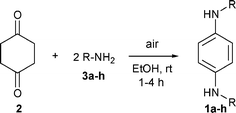
One-step synthesis of N,N′-dialkyl-p-phenylenediamines†
Luke T.
Higham
a,
Katsuya
Konno
b,
Janet L.
Scott
a,
Christopher R.
Strauss
*c and
Tatsuaki
Yamaguchi
b
aARC Special Research Centre for Green Chemistry, Monash University, Clayton, Victoria, 3800, Australia
bDepartment of Life and Environmental Sciences, Faculty of Engineering, Chiba Institute of Technology, Tsudanuma, Narashino, Chiba, 275-0016, Japan
cCSIRO Molecular and Health Technologies, Private Bag 10, Clayton South, Victoria, 3169, Australia. E-mail: chris.strauss@csiro.au; Fax: +61-3-9545-2446; Tel: +61-3-9545-2183
First published on 30th October 2006
Abstract
Condensation of 1,4-cyclohexanedione with primary alkylamines in the presence of air afforded N,N′-dialkyl-p-phenylenediamines. Reactions occurred at room temperature and were complete within a few hours, in high atom economy and with water as the major by-product.
Introduction
In recent investigations into the generation of aromatic species from masked precursors,1 we discovered a green, multi-component cascade reaction for the preparation of 2- and 4-arylmethyl N-substituted and N,N-disubstituted anilines.2 Several known product anilines have pre-existing industrial applications and some of our new compounds have shown interesting biological activities.3 Consequently, we have extended the scope of our studies into aromatisation to include substituted phenylenediamines such as N,N′-dialkyl-p-phenylenediamines (1).p-Phenylenediamines (1) are useful stabilisers for polymers, particularly as antioxidants and antiozonants for rubbers.4 They also serve as synthetic starting materials for flavins ,5 bis-Tröger's bases,6 rod-like oligomers, rotaxanes ,7 redox-active macrocycles8–10 and ladder polymers.11 Published routes to 1 often involve multiple steps,11–17 including protection and deprotection, that introduce two additional manipulations and lower the atom economy.14–16 They do not always show high chemoselectivity.18–21 Also they may employ potentially hazardous alkylating agents14,18 or polluting solvents15,17 and they may generate toxic by-products.11,17 Moreover, the majority of the syntheses start from p-phenylenediamine, which is obtained from benzene in a multi-step process that has low atom economy.22 Therefore, simpler and greener routes are desirable.
Fifty years ago, Leonard and Sauers reported the two-step synthesis of two N,N,N′,N′-tetraalkyl-p-phenylenediamines by condensation of 1,4-cyclohexanedione (2) with secondary amines.23 A two-step pathway was proposed, involving formation of a bis-enamine intermediate followed by aromatisation through aerial oxidation. Disadvantages included low to moderate yields (13–40%) and the use of benzene, which is now considered as an unacceptable solvent due to its carcinogenicity.24,25 Nonetheless, the potential for use of air for oxidative aromatisation at room temperature, without the need for added catalyst, was appealing. Dione 2 can be considered as a green starting material as it is prepared from succinic acid in three steps in good yield.26Succinic acid is a renewable resource and may find broad use as a building block for sustainable chemistry.27 Adaptation of Leonard and Sauers' strategy has been attempted elsewhere with varying success.28–30 This report, however, discloses the first one-step reactions of dione 2 with primary alkylamines (3), in the presence of air for direct, convenient and green preparations of N,N′-dialkyl-p-phenylenediamines (1).
Results and discussion
p-Phenylenediamines 1a–g were produced by condensation of dione 2 with primary alkylamines 3a–g in air at room temperature (Table 1). Reactions were conducted in absolute EtOH instead of benzene. Ethanol is greener than many other organic solvents. It has relatively low toxicity and can be produced industrially by fermentation. It is readily recyclable and is both a renewable and a biodegradable resource.27 Temperatures between ambient and 50 °C were suitable and air was introduced preferably by bubbling through the solution. Reactions proceeded in the absence of light but not under anaerobic conditions, presumably by a pathway involving aerial oxidation of putative intermediate diimines, 4a–g (please see Scheme 1 and Table 1 for a–g). Usually a stoichiometric ratio of dione 2 to amine 3 (1 ∶ 2) was employed. Reactions, monitored by GC, GC-MS and 1H NMR spectral analysis, typically were completed within 4 h, the conversions generally being greater than 95%. The by-products, which usually comprised 10% or less of the product mixture, included the tentatively identified iminones, 5a–g, and aminophenols, 6a–g. In a typical example, the reaction between dione 2 and n-propylamine (3b) to form N,N′-dipropyl-p-phenylenediamine (1b) is shown in Fig. 1.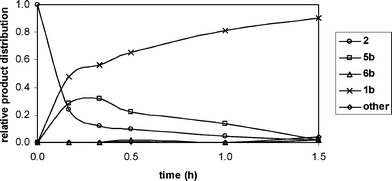 | ||
| Fig. 1 Progress of reaction between dione 2 and n-propylamine3b (1 ∶ 2 ratio) as monitored by GC-MS. | ||
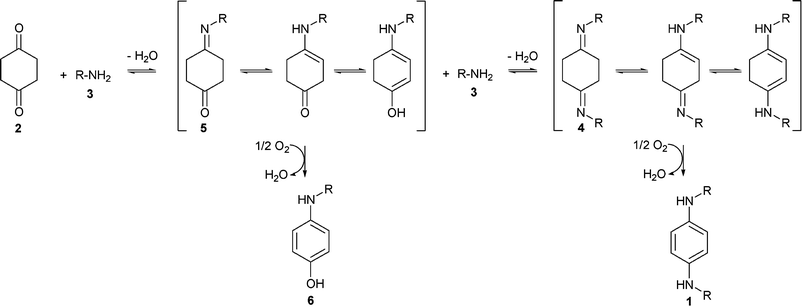 | ||
| Scheme 1 Proposed pathway for the synthesis of p-phenylenediamines (1) and by-products. | ||
|
|
||||
|---|---|---|---|---|
| Entry | R | Product | Yield (%)a | Lit. yield (%)a |
| a Isolated. b Ratio of 2 to 3 was 1 ∶ 4. c Dihydrochloride salt. d Yield starting from p-phenylenediamine. e Conversions are given (ref. 14 and 19). f Reaction performed at 50 °C. g Ratio of 2 to 3e was 1 ∶ 2.5. h Specific method for N,N′-diarylmethyl-p-phenylenediamines. i Reaction performed at reflux. j Specific method for N,N′-diaryl-p-phenylenediamines. | ||||
| a b | –CH2CH3 | 1a c | 67 | 63d,11 |
| b | –(CH2)2CH3 | 1b c | 53 | —e |
| c | –(CH2)3CH3 | 1c c | 47 | 43;d,16 59;d,20 68d,15 |
| d | –(CH2)5CH3 | 1d c | 47 | — |
| e f , g | –(CH2)7CH3 | 1e c | 62 | 7017 |
| f | –CH2Ph | 1f c | 56 | 100d,h,10 |
| g b | –(CH2)2Ph | 1g | 57 | 58d,15 |
| h i | –Ph | 1h | 43 | 92d,j,31 |
The proposed reaction pathway (Scheme 1) involves condensation of dione 2 with alkylamine 3 to give iminone 5, with the liberation of one molecule of water. Iminone 5 can then undergo aerial oxidation to aminophenol 6 or condensation with another molecule of alkylamine 3 to give diimine 4, which is then oxidised in the presence of air to give p-phenylenediamine 1. Hence the relative rates with which iminone 5 can be dehydrogenated or condensed with a second molecule of the amine 3, are critical to the distribution of final products. That diimines 4a–g were not detected in reactions between 2 and primary alkylamines 3a–g, suggests that oxidations of 4 were facile. In contrast, evidence for the formation of iminones 5a–g and aminophenols 6a–g, albeit as minor products, implies that oxidations of 5 to 6 may have been considerably slower, thereby accounting for the convergence of the process.
The p-phenylenediamines 1a–f, prepared on the 100 mg scale, were usually isolated as their dihydrochloride salts in moderate to good yields that were comparable with those from waste-generating literature methods that had required more steps and less environmentally benign reagents (Table 1). Although produced oxidatively, most of the diamines 1a–h prepared in this work were sensitive to oxidation.9,13 This was not surprising in view of the applications for diamines 1a–h as antioxidants and it implied the need for careful work-up to minimise discrepancies between conversions and isolated yields. When necessary, to limit the extent of decomposition, products were isolated as their dihydrochloride salts from organic solutions of the diamines 1a–h, which mainly were low melting point solids. It was possible, however, that some loss occurred during work-up and product isolation. The salts were stable and could be stored for months in sealed vials, including in the presence of air.
This work and that of others,29,30 suggested that the condensation of amines with dione 2 can afford alternative reaction products through relatively subtle changes in conditions. For example, Haga et al. found that under acid catalysis, dione 2 with N-alkylarylamines or diarylamines in refluxing toluene gave N-alkyl-N-arylanilines or N,N-diarylanilines, respectively.29 They proposed that an enaminone intermediate underwent dehydrative aromatisation. Up to this point in the present work, acid catalysis had not been employed. When the conditions of Haga et al. were used herein, but with a primary amine, n-octylamine (3e), instead of an N-alkylarylamine or a diarylamine, no N-n-octylaniline was detected. Instead, the present oxidative diamination occurred, with N,N′-dioctyl-p-phenylenediamine (1e) obtained as the major product in 59% yield by GC after 5 h. In the absence of acid, a 62% yield of 1e had been obtained after 4 h (Table 1). Thus, it was concluded that the process could be conducted in the presence of acid, without any significant effect on the yield.
Recently, Yudina and Bergman obtained 1,4-bis(p-methoxyphenyl)phenylenediamine in 13% yield by refluxing a 1 ∶ 2 mixture of dione 2 and p-methoxyaniline in benzene for 18 h.28 We also have produced N,N′-diaryl-p-phenylenediamines, but by adaptation of the subject process. A mixture of 2 and aniline (3h) in refluxing absolute EtOH for 4 h afforded N,N′-diphenyl-p-phenylenediamine (1h) in 43% yield. The result appeared to be superior to the above literature protocol with regard to reaction time , conditions and yield. This suggests that the approach could supplement a recent high yielding method starting from p-phenylenediamine.31
Conclusions
A new method for preparing N,N′-dialkyl-p-phenylenediamines 1 from the reaction of dione 2 and primary alkylamines 3 is reported. Green aspects included the selection of starting materials, high atom economy, the use of EtOH as a solvent and air at room temperature as an in situ oxidant in the absence of added catalyst. The in situ multi-step process was performed in one pot, with water as the major by-product and typically, was complete within a few hours.Experimental
All solvents, reagents and starting materials were purchased and used without further purification. 1H and 13C NMR spectra were recorded on a Bruker Advance DRX 400 spectrometer at 400 and 100 MHz, respectively. Samples were run in CDCl3 or CD3OD and referenced to TMS. J values are given in Hz. IR spectra were obtained on neat samples using a Bruker Equinox 55 fitted with a Specac Diamond ATR and MCT detector. Melting points were recorded on a Buchi Melting Point B-545. GC analyses were carried out using a Hewlett Packard Series 5890 GC equipped with a SGE BPX5 fused silica column (25 m × 0.32 mm id, 0.5 µm film thickness). The injector and detector temperatures were 220 and 250 °C, respectively. The oven temperature was programmed as follows: initially 70 °C, increasing to 280 °C at 10 °C min–1 and holding for 5 min. Helium was the carrier gas (2 mL min–1). GC-MS spectra were obtained with a ThermoQuest TRACE DSQ GC-MS using EI with an ionisation energy of 70 eV. The column was a SGE BPX5 (10 m × 0.1 mm id, 0.1 µm film thickness), with a temperature program of 40 °C for 2 min, then heating at 40 °C min–1 to 300 °C, at which the temperature was held for 2 min. The injector temperature was 250 °C and the transfer line was set to 250 °C. High-purity helium was used as the carrier gas with a flow rate of 0.8 mL min–1.General procedure for the synthesis of N,N′-dialkyl-p-phenylenediamines 1a–f
A mixture of 1,4-cyclohexanedione (2, 0.28 g, 2.5 mmol) and the chosen amine 3 (5.0 mmol) in EtOH (99.7%, 25 mL) were stirred in a 50 mL round bottom flask at rt with air bubbling through the solution. After reaction completion (1–4 h), as determined by GC, the EtOH was removed under reduced pressure. The residue was dissolved in a minimal amount of acetone (3–5 mL) and conc. HCl (32%, 10–20 drops) was added to form the dihydrochloride salt. The salt was characterised by mp, 1H and 13C NMR spectroscopies, and IR spectroscopy. The free base was characterised by MS and 1H NMR spectroscopy.Synthesis of N,N′-diphenethyl-p-phenylenediamine (1g)
A mixture of 1,4-cyclohexanedione (2, 0.28 g, 2.5 mmol) and 2-phenylethanamine (3g, 1.21 g, 10.0 mmol) in EtOH (99.7%, 10 mL) were stirred in a 25 mL round bottom flask at rt with air bubbling through the solution for 3 h. After cooling, a solid was collected by filtration and recrystallised from EtOH to give 1g (0.450 g, 57%) as a colourless solid; mp 127.5–128 °C (lit.,15 127–128.5 °C); δH(CDCl3, TMS) 7.32–7.27 (4 H, m), 7.23–7.19 (6 H, m), 6.56 (4 H, br s), 3.49 (2 H, br s), 3.33 (4 H, br m), 2.88 (4 H, t, J 7.0); δC(CDCl3, TMS) 148.1, 139.5, 128.8, 128.6, 126.3, 115.2, 46.5, 35.6.Synthesis of N,N′-diphenyl-p-phenylenediamine (1h)
A mixture of 1,4-cyclohexanedione (2, 0.28 g, 2.5 mmol) and aniline (3h, 0.47 g, 5.0 mmol) in EtOH (99.7%, 25 mL) were refluxed in a 50 mL round bottom flask with air bubbling through the solution for 4 h. The solution was cooled to rt and the precipitate was collected by filtration and recrystallised from toluene to give 1h (0.28 g, 43%) as a brown solid; mp 146–148 °C (lit.,34 148–149 °C); m/z 260 (M+, 100%), 183 (15), 167 (11).EI-MS for compounds tentatively identified as iminones (5) and aminophenols (6)
Acknowledgements
We thank the Australian Research Council (ARC) for funding this research through the ARC Special Research Centre for Green Chemistry (CGC) and through an Australian Postgraduate Award (to LTH). The CGC is thanked for hosting post-graduate exchange studies (for KK).References
- L. T. Higham, U. P. Kreher, C. L. Raston, J. L. Scott and C. R. Strauss, Org. Lett., 2004, 6, 3261–3264 CrossRef CAS.
- A. E. Rosamilia, J. L. Scott and C. R. Strauss, Org. Lett., 2005, 7, 1525–1528 CrossRef CAS.
- WO Pat., 012 683, 2006: Chem. Abstr., 2006, 144, 212532 Search PubMed.
- M. Braden and A. N. Gent, J. Appl. Polym. Sci., 1962, 4, 449–455 CrossRef; J. C. Andries, C. K. Rhee, R. W. Smith, D. B. Ross and H. E. Diem, Rubber Chem. Technol., 1979, 52, 823–837 CAS; R. P. Lattimer, R. W. Layer, E. R. Hooser and C. K. Rhee, Rubber Chem. Technol., 1991, 64, 780–789 CAS; G. J. Lake and P. G. Mente, Polym. Degrad. Stab., 1995, 49, 193–203 CrossRef CAS.
- Y. Yano, M. Nakazato and R. E. Vasquez, J. Chem. Soc., Chem. Commun., 1985, 226–227 RSC; F. Yoneda, M. Koga and Y. Yano, J. Chem. Soc., Perkin Trans. 1, 1988, 7, 1813–1817 RSC; H. Ohshiro, K. Mitsui, N. Ando, Y. Ohsawa, W. Koinuma, H. Takahashi, S.-I. Kondo, T. Nabeshima and Y. Yano, J. Am. Chem. Soc., 2001, 123, 2478–2486 CrossRef CAS.
- T. Mas, C. Pardo, F. Salort, J. Elguero and M. R. Torres, Eur. J. Org. Chem., 2004, 1097–1104 CrossRef CAS.
- E. Córdova, R. A. Bissell and A. E. Kaifer, J. Org. Chem., 1995, 60, 1033–1038 CrossRef CAS.
- US Pat., 6 262 258, 2001: Chem. Abstr., 2001, 135, 92662 Search PubMed; X. Liu, A. H. Eisenberg, C. L. Stern and C. A. Mirkin, Inorg. Chem., 2001, 40, 2940–2941 Search PubMed; J. W. Sibert, G. R. Hundt, A. L. Sargent and V. Lynch, Tetrahedron, 2005, 61, 12350–12357 CrossRef CAS.
- H. Takemura, K. Takehara and M. Ata, Eur. J. Org. Chem., 2004, 4936–4941 CrossRef CAS.
- F. C. Krebs and M. Jørgensen, J. Org. Chem., 2002, 67, 7511–7518 CrossRef CAS.
- K. Oyaizu, F. Mitsuhashi and E. Tsuchida, Macromol. Chem. Phys., 2002, 203, 1328–1336 CrossRef CAS.
- P. Vouros and K. Biemann, Org. Mass Spectrom., 1969, 2, 375–386 CrossRef CAS.
- L. Michaelis, M. P. Schubert and S. Granick, J. Am. Chem. Soc., 1939, 61, 1981–1992 CrossRef CAS.
- N. E. Agafonov, A. V. Dudin, A. A. Preobrazhenskii and V. M. Zhulin, Russ. Chem. Bull., 2003, 52, 273–275 CrossRef CAS.
- (a) R. Adams and K. A. Schowalter, J. Am. Chem. Soc., 1952, 74, 2597–2602 CrossRef CAS; (b) for the synthesis of N,N′-di(benzenesulfonyl)-p-phenylenediamine, see: N. Nagel, H. Bock and P. Eller, Acta Crystallogr., Sect. B: Struct. Sci., 2000, 56, 234–244 Search PubMed.
- J. V. Capinjola, J. Am. Chem. Soc., 1951, 73, 1849 CrossRef CAS.
- J. Sinnreich, Synthesis, 1979, 578–580.
- DE Pat., 2 055 208, 1972: Chem. Abstr., 1972, 77, 61522 Search PubMed.
- M. P. Reynolds and H. Greenfield, in Catalysis of Organic Reactions, ed. R. E. Malz, Jr, Marcel Dekker, New York, 1996, ch. 30, pp. 343–351 Search PubMed.
- J. Horyna and O. Cerny, Collect. Czech. Chem. Commun., 1956, 21, 906–911 CAS.
- CS Pat., 87 381,1957: Chem. Abstr., 1959, 53, 6827 Search PubMed.
- A. I. Vogel, A. R. Tatchell, B. S. Furnis, A. J. Hannaford and P. W. G. Smith, Vogel's Textbook of Practical Organic Chemistry, Longman Scientific and Technical, London, 5th edn, 1989 Search PubMed.
- N. J. Leonard and R. R. Sauers, J. Org. Chem., 1956, 21, 1187–1188 CrossRef CAS.
- D. L. Bayliss, C. Chen, A. Jarabek, B. Sonawane and L. Valcovic, Carcinogenic Effects of Benzene: An Update, National Center for Environmental Assessment, Washington Office, Washington, DC, 1998, http://www.epa.gov/ncea/pdfs/benzenef.pdf Search PubMed.
- P. T. Anastas and J. W. Warner, Green Chemistry – Theory and Practice, Oxford University Press, Oxford, New York, 1998 Search PubMed.
- A. T. Nielsen and W. R. Carpenter, Org. Synth., 1965, 45, 25–28 CAS.
- H. Danner and R. Braun, Chem. Soc. Rev., 1999, 28, 395–405 RSC.
- L. N. Yudina and J. Bergman, Tetrahedron, 2003, 59, 1265–1275 CrossRef CAS.
- K. Haga, M. Oohashi and R. Kaneko, Bull. Chem. Soc. Jpn., 1984, 57, 1586–1590 CrossRef CAS; K. Haga, K. Iwaya and R. Kaneko, Bull. Chem. Soc. Jpn., 1986, 59, 803–807 CAS.
- WO Pat., 092 832, 2005: Chem. Abstr., 2005, 143, 367079 Search PubMed.
- S. Kuhl, Y. Fort and R. Schneider, J. Organomet. Chem., 2005, 690, 6169–6177 CrossRef CAS.
- M. Sekiya, M. Tomie, K. Ito, J. Suzuki, K. Suzuki and Y. Terao, Chem. Pharm. Bull., 1973, 21, 1625–1631 CAS.
- GB Pat., 787 659, 1957: Chem. Abstr., 1958, 52, 56190 Search PubMed.
- D. D. Perrin, W. L. F. Armarego and D. R. Perrin, Purification of Laboratory Chemicals, Pergamon, New York, 2nd edn, 1980 Search PubMed.
- N. Takahashi, T. Honda and T. Ohba, Bioorg. Med. Chem., 2006, 14, 409–417 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR spectra of compounds 1a–f·2HCl and 1g. See DOI: 10.1039/b607632g |
| This journal is © The Royal Society of Chemistry 2007 |