Ionic liquids via reaction of the zwitterionic 1,3-dimethylimidazolium-2-carboxylate with protic acids. Overcoming synthetic limitations and establishing new halide free protocols for the formation of ILs
Marcin
Smiglak
a,
John D.
Holbrey†
a,
Scott T.
Griffin
a,
W. Matthew
Reichert
a,
Richard P.
Swatloski
a,
Alan R.
Katritzky
*b,
Hongfang
Yang
b,
Dazhi
Zhang
b,
Kostyantyn
Kirichenko
b and
Robin D.
Rogers
*a
aCenter for Green Manufacturing and Department of Chemistry, The University of Alabama, Tuscaloosa, AL 35487, USA. E-mail: rdrogers@bama.ua.edu
bCenter for Heterocyclic Compounds, Department of Chemistry, University of Florida, Gainesville, FL 32611, USA. E-mail: katritzky@chem.ufl.edu
First published on 5th December 2006
Abstract
The previously reported preparation of 1,3-dimethylimidazolium salts by the reaction of 1,3-dialkylimidazolium-2-carboxylate zwitterions with protic acids has been reinvestigated in detail, leading to the identification of two competing reactions: isomerisation and decarboxylation. The ability to control both pathways allows this methodology to be used as an effective, green, waste-free approach to readily prepare a wide range of ionic liquids in high yields. Additionally, this reaction protocol opens new possibilities in the formation of other imidazolium salts, whose syntheses were previously either very expensive (due to ion exchange protocols involving metals like Ag) or difficult to achieve (due to multiple extractions and large quantities of hard to remove inorganic by-products).
Introduction
Ionic liquids (ILs; conventionally defined as organic salts with a melting point below 100 °C1) have been extensively investigated in recent years. Their often unique physical and chemical properties, such as potential non-volatility, thermal stability, and large liquid ranges are increasing their potential applications far beyond the initial investigation of IL electrolytes.2,3 New solvent applications (e.g., electrochemistry,4–6 separation science,7–10 chemical synthesis,11–15 and catalysis13,16,17), as well as new materials applications of the ILs themselves (e.g., energetic materials,18–24 thermal fluids, lubricants, and fuel cell electrolytes25–27) are continuously being reported.This current, growing academic and industrial interest in diverse IL applications and technologies,1–23,28–32 calls for the development of approaches for the preparation and purification of ILs which are cost/time efficient and at the same time atom efficient and limit the use of hazardous reagents. One such example, the synthesis of 1,3-dialkylimidazolium alkyl sulfates from 1-alkylimidazole with dimethyl or diethyl sulfate, excluding contamination by halides has been described by Holbrey et al.33 and Wasserscheid et al.34
Unfortunately, most of the current synthetic protocols for the preparation of ILs still struggle with multi-step synthesis, complex purification and, in many cases, formation of undesirable halide-containing by-products. Thus, new synthetic strategies for obtaining imidazolium-based organic salts (IL precursors or ILs themselves) allowing flexible design capabilities, are still necessary in order to introduce ILs into routine usage.
Since the mid 1980s, there has been considerable interest in the use of dimethyl carbonate (DMC) as a clean methylating agent which could be used as safe replacement of current reagents such as methylhalides, dimethyl sulfate, and phosgene.35 Additionally, a new and improved synthesis of DMC, developed by Enichem,36,37 and UBE38 (catalytic oxidative carbonylation of methanol with oxygen instead of synthesis from phosgene), very low toxicity of this reagent,39,40 the electrophilic character of DMC,41 and environmentally benign by-products of the methylation reaction (i.e., MeOH and CO2)41,42 make this an attractive alternative methylating agents in organic synthesis.
As recently reported by several research groups,43–46 efforts have been made to investigate the properties of DMC as an alkylating agent for the preparation of imidazolium-based organic salts. It was suggested that, when using DMC in reaction with N-alkylimidazoles, new halide-free ionic liquids can be formed. According to those reports, alkylation of 1-methylimidazole with DMC, depending on the reaction conditions, resulted in the formation of either 1,3-dimethylimidazolium-2-carboxylate ([1,3-diMIM-2-COO]) (1) or 1,3-dimethylimidazolium-4-carboxylate ([1,3-diMIM-4-COO]) (2). Aresta et al.44 reported simultaneous formation of both products with some selectivity for [1,3-diMIM-2-COO] (1). They also noted that upon heating to 140 °C, complete isomerization of [1,3-diMIM-2-COO] (1) into [1,3-diMIM-4-COO] (2) occurs. These results are consistent with BASF's47 reported formation of [1,3-diMIM-4-COO] (2) in the reaction of DMC with 1-methylimidazole at 140 °C.
Aresta et al.44 further reported that the reaction of 1,3-dimethylimidazolium-2-carboxylate (1) with tetrafluoroboric acid leads to the formation of two isomeric products: 2-carboxy-1,3-dimethylimidazoliumtetrafluoroborate ([2-(COOH)-1,3-diMIM][BF4]) (4b) and 4-carboxy-1,3-dimethylimidazoliumtetrafluoroborate ([4-(COOH)-1,3-diMIM][BF4]) (5b), depending on the concentration of acid used. Reaction of carboxylate zwitterion salt [1,3-diMIM-2-COO] (1) with equimolar acid gave [2-(COOH)-1,3-diMIM][BF4] (4b), while with excess imidazolium carboxylate ([1,3-diMIM-2-COO]/[HBF4] = 1.25 : 1.0) or upon slow addition of the acid to the imidazolium salt, decarboxylation occurred resulting in the formation of 1,3-dimethylimidazoliumtetrafluoroborate ([1,3-diMIM][BF4]) (3b). The treatment of ([2-(COOH)-1,3-diMIM][BF4]) (4b) with triethylamine also led to decarboxylation to give [1,3-diMIM][BF4] (3b). In contrast, [4-(COOH)-1,3-diMIM][BF4]) (5b) appeared to be stable under these conditions. The multiple reaction pathways and outcomes, could prevent utilization of these techniques in routine preparation of 1,3-dialkylimidazolium ILs, unless they can be fully understood and controlled.
Louie et al.48,49 have reported the development of protocols for efficiently coupling various diynes with CO2, under mild conditions to produce pyrones, via utilization of Ni/imidazolylide complexes. Additionally, Tommasi and Sorrentino50 recently described aspects of the reactivity of 1,3-dialkylimidazolium-2-carboxylates toward organic substrates (methanol, acetophenone, and benzaldehyde) in the presence of Na+ and K+ cations. The authors described the reaction of CO2 transfer from the 1,3-dialkylimidazolium-2-carboxylates and the formation of new organic carboxylate products.
We had previously proposed43 that the simple reaction of the 1,3-dialkylimidazolium-2-carboxylate zwitterion with protic acids should yield a variety of halide-free ILs. Here we report the results of investigation of this approach, especially its variable reactivity with acids (as a route to ion-exchange and fast formation of ILs), as well as, the thermal and chemical stability of the products. Our investigation focused on (i) overcoming limitations and difficulties associated with this synthesis, and (ii) the use of this reaction as a protocol to prepare new ILs, or their precursors, via both halide and by-product free routes, by utilization of previously mentioned isomeric products: kinetic [2-(COOH)-1,3-diMIM] (4) and thermodynamic [4-(COOH)-1,3-diMIM] (5) (Table 1).
| Component number | Component name | Component structure | Used component abbreviation |
|---|---|---|---|
| a The counter ions used for pairing with the cations include: a, nitrate[NO3]; b, tetrafluoroborate[BF4];44c, hexafluorophosphorate[PF6]; d, chloride[Cl]; e, hydrogen sulfate[HSO4]; f, picrate[Pic]. | |||
| 1 | 1,3-dimethylimidazolium-2-carboxylate |
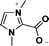
|
[1,3-diMIM-2-COO] |
| 2 | 1,3-dimethylimidazolium-4-carboxylate |
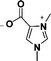
|
[1,3-diMIM-4-COO] |
| 3 | 1,3-dimethylimidazolium |
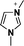
|
[1,3-diMIM] |
| 4 | 2-carboxy-1,3-dimethylimidazolium |
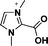
|
[2-(COOH)-1,3-diMIM] |
| 5 | 4-carboxy-1,3-dimethylimidazolium |
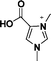
|
[4-(COOH)-1,3-diMIM] |
Results and discussion
As the starting point for this investigation we repeated the reaction of dimethylcarbonate (DMC) with 1-methylimidazole,43 and obtained [1,3-diMIM-2-COO] (1) in 85% yield as crystalline material. The product was characterized by 1H and 13C NMR techniques, and no [1,3-diMIM-4-COO] (2) was detected.A 0.01 mol stirred solution of [1,3-diMIM-2-COO] (1) in 50% aqueous ethanol (v/v, 20 mL) was then treated with various acids (HPF6, H2SO4, HCl, HNO3, picric acid) via dropwise addition of an equimolar amount of the appropriate acid (10 mL of 1 M aqueous solution; ethanolic solution in the case of picric acid), at room temperature. In all cases except one (reaction with HNO3) the reaction proceeded as expected (Scheme 1, path A); the zwitterion 1 underwent decarboxylation, leading to the formation of the [1,3-diMIM] salt (3c–f) and evolution of gaseous CO2. All acids used are considered strong acids with pKa < 0, with the exception of picric acid with pKa = 0.3. All salts were characterized by NMR, TGA, and DSC techniques, and the results were compared with the available literature data for known salts (Table 2).
![Reactions of [1,3-diMIM-2-COO] (1) with different acids. Reaction with nitric acid (depending upon thermal conditions) resulted in the exclusive formation of one of the isomeric [2-(COOH)-1,3-diMIM][NO3] (4a) or [4-(COOH)-1,3-diMIM][NO3] (5a).](/image/article/2007/GC/b610421e/b610421e-s1.gif) | ||
| Scheme 1 Reactions of [1,3-diMIM-2-COO] (1) with different acids. Reaction with nitric acid (depending upon thermal conditions) resulted in the exclusive formation of one of the isomeric [2-(COOH)-1,3-diMIM][NO3] (4a) or [4-(COOH)-1,3-diMIM][NO3] (5a). | ||
| Sample | Compound | Mp/°C | T 5%dec/°C |
|---|---|---|---|
| a Melting points (mp) (°C) were measured from transition onset temperature determined by DSC from the second heating cycle at 5 °C min–1, after initially melting and then cooling samples to –100 °C. Decomposition temperatures (T5%dec) were determined by TGA from onset to 5 wt% mass loss, heating at 5 °C min–1 under air. b Melting point of 3c was checked visually using hot stage apparatus due to reported reactivity and catalyzed decomposition of the PF6 anion-based ionic liquids while in contact with aluminium.53 c Compound was obtained later in the study as the result of the developed synthetic protocol presented below in the article. | |||
| 3c | [1,3-diMIM] [PF6] | 67–68b | 298 |
| 3d | [1,3-diMIM] [Cl] | 147 (lit ≈ 127)51 | 202 |
| 3e | [1,3-diMIM] [HSO4] | 98 | 264 |
| 3f | [1,3-diMIM] [Pic] | 144 (lit ≈ 151)52 | 210 |
| 3a c | [1,3-diMIM] [NO3] | 58 | 253 |
Unexpected behavior (Scheme 1, path B) was observed in the reaction with HNO3 leading to the formation of different products, similar to results by Aresta et al.44 Depending on the reaction conditions, the isomeric salts, either [2-(COOH)-1,3-diMIM][NO3] (4a) or [4-(COOH)-1,3-diMIM][NO3] (5a), were formed with no detectable decarboxylation in contrast to the examples which followed path A. Neither variation of the reaction conditions (concentrations of acid), nor order of addition of the substrates (acid to imidazolium zwitterion or imidazolium zwitterion to acid) resulted in decarboxylation. The structures of both isomers were confirmed by 1H (Fig. 1), 13C, and NOESY NMR, and unambiguously by both single crystal (Fig. 2) and powder X-ray diffraction (Fig. 3).
![1H NMR (360 MHz, DMSO-d6) evidence for the presence of two distinguishable isomers of carboxy-1,3-dimethylimidazolium nitrates: [2-(COOH)-1,3-diMIM][NO3] (4a) and [4-(COOH)-1,3-diMIM][NO3] (5a).](/image/article/2007/GC/b610421e/b610421e-f1.gif) | ||
| Fig. 1 1H NMR (360 MHz, DMSO-d6) evidence for the presence of two distinguishable isomers of carboxy-1,3-dimethylimidazolium nitrates: [2-(COOH)-1,3-diMIM][NO3] (4a) and [4-(COOH)-1,3-diMIM][NO3] (5a). | ||
Single crystals of both isomers were prepared by dissolution of the product in 50% aqueous ethanol (v/v) followed by slow evaporation of the solvents. The crystal structure of [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O) (Fig. 2, top) contains water of crystallization which participates in strong hydrogen bonds with the carboxylic acid group of the cation (O2–H2A⋯O1W = 1.48(6) Å, 170(4)°) and with the anion (O1W–H10⋯O1 at 1.86(5) Å, 168(5)°, with O1 at 2 – x, 1 – y, –z). The structure of [4-(COOH)-1,3-diMIM][NO3] (5a) (Fig. 2, bottom) shows the expected conformation for the cation. The closest contact in the structure is between the carboxyl group and the anion (O2–H20⋯O4 1.79(4) Å, 167(4)°).
![ORTEP and packing diagrams of [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O) (top) and [4-(COOH)-1,3-diMIM][NO3] (5a) (bottom).](/image/article/2007/GC/b610421e/b610421e-f2.gif) | ||
| Fig. 2 ORTEP and packing diagrams of [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O) (top) and [4-(COOH)-1,3-diMIM][NO3] (5a) (bottom). | ||
To ensure that the single crystal data indeed represented the overall bulk structure, powder X-ray diffraction spectra were obtained. This evidence is supportive of the assumption that the two isomeric carboxy-1,3-dimethylimidazolium nitrates (4a and 5a) can be selectively synthesized. First, the theoretical powder diffraction spectra were generated using the single crystal data for each of the samples {Fig. 3(B) for the [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O) isomer, and (D) for the [4-(COOH)-1,3-diMIM][NO3] (5a) isomer} and these were compared with the experimental spectra {Fig. 3(A) for [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O), and (C) for [4-(COOH)-1,3-diMIM][NO3] (5a)}. The simulated (Fig. 3(D)) and experimental patterns (Fig. 3(C)) for [4-(COOH)-1,3-diMIM][NO3] (5a) match well, indicating a pure phase. However, a comparison of the simulated (Fig. 3(B)) and experimental spectra (Fig. 3(A)) for [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O), while matching reasonably well, reveals the presence of a small amount of [4-(COOH)-1,3-diMIM][NO3] (5a) in the bulk phase.
![Powder X-ray diffraction patterns for isomers of carboxy-1,3-dimethylimidazolium nitrates (4a·H2O and 5a) compared with the simulated patterns generated from the single crystal data. (A) Experimental [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O); (B) simulated [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O); (C) experimental [4-(COOH)-1,3-diMIM][NO3] (5a); (D) simulated [4-(COOH)-1,3-diMIM][NO3] (5a).](/image/article/2007/GC/b610421e/b610421e-f3.gif) | ||
| Fig. 3 Powder X-ray diffraction patterns for isomers of carboxy-1,3-dimethylimidazolium nitrates (4a·H2O and 5a) compared with the simulated patterns generated from the single crystal data. (A) Experimental [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O); (B) simulated [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O); (C) experimental [4-(COOH)-1,3-diMIM][NO3] (5a); (D) simulated [4-(COOH)-1,3-diMIM][NO3] (5a). | ||
Selective isomer formation
In order to more fully characterize the system, pure [2-(COOH)-1,3-diMIM][NO3] (4a) and [4-(COOH)-1,3-diMIM][NO3] (5a) salts were prepared by two different procedures allowing the selective formation of each isomer in high yields and isomeric purity at the appropriate reaction temperature (Scheme 2).![Formation of [1,3-diMIM][NO3] (3a) via two distinctive pathways. Top: reaction of [1,3-diMIM-2-COO] (1) with HNO3 at low temperature (kinetic product) and further thermal or DMSO-catalyzed decarboxylation via the [2-(COOH)-1,3-diMIM][NO3] intermediate (4a). Bottom: reaction of [1,3-diMIM-2-COO] (1) with HNO3 at higher temperature (thermodynamic product) and further thermal decarboxylation of [4-(COOH)-1,3-diMIM][NO3] (5a).](/image/article/2007/GC/b610421e/b610421e-s2.gif) | ||
| Scheme 2 Formation of [1,3-diMIM][NO3] (3a) via two distinctive pathways. Top: reaction of [1,3-diMIM-2-COO] (1) with HNO3 at low temperature (kinetic product) and further thermal or DMSO-catalyzed decarboxylation via the [2-(COOH)-1,3-diMIM][NO3] intermediate (4a). Bottom: reaction of [1,3-diMIM-2-COO] (1) with HNO3 at higher temperature (thermodynamic product) and further thermal decarboxylation of [4-(COOH)-1,3-diMIM][NO3] (5a). | ||
A series of reactions at a constant temperature of ∼5 °C including: (i) using various concentrations of nitric acid (from 0.001 M to 0.1 M), (ii) using different ratios of [1,3-diMIM-2-COO] (1) and acid (1 : 0.8, 1 : 1, 1 : 1.5, 1 : 3), and (iii) changing the order of addition of substrates, gave exclusively [2-(COOH)-1,3-diMIM][NO3] (4a). On the other hand, reaction at elevated temperature (>75 °C) produced exclusively [4-(COOH)-1,3-diMIM][NO3] (5a) (Scheme 2).
From analysis of these results and literature reports,44 we suggest that the thermal rearrangement of the [1,3-diMIM-2-COO] (1) to [1,3-diMIM-4-COO] (2) (thermodynamic product) occurs prior to contact with the acid, and this process is immediately followed by the proton transfer from HNO3 to the zwitterions (2) resulting in the formation of [4-(COOH)-1,3-diMIM][NO3] (5a) (Scheme 3).
![Thermal rearrangement of [1,3-diMIM-2-COO] (1) to [1,3-diMIM-4-COO] (2), followed by the addition of acid and proton transfer, resulting in the formation of [4-(COOH)-1,3-diMIM][NO3] (5a).](/image/article/2007/GC/b610421e/b610421e-s3.gif) | ||
| Scheme 3 Thermal rearrangement of [1,3-diMIM-2-COO] (1) to [1,3-diMIM-4-COO] (2), followed by the addition of acid and proton transfer, resulting in the formation of [4-(COOH)-1,3-diMIM][NO3] (5a). | ||
Thermal decarboxylation
Having ascertained that control over the formation of the [2-(COOH)-1,3-diMIM][NO3] (4a) and [4-(COOH)-1,3-diMIM][NO3] (5a) isomers was possible, we turned our attention to general routes from either isomer to the desired ILs. The thermal stability of 4a and 5a was measured on samples taken before re-crystallization and were compared to 1 and 3a (Table 3, Fig. 4). Both of the isomeric salts (4a and 5a) decompose following a similar two-step decomposition pathway. The first decomposition step (∼170–180 °C) is probably the result of the decarboxylation process (and also loss of bound water in the case of [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O)), leading to the formation of [1,3-diMIM][NO3] (3a); which then decomposes on further heating in a second step around 300 °C.![Thermal stabilities of analyzed salts determined by TGA with a heating rate of 5 °C min–1 in the range 50 °C to 400 °C: (– – –) [1,3-diMIM-2-COO] (1); (–□–) [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O), (–○–) [4-(COOH)-1,3-diMIM][NO3] (5a); (•••) [1,3-diMIM][NO3] (3a).](/image/article/2007/GC/b610421e/b610421e-f4.gif) | ||
| Fig. 4 Thermal stabilities of analyzed salts determined by TGA with a heating rate of 5 °C min–1 in the range 50 °C to 400 °C: (– – –) [1,3-diMIM-2-COO] (1); (–□–) [2-(COOH)-1,3-diMIM][NO3]·H2O (4a·H2O), (–○–) [4-(COOH)-1,3-diMIM][NO3] (5a); (•••) [1,3-diMIM][NO3] (3a). | ||
| Sample | Compound | T 5%dec/°C | |
|---|---|---|---|
| 1st decomposition step | 2nd decomposition step | ||
| a Decomposition temperatures (T5%dec) were determined by TGA from onset to 5 wt% mass loss, heating at 5 °C min–1 under air in the range 50 °C to 400 °C which provides a more realistic representation of thermal stability at elevated temperatures. | |||
| 1 | [1,3-diMIM-2-COO] | 147 | — |
| 4a | [2-(COOH)-1,3-diMIM] [NO3] | 194 | 265 |
| 5a | [4-(COOH)-1,3-diMIM] [NO3] | 197 | 275 |
| 3a | [1,3-diMIM] [NO3] | 253 | — |
This hypothesis is supported by results from heating samples initially to 150 °C, and then maintaining isothermal conditions for 20 h. The 1H NMR analysis of the product extracted after heating clearly indicated the presence of pure [1,3-diMIM][NO3] (3a) in both cases, as a result of thermal decarboxylation at ∼140–160 °C. With time, a proton signal corresponding to hydrogens on the respective C2 or C4 carbon of the aromatic ring of 4a and 5a was observed to appear and grow, indicating thermal conversion of the 2- or 4-carboxy-1,3-dimethylimidazolium cation (4 and 5) to the 1,3-dimethylimidazolium cation (3). A strong, single signal in the mass spectrum at 97.07 m/z also confirms formation of the [1,3-diMIM][NO3] (3a) salt.
NMR and mass spectrometry analyses of the residues, recovered from the TGA experiment, after thermal decomposition of 60% of the starting mass of the analyzed sample, also showed the presence of pure [1,3-diMIM][NO3] (3a). These results suggest the likely initial decarboxylation of products, followed by a retro-SN2 reaction (opposite to the formation of 1,3-dialkylimidazolium salts); producing volatile decomposition products, which are evacuated from the sample and therefore not detectable in the residue.
![Chemically induced decarboxylation of [2-(COOH)-1,3-diMIM][NO3] (4a).](/image/article/2007/GC/b610421e/b610421e-s4.gif) | ||
| Scheme 4 Chemically induced decarboxylation of [2-(COOH)-1,3-diMIM][NO3] (4a). | ||
DMSO catalyzed decarboxylation
Upon treatment of [2-(COOH)-1,3-diMIM][NO3] (4a) (but not [4-(COOH)-1,3-diMIM][NO3] (5a)) with DMSOat room temperature, solvent promoted decarboxylation (similar to, or based on, the Krapcho reaction)54–59 was observed to give pure [1,3-diMIM][NO3] (3a) (Scheme 4) with 90% conversion in a matter of hours. The attempts to perform the same reaction in DMF or H2O as solvent did not result in formation of desired product at any significant level.Kinetic studies of the decarboxylation of [2-(COOH)-1,3-diMIM][NO3] (4a) were conducted by NMR with DMSO-d6 (Fig. 5). The 1H NMR spectra (acquired after 3, 5, 10, 15, 25, 45, and 60 min and again after 72 h) show that 60% conversion of 4a to 3a was reached within 1 h, and complete conversion after 72 h with no evidence for any other impurity or by-product. The 1H NMR spectra indicated the appearance and gradual increase in relative intensity of the new distinctive signals at 9.04 ppm and 3.86 ppm corresponding to the C-2 proton in the imidazolium ring and to the N-CH3 protons of [1,3-diMIM][NO3] (3a), respectively, while the intensity of the N-CH3 signal at 4.05 ppm of [2-(COOH)-1,3-diMIM][NO3] (4a) proportionally decreased (Fig. 5).
![(a) Solvent (DMSO) induced decarboxylation of [2-(COOH)-1,3-diMIM][NO3] (4a) observed by 1H NMR (360 MHz, DMSO-d6). (b) The NMR indicates the formation of pure [1,3-diMIM][NO3] (3a) obtained after performing the decarboxylation reaction of [2-(COOH)-1,3-diMIM][NO3] (4a) in DMSO at room temperature for 72 h.](/image/article/2007/GC/b610421e/b610421e-f5.gif) | ||
| Fig. 5 (a) Solvent (DMSO) induced decarboxylation of [2-(COOH)-1,3-diMIM][NO3] (4a) observed by 1H NMR (360 MHz, DMSO-d6). (b) The NMR indicates the formation of pure [1,3-diMIM][NO3] (3a) obtained after performing the decarboxylation reaction of [2-(COOH)-1,3-diMIM][NO3] (4a) in DMSO at room temperature for 72 h. | ||
Interestingly, the treatment of the [4-(COOH)-1,3-diMIM][NO3] (5a) isomer does not result in decarboxylation even in DMSO solution for 48 h at 60 °C. From this, we confirm the previous conclusion that the 4-carboxy isomer (5a) is the more stable (thermodynamic) product and does not undergo decarboxylation for this reason.
It is worth considering the differences between the two solid state structures of the isomeric salts, 4a and 5a, as observed in the crystal structures described earlier. The water molecule found in the structure of 4a, but not in 5a, is strongly hydrogen-bonded to the carboxylic acid group of the cation. While the presence, or absence, of this hydrate in the crystal form may be due to the crystallization procedure, it is possible that its presence in 4a·H2O reflects a slightly greater acidity of the carboxylic acid group in 4a compared to 5a, and that this might account for the differences of reactivity, with the more acidic 4a being both thermodynamically and chemically more reactive. However, ab initio calculations (GAMESS, RHF 6-311 + G(d,p) basis set)60 of the electrostatic charge distribution about isolated cations do not reveal any differences between the two cations which could support this hypothesis, and the explanation for the differences in reactivity remains unresolved at present.
In a final study to support either the decarboxylation or rearrangement reaction of [2-COOH-1,3-diMIM][NO3] (4a), this isomer was held in aqueous solution for 72 h at room temperature, followed by heating to 60 °C for 12 h. NMR indicated that neither decarboxylation, nor rearrangement to the 4-carboxy isomer (5a) takes place under these conditions. Any increase in the concentration of [1,3-diMIM][NO3] (3a) was below 2%. It appears that the [2-(COOH)-1,3-diMIM][NO3] (4a) isomer is stable in aqueous media, whereas the decarboxylation reaction occurs quite readily in the polar, aprotic solvent, DMSO.
Conclusions
Previously reported difficulties with the utilization of the synthesis of halide-free ILs, via the reaction of alkylimidazole with dimethylcarbonate, and further reaction with protic acids were investigated here in detail. A new, improved synthetic protocol for the formation of 1,3-dialkylimidazolium salts, via the reaction of 1,3-dialkylimidazolium-2-carboxylate and protic acid (supported by either thermally or chemically induced decarboxylation of carboxy-1,3-dialkylimidazolium intermediate) was designed to overcome these limitations; thus allowing for the wider usage of this technique as a viable route to halide-free, one-pot syntheses of ILs or their precursors. As the results demonstrate, the reaction of [1,3-diMIM-2-COO] (1) with protic acid leads to the formation of protonated isomeric 2- and 4-carboxy-1,3-dimethylimidazolium salts (4a and 5a), which previously was a limitation of this protocol for the preparation of pure ILs. Decarboxylation to imidazolium salts can be achieved for both isomers via thermal decarboxylation heating to 150 °C, and additionally, at room temperature, chemically-induced, DMSO catalyzed decarboxylation is also possible, and occurs only for the [2-(COOH)-1,3-diMIM][NO3] (4a) isomer. These results support the earlier hypothesis that the [4-(COOH)-1,3-diMIM][NO3] salt (5a) is the more thermodynamically stable product of the reaction of the zwitterionic substrate with nitric acid.Experimental
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) and were used as obtained.1,3-dimethylimidazolium-2-carboxylate (1)
This zwitterion was obtained according to the literature procedure.43 Colorless crystals of the product, with melting at the decomposition temperature, were obtained in 85% yield and >99% purity. The NMR data were consistent with literature data.43General procedure for the preparation of 1,3-dimethylimidazoliumhexafluorophosphate[PF6]– (3c), chloride[Cl]– (3d), hydrogen sulfate[HSO4]– (3e), and picrate[Pic]– (3f) salts
All reactions were performed using the same procedure: 0.01 mol of the appropriate acid (HPF6, HCl, H2SO4, picric acid) was dissolved in 10 mL of H2O (ethanol in the case of picric acid) and added dropwise, at room temperature, to a stirred solution of 0.01 mol of [1,3-diMIM-2-COO] (1) in 50% aqueous ethanol (v/v, 20 mL). The mixture was stirred for an additional 24 h in closed flask (to avoid inorganic acid evaporation) and the solvent was evaporated on a rotary evaporator under vacuum. All samples were then dried under high vacuum at room temperature. 1,3-dimethylimidazolium salts (3c–f) were recovered and checked for the presence of starting material using 1H and 13C NMR.1,3-dimethylimidazolium hexafluorophosphate (3c)
White solid, water insoluble, 98% yield, melting point (hot stage apparatus) 67–68 °C, onset for the 5% decomposition T5%dec = 298 °C; δH (360 MHz, DMSO-d6): 3.84 (s, 6H, N-CH3), 7.67 (d, 2H, C(4/5)H), 9.01 (s, 1H, C(2)H); δC (90 MHz, DMSO-d6): 35.55, 123.34, 136.92.1,3-dimethylimidazolium chloride (3d)
White solid, very hygroscopic, 99% yield, melting point (DSC) 147 °C, onset for the 5% decomposition T5%dec = 202 °C; δH (360 MHz, D2O): 3.88 (s, 6H, N-CH3), 7.40 (d, 2H, C(4/5)H), 8.64 (s, 1H, C(2)H); δH (360 MHz, DMSO-d6): 3.86 (s, 6H, N-CH3), 7.75 (d, 2H, C(4/5)H), 9.33 (s, 1H, C(2)H); δC (90 MHz, DMSO-d6): 35.63, 123.37, 137.12.1,3-dimethylimidazolium hydrogen sulfate (3e)
White solid, hygroscopic, 99% yield, melting point (DSC) 98 °C, onset for the 5% decomposition T5%dec = 264 °C; δH (360 MHz, D2O): 3.84 (s, 6H, N-CH3), 7.67 (s, 2H, C(4/5)H), 8.60 (s, 1H, C(2)H); δH (360 MHz, DMSO-d6): 3.82 (s, 6H, N-CH3), 7.64 (d, 2H, C(4/5)H), 9.00 (s, 1H, C(2)H); δC (90 MHz, DMSO-d6): 35.79, 123.56, 136.26.1,3-dimethylimidazolium picrate (3f)
Yellow solid, 95% yield, melting point (DSC) 144 °C, onset for the 5% decomposition T5%dec = 210 °C; δH (360 MHz, D2O): 3.87 (s, 6H, N-CH3), 7.39 (s, 2H, C(4/5)H), 8.62 (s, 1H, C(2)H), 8.95 (s, 2H, picrate); δH (360 MHz, DMSO-d6): 3.84 (s, 6H, N-CH3), 7.67 (d, 2H, C(4/5)H), 8.59 (s, 2H, picrate), 9.03 (s, 1H, C(2)H); δC (90 MHz, DMSO-d6): 35.57, 123.35, 124.05, 125.11, 136.95, 141.75, 160.71.2-carboxy-1,3-dimethylimidazolium nitrate (4a)
To a solution of [1,3-diMIM-2-COO] (1) (0.01 mol) dissolved in 20 mL of a 50% aqueous ethanol (v/v), 5.15 mL of 2 M HNO3 (0.0103 mol) was added dropwise. The reaction mixture was stirred at ∼10 °C for 24 h. The product crystallized during slow solvent evaporation at room temperature and under atmospheric pressure, as colorless crystals. The product melts at the first decomposition temperature (∼200 °C). Onset for the 5% decomposition T5%dec = 194 °C; δH (360 MHz, D2O): 3.99 (s, 6H, N-CH3), 7.39 (s, 2H, C(4/5)).Note: changes in the order of added substrates, as well as, different concentrations of acid did not influence the final product. The evaporation of the solvent under vacuum does not cause isomerization of [2-(COOH)-1,3-diMIM][NO3] (4a) to [4-(COOH)-1,3-diMIM][NO3] (5a).
4-carboxy-1,3-dimethylimidazolium nitrate (5a)
To a solution of [1,3-diMIM-2-COO] (1) (0.01 mol) in 20 mL of a 50% aqueous ethanol (v/v), at 75 °C, was added dropwise 5.15 mL of 2 M HNO3 (0.0103 mol). The reaction mixture was stirred at ∼10 °C for 24 h and the solvent was removed under vacuum without heating. The product crystallized during slow solvent evaporation as white crystals. The product melts at the first decomposition step temperature (∼200 °C). Onset for the 5% decomposition T5%dec = 197 °C; δH (360 MHz, D2O): 3.90 (s, 3H, N1-CH3), 4.04 (s, 3H, N3-CH3), 8.00 (d, 1H, C(5)H), 8.79 (s, 1H, C(2)H); δH (360 MHz, DMSO-d6): 3.86 (s, 3H, N1-CH3), 3.98 (s, 3H, N3-CH3), 8.38 (d, 1H, C(5)H), 9.28 (s, 1H, C(2)H), 14.24 (broad, 1H, COOH); δC (90 MHz, DMSO-d6): 30.66, 35.94, 124.66, 129.10, 140.52, 158.95. Additionally, NOE NMR confirmed the structure of this isomer.1,3-dimethylimidazolium nitrate (3a)
Analyses
CCDC reference numbers 605759 and 605760. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b610421e.
Acknowledgements
This research was supported by the Air Force Office of Scientific Research (grant F49620-03-1-0357).References
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef
.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS
.
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS
.
- S. Zein El Abedin, H. K. Farag, E. M. Moustafa, U. Welz-Biermann and F. Endres, Phys. Chem. Chem. Phys., 2005, 7, 2333 RSC
.
- P. Wang, S. M. Zakeeruddin, I. Exnarb and M. Grätzel, Chem. Commun., 2002, 2972 RSC
.
-
T. Sato, G. Masuda, M. Kotani and S. Iizuka, PCT Int. Appl., 2004019356, 2004 Search PubMed
; Chem. Abstr., 2004, 140, 245004 Search PubMed
.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, S. T. Griffin and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3596 CrossRef CAS
.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis, Jr. and R. D. Rogers, Chem. Commun., 2001, 135 RSC
.
- S. Dai, Y. H. Ju and C. E. Barnes, J. Chem. Soc., Dalton Trans., 1999, 1201 RSC
.
-
P. Wasserscheid, A. Boesmann, A. Jess, L. Datsevitch, C. Schmitz and A. Lauter, PCT Int. Appl., 2003037835, 2003 Search PubMed
; Chem. Abstr., 2003, 138, 370660 Search PubMed
.
- C. Chiappe and D. Pieraccini, ARKIVOC, 2002, 11, 249
.
- L. Kiss, T. Kurtán, S. Antus and H. Brunner, ARKIVOC, 2003, 5, 69
.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459 CrossRef CAS
.
- J. Mo, L. Xu and J. Xiao, J. Am. Chem. Soc., 2005, 127, 751 CrossRef CAS
.
- J. Ding, V. Desikan, X. Han, T. L. Xiao, R. Ding, W. S. Jenks and D. W. Armstrong, Org. Lett., 2005, 7, 335 CrossRef CAS
.
- C. M. Gordon, Appl. Catal., A, 2001, 222, 101 CrossRef CAS
.
- H. Olivier-Bourbigou and L. Magna, J. Mol. Catal. A: Chem., 2002, 182–183, 419 CrossRef CAS
.
- G. Drake, T. Hawkins, A. Brand, L. Hall, M. Mckay, A. Vij and I. Ismail, Propellants, Explos., Pyrotech., 2003, 28, 174 CrossRef CAS
.
- A. R. Katritzky, S. Singh, K. Kirichenko, J. D. Holbrey, M. Smiglak, W. M. Reichert and R. D. Rogers, Chem. Commun., 2005, 868 RSC
.
- A. R. Katritzky, H. Yang, D. Zhang, K. Kirichenko, M. Smiglak, J. D. Holbrey, W. M. Reichert and R. D. Rogers, New J. Chem., 2006, 30, 349 RSC
.
- H. Xue, B. Twamley and J. M. Shreeve, J. Mater. Chem., 2005, 15, 3459 RSC
.
- C. Ye, J. Xiao, B. Twamley and J. M. Shreeve, Chem. Commun., 2005, 2750 RSC
.
- H. Xue, Y. Gao, B. Twamley and J. M. Shreeve, Inorg. Chem., 2005, 44, 5068 CrossRef CAS
.
- H. Xue and J. M. Shreeve, Adv. Mater., 2005, 17, 2142 CrossRef CAS
.
- Q. Lu, H. Wang, C. Ye, W. Liu and Q. Xue, Tribol. Int., 2004, 37, 547 CrossRef CAS
.
- Z. Mu, W. Liu, S. Zhang and F. Zhouy, Chem. Lett., 2004, 33, 524 CrossRef CAS
.
- C. Ye, W. Liu, Y. Chen and L. Yu, Chem. Commun., 2001, 2244 RSC
.
- M. T. Carter, C. L. Hussey, S. K. D. Strubinger and R. A. Osteryoung, Inorg. Chem., 1991, 30, 1149 CrossRef CAS
.
- J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223 CrossRef
.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC
.
- K. N. Marsh, J. A. Boxall and R. Lichtenthaler, Fluid Phase Equilib., 2004, 219, 93 CrossRef CAS
.
- S. A. Forsyth, J. M. Pringle and D. R. MacFarlane, Aust. J. Chem., 2004, 57, 113 CrossRef
.
- J. D. Holbrey, W. M. Reichert, R. P. Swatloski, G. A. Broker, W. R. Pitner, K. R. Seddon and R. D. Rogers, Green Chem., 2002, 4, 407 RSC
.
-
P. Wasserscheid, R. van Hal, A. Bosmann, J. Esser and A. Jess, New ionic liquids based on alkylsulfate and alkyl oligoether sulfate anions: synthesis and applications, in Ionic Liquids as Green Solvents, ed. R. D. Rogers and K. R. Seddon, American Chemical Society Symposium Series 856, Washington, DC, USA, 2003, p. 57 Search PubMed
.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706 CrossRef CAS
.
-
U. Romano, F. Rivetti and N. Di Muzio, U.S. Pat., 4318862, 1979 Search PubMed
; U. Romano, F. Rivetti and N. Di Muzio, Chem. Abstr., 1981, 95, 80141w Search PubMed
.
-
F. Rivetti, U. Romano and D. Delledonne, Dimethylcarbonate and its production technology, in Green Chemistry: Designing Chemistry for the Environment, ed. P. Anastas and T. C. Williamson, American Chemical Society Symposium Series 626, Washington, DC, USA, 1996, p. 70 Search PubMed
.
-
K. Nisihra, K. Mizutare and S. Tanaka, UBE Industries, Japan, U.S. Pat., 5162563, 1992 Search PubMed
.
-
F. Rivetti, Dimethylcarbonate: an answer to the need for safe chemicals, in Green Chemistry: Challenging Perspectives, ed. P. Tundo and P. Anastas, Oxford University Press, Oxford, UK, 2000, p. 201 Search PubMed
.
-
Registry of Toxic Effects of Chemical Substances, ed. D. V. Sweet, U.S. GPO, Washington, DC, USA, 1986, vol. 2, p. 186 Search PubMed
.
- P. Tundo, L. Rossi and A. Loris, J. Org. Chem., 2005, 70, 2219 CrossRef CAS
.
- F. Trotta, P. Tundo and G. Moraglio, J. Org. Chem., 1987, 52, 1300 CrossRef
.
- J. D. Holbrey, W. M. Reichert, I. Tkatchenko, E. Bouajila, O. Walter, I. Tommasi and R. D. Rogers, Chem. Commun., 2003, 28 RSC
.
-
M. Aresta, I. Tkatchenko and I. Tommasi, Unexpected synthesis of 1,3-dialkylimidazolium-2-carboxylate, in Ionic Liquids as Green Solvents, R. D. Rogers and K. R. Seddon, American Chemical Society Symposium Series 856, Washington, DC, USA, 2002, 93 Search PubMed
.
-
S. Mori, K. Ida and M. Ue, Mitsubishi Chemical Co, Ltd, U.S. Pat., 4892944, 1990 Search PubMed
.
-
M. Ue, M. Takeda, T. Takahashi and M. Takehara, U.S. Pat., 5856513, 1997 Search PubMed
.
-
J. Fisher, W. Siegel, V. Bomm, M. Fisher and K. Mundinger, U.S. Pat., 6175019, 1999 Search PubMed
.
- J. Louie, J. E. Gibby, M. V. Farnworth and T. N. Tekavec, J. Am. Chem. Soc., 2002, 124, 15188 CrossRef CAS
.
- T. N. Tekavec, A. M. Arif and J. Louie, Tetrahedron, 2004, 60, 7431 CrossRef CAS
.
- I. Tommasi and F. Sorrentino, Tetrahedron Lett., 2005, 46, 2141 CrossRef CAS
.
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263 CrossRef CAS
.
- D. W. Karkhanis and L. Field, Phosphorus Sulfur, 1985, 22, 49 Search PubMed
.
- H. L. Ngo, K. LeCompte, L. Hargens and A. B. McEwen, Thermochim. Acta, 2000, 357–358, 97 CrossRef CAS
.
- A. P. Krapcho, G. A. Glynn and B. J. Grenon, Tetrahedron Lett., 1967, 8, 215 CrossRef
.
- A. P. Krapcho, J. F. Weimaster, J. M. Eldridge, E. G. E. Jahngen, A. J. Lovey and W. P. Stephens, J. Org. Chem., 1978, 43, 138 CrossRef CAS
.
- A. M. Bernard, G. Cerioni and P. P. Piras, Tetrahedron, 1990, 46, 3929 CrossRef CAS
.
- P. J. Gilligan and P. J. Krenitsky, Tetrahedron Lett., 1994, 35, 3441 CrossRef CAS
.
- A. P. Krapcho, Synthesis, 1982, 10, 805 CrossRef
.
- A. P. Krapcho, Synthesis, 1982, 11, 893 CrossRef
.
- M. W. Schmidt, K. K. Balsrige, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, M. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis and J. A. Montgomery, J. Comput. Chem., 1993, 14, 1347 CrossRef CAS
.
-
G. M. Sheldrick, SHELXTL, version 5.05, Siemens Analytical X-Ray Instruments Inc., Madison, WI, USA, 1996 Search PubMed
.
-
G. M. Sheldrick, Program for Semiempirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996 Search PubMed
.
Footnote |
| † Present address: The QUILL Research Centre, Queen's University Belfast, Northern Ireland, UK BT9 5AG. |
| This journal is © The Royal Society of Chemistry 2007 |
