruker, Rheinstetten, Germany) at a frequency of 125 MHz and a 5 mm dual probe. The sample solutions were prepared in deuterochloroform (CDCl3, 99.8% D, Cambridge Isotope Laboratories, Inc., Andover, MA, USA). Standard operating conditions were used with 30° pulse angle, 3 seconds between pulses, and 1H decoupling. Three two-dimensional NMR experiments were run: COSY (correlation spectroscopy), HSQC (heteronuclear single quantum correlation), and HMBC (heteronuclear multiple bond correlation).Size exclusion chromatography (SEC)
Molecular weights were measured on a PL-GPC 120 high temperature chromatograph (Polymer Laboratories, Amherst, MA, USA) equipped with autosampler and in-built differential refractometer detector. Two PL gel 3 µm mixed E columns (300 mm × 7.5 mm, Polymer Laboratories) were used in series to resolve the samples. The injection volume was 100 µL. The samples were eluted using a 1.00 mL min−1 flow rate of THF at 40 °C. The SEC was calibrated using a mixture of linear polystyrene standards (Mn 1700, 2450, 5050, 7000, 9200, and 10665) obtained from Polymer Laboratories (Amherst, MA, USA), and methyl oleate (Mn 296.48), methyl linoleate (Mn 294.48), monoolein (Mn 353), diolein (Mn 619.2), and triolein (Mn 885.4) obtained from Aldrich Chemical (Milwaukee, WI, USA).Results and discussion
In this work, we sought a “green” approach to modify vegetable oils that is fast and facile and does not involve any catalysts or solvents. We discovered that soybean oil and DEAD can readily react with each other. Although this work was focused on soybean oil, any vegetable oil with linoleic or linolenic moieties will readily undergo this reaction.Reactions in a microwave oven
An interesting feature of this reaction is its ability to be carried out in a microwave oven in 5–15 minutes. As far as we know, this is the first report of such a reaction on soybean oil. Some typical reactions are given in Table 1.| Sample | Reactant | Molar ratio FA : DEAD | Reaction time/min | Temp/°C | % product | % ene productd |
|---|---|---|---|---|---|---|
| a Also contains 15% soybean oil dimer, 4% soybean oil trimer. b Also contains 1% methyl linoleate dimer. c From SEC. d From NMR. | ||||||
| 17865-33-2 | Soybean oil | 1 : 1.00 | 10 | 115 | 81ac | 31 |
| 17865-33-1 | Soybean oil | 1 : 0.86 | 5 | 115 | 46c | 31 |
| 17865-23-1 | Me linoleate | 1 : 0.80 | 10 | 115 | 82d | 62 |
| 17865-32-1 | Me linoleate | 1 : 1.20 | 5 | 90 | 99bc | 71 |
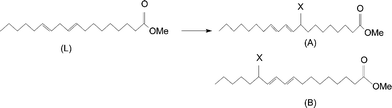
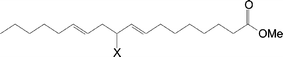
In order to elucidate the reaction mechanism, we also carried out the microwave reaction with methyl linoleate (Table 1). For sample 32-1, the 1H spectrum is given in Fig. 1a. The spectrum can be assigned mostly to the 1 : 1 ene reaction adduct. The ene reaction is well known and well documented.11,12 The 13C NMR spectrum is given in Fig. 1b. To help with interpretation, we obtained the appropriate two-dimensional spectra (COSY, HSQC, and HMBC). The reaction was shown to give two products with the following structures:
 | ||
| Fig. 1 (a) 1H NMR spectrum of the microwave reaction products between methyl linoleate and DEAD. (b) 13C NMR spectrum of the microwave reaction products between methyl linoleate and DEAD. | ||
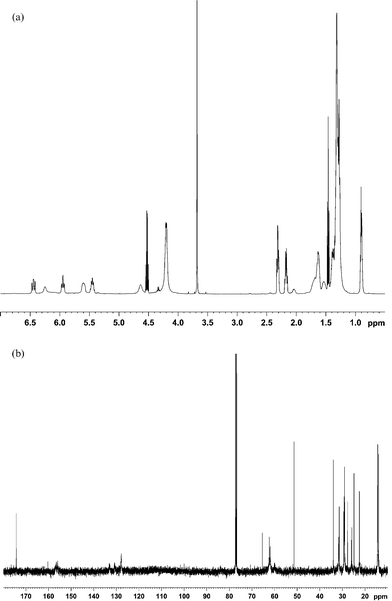
A preliminary structure elucidation was first obtained through literature values on similar materials13–16 and empirical additive shift rules.17–19 With the addition of the COSY spectrum, 1H assignments were positively obtained. From HSQC spectrum, further 13C and 1H assignments were made through 13C–1H shift correlation. The assignments were confirmed with the HMBC spectrum. For illustration, the COSY and the HSQC spectra are shown in Fig. 2. The full assignments are summarized in Table 2. It is of interest that despite the spectral complexity, all major peaks are assigned. Note that the peaks next to the point of attachment of DEAD on the linoleate show up as doublet in both 1H and 13C spectra due to asymmetry at that point.
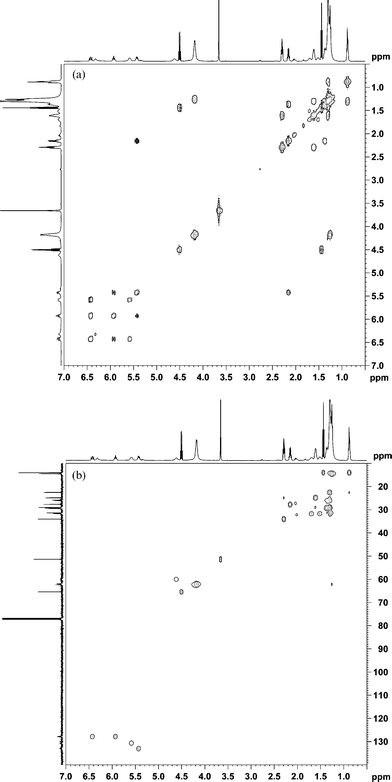 | ||
| Fig. 2 (a) 1H COSY spectrum of the microwave reaction products between methyl linoleate and DEAD. (b) 1H–13C HSQC spectrum of the microwave reaction products between methyl linoleate and DEAD. | ||
| Chemical shift of carbon or proton number | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | OMe | NH | |
| Methyl linoleate (L) | ||||||||||||||||||||
| 1H | — | 2.26 | 1.58 | 1.28 | 1.28 | 1.28 | 1.3 | 2.02 | 5.33 | 5.33 | 2.73 | 5.33 | 5.33 | 2.02 | 1.28 | 1.28 | 1.28 | 0.85 | 3.62 | — |
| 13C | 174.0 | 34.0 | 24.9 | 29.1 | 29.1 | 29.1 | 30 | 27.1 | 130.0 | 128.0 | 25.6 | 127.9 | 130.1 | 27.1 | 29.3 | 31.5 | 22.5 | 14.0 | 51.0 | — |
| Methyl linoleate–DEAD (A) | ||||||||||||||||||||
| 1H | — | 2.28 | 1.60 | 1.28 | 1.28 | 1.28 | 1.3 | 1.50 | 4.60 | 5.57 | 6.41 | 5.91 | 5.41 | 2.14 | 1.35 | 1.28 | 1.28 | 0.87 | 3.62 | 6.21 |
| 1.68 | ||||||||||||||||||||
| 13C | 174.0 | 34.0 | 24.8 | 29.1 | 29.1 | 29.2 | 26 | 31.6 | 60.1 | 130.9 | 127.9 | 127.9 | 133.3 | 27.7 | 29.3 | 31.4 | 22.5 | 13.9 | 51.1 | |
| 31.7 | ||||||||||||||||||||
| Methyl linoleate–DEAD (B) | ||||||||||||||||||||
| 1H | — | 2.28 | 1.60 | 1.28 | 1.28 | 1.28 | 1.4 | 2.14 | 5.41 | 5.91 | 6.41 | 5.57 | 4.60 | 1.50 | 1.28 | 1.28 | 1.28 | 0.87 | 3.62 | 6.21 |
| 1.68 | ||||||||||||||||||||
| 13C | 174.0 | 34.0 | 24.9 | 29.1 | 29.1 | 29.1 | 30 | 27.7 | 133 | 127.9 | 127.9 | 130.9 | 60.1 | 31.6 | 25.8 | 31.4 | 22.5 | 13.9 | 51.1 | |
| 31.7 | ||||||||||||||||||||
| Methyl oleate (O) | ||||||||||||||||||||
| 1H | — | 2.30 | 1.62 | 1.30 | 1.30 | 1.30 | 1.30 | 2.00 | 5.34 | 5.34 | 2.00 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 0.88 | 3.66 | |
| 13C | 174.1 | 34.0 | 24.9 | 29.0 | 29.0 | 29.0 | 29.6 | 27.1 | 129.7 | 129.9 | 27.1 | 29.6 | 29.3 | 29.5 | 29.3 | 31.9 | 22.6 | 14.0 | 51.2 | |
| Methyl oleate–DEAD adduct (C) | ||||||||||||||||||||
| 1H | — | 2.31 | 1.62 | 1.29 | 1.29 | 1.29 | 1.3 | 1.50 | 4.51 | 5.38 | 5.60 | 2.00 | 1.31 | 1.29 | 1.29 | 1.29 | 1.29 | 0.88 | 3.67 | 6.25 |
| 13C | 174.3 | 34.0 | 24.9 | 29 | 29 | 29.6 | 26 | 32.2 | 60.0 | 128 | 134 | 27.1 | 29.5 | 29.5 | 29.3 | 31.9 | 22.6 | 14.0 | 51.3 | |
| 32.4 | ||||||||||||||||||||
| Methyl oleate–DEAD adduct (D) | ||||||||||||||||||||
| 1H | 174.3 | 2.31 | 1.62 | 1.29 | 1.29 | 1.31 | 2.00 | 5.60 | 5.38 | 4.51 | 1.50 | 1.31 | 1.29 | 1.29 | 1.29 | 1.29 | 1.29 | 0.88 | 36.7 | 6.25 |
| 1.61 | ||||||||||||||||||||
| 13C | — | 34.0 | 24.9 | 29.0 | 29.0 | 29.5 | 27.1 | 134.0 | 128 | 60.0 | 32.2 | 26.2 | 29.6 | 29.5 | 29.3 | 31.9 | 22.6 | 14.0 | 51.3 | |
| 32.4 | ||||||||||||||||||||
| Unreacted DEAD | COO | CH2 | CH3 | |||||||||||||||||
| 1H | — | 4.53 | 1.44 | |||||||||||||||||
| 13C | 160.3 | 65.4 | 14.1 | |||||||||||||||||
| Reacted DEAD | ||||||||||||||||||||
| 1H | — | 4.17 | 1.26 | |||||||||||||||||
| 13C | 156.7 | 62.3 | 14.3 | |||||||||||||||||
| 156.0 | 61.9 | 14.2 | ||||||||||||||||||
In the ene reaction, DEAD can add to the double bond in two ways. It is important to note that only the conjugated structures are observed. The alternative ene reaction products were not found, e.g.,
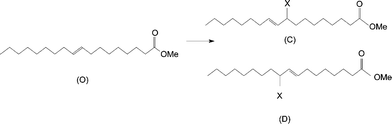
A similar study was done for methyl oleate and DEAD. The reaction was more sluggish, but the reaction products were exactly what we would expect for the ene reaction.
Again, the NMR assignments were made using COSY, HSQC, and HMBC. For convenience, the complete spectral assignments are summarized in Table 2.
Likewise, the structures of the SBO–DEAD adducts were also elucidated using NMR. Soybean oil contains a distribution of fatty esters, typically about 10% palmitate, 5% stearate, 25% oleate, 51% linoleate, and 7% linoleneate. Obviously palmitate and stearate cannot undergo ene reaction. Thus, we would expect the main reactions to occur among linoleate, oleate, (and linolenate) and DEAD. Indeed, the 1H and 13C spectra appear to be composites of the linoleate–DEAD and oleate–DEAD adducts (Fig. 3). Accordingly, the same structures shown above for A, B, C, D are also found in the soybean oil–DEAD reaction.
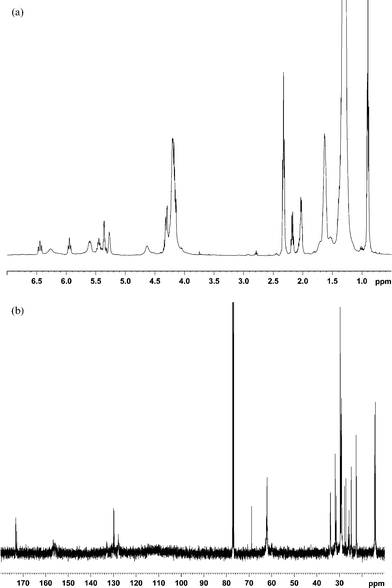 | ||
| Fig. 3 (a) 1H NMR spectrum of the microwave reaction products between soybean oil and DEAD. (b) 13C NMR spectrum of the microwave reaction products between soybean oil and DEAD. | ||
Note that in Table 1, the ene reaction products account for only 30–40% of the yield for soybean oil–DEAD. In addition to ene reaction, Diels–Alder reaction occurs with the diene in the fatty acid moiety of the SBO–aza-dicarboxylate ester. Two reactions are shown below.
1. Reaction with DEAD
2. Reaction with another SBO unit
The second reaction gives rise to dimers, trimers, and polymers of SBO. This is especially likely when the microwave reaction is carried to high conversions. Sample 33-2 in Table 1, for example, contains up to 20% dimers and trimers (according to SEC), and the material is very viscous. If the reaction conditions are optimized, higher yields of polymers can be obtained.20
Reactions using conventional heat
The ene reaction can also be carried out using conventional heat treatment. A typical reaction requires 4 hours of reaction time at 60 °C under nitrogen. Some examples with different molar ratios of SBO/DEAD are given in Table 3.| Sample | Molar ratio SBO : DEAD | NMR data | SEC data | |||
|---|---|---|---|---|---|---|
| Mole% DEAD reacted | % ene reaction | SBO adducts | SBO dimer adducts | SBO trimer and polymer | ||
| 17865-3-1 | 1 : 1.0 | 33 | 17 | 66 | 17 | 17 |
| 17865-4-1 | 1 : 1.7 | 56 | 31 | |||
| 17865-8-1 | 1 : 2.0 | 62 | 37 | 75 | 13 | 12 |
| 17865-9-1 | 1 : 2.5 | 64 | 37 | |||
| 17865-7-1 | 1 : 3.0 | 80 | 38 | 72 | 17 | 11 |
Just as in the case of the microwave reaction, the ene reaction proceeds up to a point and then Diels–Alder takes over, reacting (1) with DEAD and (2) with the double bond in soybean oil. The latter reaction produces dimers, trimers, and polymers. As more ene reaction proceeds, more Diels–Alder reaction proceeds as well, so that we reach an equilibrium of about 30–40% ene reaction product only.
Applications
The reaction described herein is a good method to introduce electrophilic nitrogen onto the fatty acid triglyceride structure. The soybean oil–DEAD adduct should be a good synthon for further reactions. As indicated above, the reaction can be carried to higher polymers. The triglyceride can be hydrolyzed chemically or enzymatically to give free carboxylic acid.20 Alkaline hydrolysis can provide the hydrazino fatty acid. Furthermore, in this reaction the double bonds are not destroyed and simply shifted. Thus, the various modification reactions described for vegetable oils by a number of previous workers can be readily implemented, e.g., epoxidation,21,22 maleation,23,24 polymer formation,25,26 and others.27–29Perhaps another possible area of application is pharmaceutical. The hydrazide functionality is found in some drug molecules (e.g., Iproniazid and hydralazane). An α-hydrazino acid (Carbidopa) is a well-known anti-Parkinsons drug. It remains to be seen if the soybean oil derivatives or their hydrolyzates contain pharmaceutical activity.
Future work
It is noteworthy that even at room temperature when DEAD and soybean oil are mixed, the viscosity increases over time. NMR also shows the presence of ene products. This phenomenon may find applications where a self-thickening agent is needed in a solution. We are further investigating this room temperature reaction in detail, and results will be reported in the future.Following the work of Sharpless,30 we also tried to react DEAD and soybean oil in water. However, we found that DEAD underwent competing reactions with water and vegetable oil to give both ene product and DEADH2. Although we could separate the two materials, the yield suffered. The reaction of DEAD with water to give the reduced form (DEADH2) was known.31
Acknowledgements
The authors wish to thank Janet Berfield for expert technical assistance.References
- I. S. Rhee, NLGI Spokesman, 1996, 60(5), 28 Search PubMed.
- S. J. Randles and M. Wright, J. Synth. Lubr., 1992, 9, 145–161 Search PubMed.
- S. Asadauskas, J. M. Perez and J. L. Duda, Lubr. Eng., 1996, 52, 877–882 CAS.
- N. S. Battersby, S. E. Pack and R. J. Watkinson, Chemosphere, 1998, 24, 1998–2000.
- R. E. Gapinski, I. E. Joseph and B. D. Layzell, SAE Tech. Pap. Ser., 1994, 941785, pp. 1–9 Search PubMed.
- R. Becker and A. Knorr, Lubr. Sci., 1996, 8, 95–117 Search PubMed.
- S. A. Ohkawa, H. Konishi, K. Hatano, K. Tanaka and M. Iwamura, SAE Tech. Pap. Ser., 1995, 951038, pp. 55–63 Search PubMed.
- I. S. Rhee, C. Valez and K. Bernewitz, TARDEC Tech Report 13640, US Army Tank-Automotive Command Research, Development and Engineering Center, Warren, MI, USA, 1995, 1 Search PubMed.
- E. Kassfeldt and D. Goran, Environmentally adapted hydraulic oils, Wear, 1997, 207, 41 CrossRef CAS.
- A. Biswas, A. Adhvaryu, S. H. Gordon, S. Z. Erhan and J. L. Willett, J. Agric. Food Chem., 2005, 53(24), 9485 CrossRef CAS.
- K. Mikami and M. Shimizu, Chem. Rev., 1992, 92, 1021 CrossRef CAS.
- S. H. Nahm and H. N. Cheng, J. Org. Chem., 1986, 51, 5093 CrossRef CAS.
- C. J. Pouchert and J. Behnke, The Aldrich Library of 13C and 1H FT NMR Spectra, Aldrich Chemical Co., Inc, 1993 Search PubMed.
- J. G. Batchelor, R. J. Cushley and J. H. Prestegard, J. Org. Chem., 1974, 39, 1698 CrossRef CAS.
- J. Bus, I. Sies and A. Jie, Chem. Phys. Lipids, 1976, 17, 501 CrossRef CAS.
- A. R. Cox, B. Vincent, S. Harley and S. E. Taylor, Colloids Surf., A, 1999, 146, 153 CrossRef CAS.
- E. G. Paul and D. M. Grant, J. Am. Chem. Soc., 1964, 86, 2984 CrossRef CAS.
- E. Pretsch, J. T. Clerc, J. Seibl and W. Simon, Tables of Spectral Data for Structure Elucidation of Organic Compounds, Springer, Berlin, 2nd edn, 1989 Search PubMed.
- H. N. Cheng and M. A. Bennett, Anal. Chim. Acta, 1991, 242, 43 CrossRef CAS.
- A. Biswas, R. L. Shogren, J. L. Willet, S. Z. Erhan and H. N. Cheng, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2006, 47(2), 259 CAS.
- Z. S. Petrovic, A. Zlatanic, C. C. Lava and S. Sinadinovic-Fiser, Eur. J. Lipid Sci. Technol., 2002, 104, 293 CrossRef CAS.
- M. C. Kuo and T. C. Chou, Ind. Eng. Chem. Res., 1987, 26, 277 CrossRef CAS.
- H. Warth, R. Mulhaupt, B. Hoffmann and S. Lawson, Angew. Makromol. Chem., 2003, 249, 79.
- P. Tran, K. Seybold, D. Graiver and R. Narayan, J. Am. Oil Chem. Soc., 2005, 82, 189 CAS.
- F. Li, M. V. Hanson and R. C. Larock, Polymer, 2001, 42, 1567 CrossRef CAS.
- S. N. Khot, J. J. Lascala, E. Can, S. S. Morye, G. I. Williams, G. R. Palmese, S. H. Kusefoglu and R. P. Wool, J. Appl. Polym. Sci., 2001, 82, 703 CrossRef CAS.
- T. M. Baber, D. Graiver, C. T. Lira and R. Narayan, Biomacromolecules, 2005, 6, 1334 CrossRef CAS.
- U. Biermann and J. O. Metzger, Angew. Chem., Int. Ed., 2000, 39, 2206 CrossRef CAS.
-
H. P. Benecke, B. R. Vijayendran and J. D. Elhard, U. S. Pat., 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797
797![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 753, 2004 Search PubMed.
753, 2004 Search PubMed. - S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 21, 3157 CrossRef.
- J. C. Kauer, Org. Synth., CV 4, 411 Search PubMed.
| This journal is © The Royal Society of Chemistry 2007 |