Synthesis of polyaniline derivatives via biocatalysis†
Seong-Cheol
Kim
a,
Pilho
Huh
a,
Jayant
Kumar
*a,
Bongsoo
Kim
b,
Jang-Oo
Lee
b,
Ferdinando F.
Bruno
c and
Lynne A.
Samuelson
*c
aThe Center for Advanced Materials, Polymer Science Program, Departments of Chemistry and Physics, University of Massachusetts, Lowell, MA 01854, USA. E-mail: Jayant_Kumar@uml.edu; Fax: +1-978-458-9571; Tel: +1-978-934-3687
bDepartment of Polymer Sci. & Eng., Pusan National University, Pusan, 609-735, Korea. Fax: +82-51-513-7720; Tel: +82-51-510-3637
cU. S. Army RDECOM, Natick Soldier Center, Natick, Massachusetts 01760. E-mail: Lynne_Samuelson@natick.army.mil; Fax: +1-978-458-9571; Tel: +1-978-934-3972
First published on 9th October 2006
Abstract
Three structurally different aniline monomers, which can not be polymerized by chemical methods, have been polymerized with horseradish peroxidase. Enzymatic synthesis of linear polyaniline requires template molecules to minimize branching in the polyaniline backbone. Monomers having methoxy and methyl blocking groups at the ortho or meta position could induce the conducting form of para-linked polyaniline without the use of an anionic template, such as SPS. A new mild peroxide, peroxyacetic acid, was identified and used to oxidize horseradish peroxidase in water.
Introduction
In recent years there have been extensive studies in the use of conducting polyanilines because of their wide range of electrical, electrochemical, and optical properties as well as their good stability.1–5 Polyaniline is commonly synthesized by oxidizing aniline monomers using electrochemical or chemical methods.6–10 This polymer can be doped either by a protonic acid or charge-transfer agents. In addition, the electronic and optical properties can be controlled reversibly by varying the doping level.11,12Synthesis of polyaniline via biocatalysis with an anionic template has been shown to enhance the water solubility and the stability of the final polyaniline-template complex, and is a more environmentally benign route.13–17 Using enzymes as a catalyst for the synthesis of polyaniline could also improve the toxic chemical process conditions to a mild and environmentally benign route.
A major drawback of enzymatic polymerization without a template was the precipitation of highly branched low MW polyaniline. Non-conducting forms of branched polymers (oligomers) were formed due to the various resonance structure of aniline radical.18,19
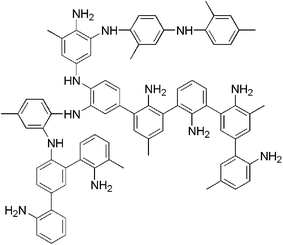 | ||
| Scheme 1 | ||
Several types of anionic templates have been studied to synthesize the para-linked conducting form of polyaniline, such as polyacrylic acid, sulfonated polystyrene, polyvinyl phosphate, lignin sulfonate, RNA and DNA.13–17 Here the anionic polyelectrolytes serve three critical functions. First, they preferentially align the aniline monomers and promote a more ordered para-linked reaction. Furthermore, they provide counterions for doping of the synthesized polyaniline. Finally, they maintain water solubility, consequently, increasing processability of the final polymer.
Although template assisted polymerization of polyaniline has many advantages, there are still limitations which need to be addressed. Generally template molecules are not suitable dopants for polyanilines, and thus the conductivities of corresponding anionic template/polyaniline complexes are relatively low compared with camphorsulfonic acid-doped polymers. Even though polyaniline shows increased water-solubility due to the anionic template, the wettability of the polyaniline/template solution is poor because most of the substrate is hydrophobic. Once the polyaniline complex is coated and dried on the substrate, it does not dissolve again, which is advantageous for permanent processing but limits the recyclability of the materials. In addition, the removal of the anionic template by ion exchange is quite difficult and time-consuming. Therefore, the complexed polyaniline is hard to modify or to functionalize for certain applications. In this article, we present the enzymatic synthesis of polyaniline derivatives without using templates. Furthermore, we will demonstrate that peroxyacetic acid successfully activates horseradish peroxidase in aqueous solution.
Results and discussion
The aniline radical has several intermediate resonance structures. These intermediates generate parasitic branches, disrupting, consequently, polyaniline conjugation. The monomer is expected to be primarily positively charged at pHs lower than 4.5, since aniline has a pKa of 4.63.15 The solubility of monomers and oligomers are not high in aqueous solution. To increase the solubility of monomer, at mild acidic conditions, various polar organic solvents have been mixed up to 40 vol%. Methyl and methoxy groups reduce the oxidation potential and can increase the solubility of the corresponding polyaniline in common organic solvents.202,5-Dimethoxyaniline has two methoxy groups in the ortho and meta positions. 2-Methyl-5-methoxyaniline and 2-methoxy-5-methylaniline may have a higher chance of ortho linkage than 2,5-dimethoxyaniline due to reduced steric hinderance generating a non-conducting form of the polyaniline, as shown before.Fig. 1 shows the UV-Vis spectra of the substituted polyanilines synthesized in 10 vol% ethanol. As no template was used, several minutes are necessary to generate a polaron transition peak at 800 nm due to a relatively slow reaction. No significant change of intensity of the polaron peak was observed in two hours for 2,5-dimethoxyaniline and 2-methyl-5-methoxyaniline and 2 days for 2-methoxy-5-methylaniline. As expected, 2,5-dimethoxyaniline shows the strongest intensity of polaron transition and 2-methyl-5-methoxyaniline shows a very weak polaron peak after two hours. However, although the polaron transition at 900 nm appeared in two days, 2-methoxy-5-methylaniline does not generate a polaron after a similar time. Poly(2-methoxy-5-methylaniline) also generates an excitonic transition peak at 520 nm, which is assigned to branched low molecular weight polyaniline. This difference in the intensity of each polaron peak is expected, and attributed to the pendent groups of aniline monomers. The 2,5-dimethoxyaniline structure provides better solubility and steric hindrance to minimize ortho coupling. Thus, the para directed reaction can proceed, as indicated by the strong absorption near 800 nm. The other two monomers, 2-methyl-5-methoxyaniline and 2-methoxy-5-methylaniline, however, are less soluble and more prone to ortho coupling. Therefore, this figure proves that the limitation of enzyme-catalyzed polyaniline synthesis can be overcome by a careful design of monomers.
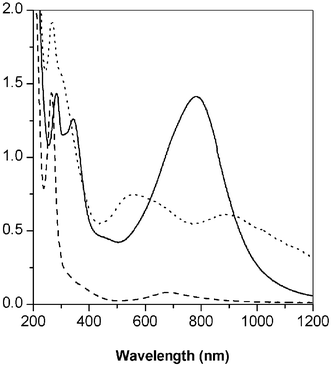 | ||
Fig. 1 UV-Vis spectra of polyanilines in a mixed solvent system; pH 3; reaction time : 2,5-dimethoxyaniline and 2-methyl-5-methoxyaniline (2 hours), 2-methoxy-5-methylaniline (2 days); dopant: HCl; solid line (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ): poly(2,5-dimethoxyaniline), broken line ( ): poly(2,5-dimethoxyaniline), broken line (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ): poly(2-methyl-5-methoxyaniline), dashed line (⋯): poly(2-methoxy-5-methylaniline). ): poly(2-methyl-5-methoxyaniline), dashed line (⋯): poly(2-methoxy-5-methylaniline). | ||
Scheme 2 shows the possible emeraldine salt forms of the polymers.11,12 The conductivity of substituted polyanilines was measured by four point probe method in two days of a reaction. The measured conductivity of the substituted polyanilines was 0.3 S cm–1 for poly(2,5-dimethoxyaniline) after additional doping with HCl vapor. An order of decrease in conductivity of poly(2,5-dimethoxyaniline) compared to unsubstituted polyaniline could be due to the decreased conjugation caused by two pendent methoxy groups. The intensity of the polaron transition and, consequently, the conductivity, can be modulated by changing the dopant counter ions. Camphorsulfonic acid is known as a stronger dopant than hydrochloric acid.21 Therefore, the higher intensity of a polaron transition was observed when poly(2-methyl-5-methoxyaniline) was doped with camphorsulfonic acid (see ESI† ).
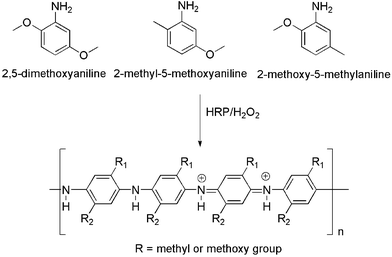 | ||
| Scheme 2 | ||
To find the best solvent composition for the enzymatic polymerization, the polymerization reaction was performed at different vol% of ethanol. As the content of organic solvent increases, the solubility of monomers and oligomers increases. However, the activity of horseradish peroxidase decreases because the suitable molecular conformation of an enzyme may change with increasing organic solvent composition. No measurable intensity of a polaron peak at 750 nm was detected in several days when more than 50 vol% of ethanol was added in water. The optimum conditions for polymerization was found at a concentration of 15 vol% ethanol solution. Other polar organic solvents such as DMF, DMSO, 1,4-dioxane and methanol, have similar trends. The enzyme loses its activity gradually with increasing amounts of organic solvent, while the solubility of aniline derivatives increases with the ratio of organic solvent.22 These two opposing effects give rise to optimum conditions at 15 vol% of organic co-solvent.
In situ polymerizations of these anilines were monitored by a UV-vis spectrometer. Real-time monitoring shows that the monomers are slightly soluble in the ethanol–water co-solvent. However, the solubility of the oligomers generated during the reaction increases with reaction time due to the positive charges generated in oligoaniline intermediates by proton doping. At the beginning of the polymerization the π-π* transition peak at 330 nm increases due to the increased solubility of undoped oligo(2,5-dimethoxyaniline).14,15 As the polymerization proceeds, a gradual increase of the polaron peaks of poly(2,5-dimethoxyaniline) is observed at 750 nm with a decrease of the π-π* transition peak at 330 nm. When the acid-doped polyaniline is generated, its solubility in water gradually increases and it becomes fully soluble after polymerization. It was found that the peak maximum of the polaron transition in poly(2,5-dimethoxyaniline) shifted from 750 to 805 nm in two days, which suggests that the reaction might have continued gradually as time went on.
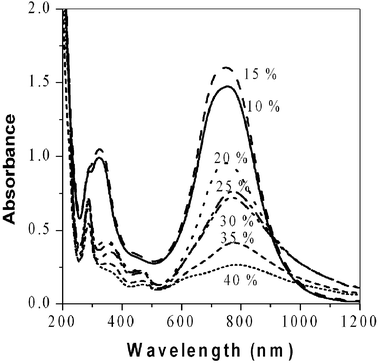 | ||
| Fig. 2 UV-Vis spectra of poly(2,5-dimethoxyaniline) as a function of %-ethanol. Polaron peak shows the highest intensity at 15 vol% of organic solvent in two hours. | ||
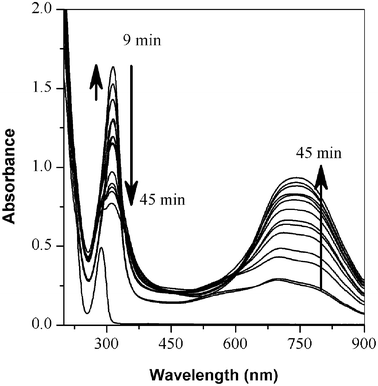 | ||
| Fig. 3 UV-Vis spectral change of poly(2,5-dimethoxyaniline) as function of time. Monomer shows a weak π-π* transition at 280 nm due to partial solubility in mixed solvent system. | ||
To determine the reversible redox behavior of the poly(2,5-dimethoxyaniline), the absorption spectra of a product prepared at pH 3 was investigated by varying pH. UV-Vis spectra, as shown in Fig. 4, show the peak shift in absorption spectra of poly(2,5-dimethoxyaniline) with increasing pH from pH 3 to 12.5 by titration with 1 M NaOH (aq). As the pH increases, the intensity of a polaron peak at 750 nm gradually decreases and a new excitation peak at 560 nm appears with a strong π-π* transition peak at 330 nm. At pH 11, the polymer solution changes to a purple color from the original dark green, which indicates that the poly(2,5-dimethoxyaniline) is fully dedoped by NaOH (aq).14–16 The titration from pH 12.5 to pH 1 shows the opposite behavior.
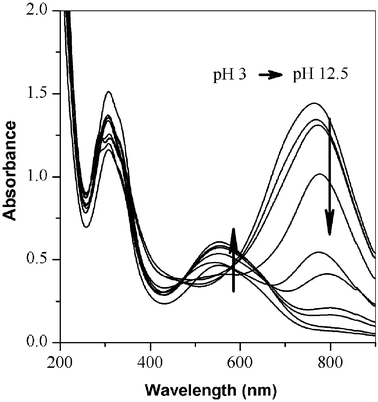 | ||
| Fig. 4 UV-Vis spectral change of poly(2,5-dimethoxyaniline) during titration by 1 M NaOH solution. The pH ranged from 3 to 12.5. The pH was monitored by a pH meter during titration. | ||
An interesting and unexpected phenomenon was observed during the enzymatic polymerization of anilines with polar organic solvents. Hydrogen peroxide has been widely used to oxidize the monomer in the presence of an enzyme, horseradish peroxidase. Methyl peroxide, ethyl peroxide and hydrogen peroxide are the only known peroxides that can bind to horseradish peroxidase in water.23,24 However, in these reactions, it was found that when 1,4-dioxane was used as the co-solvent, the polymerization of aniline could proceed without the addition of hydrogen peroxide. It was determined that acetyl acetal in dioxane, used as a stabilizer,25 is transformed to peroxyacetic acid which in turn could initiate the polymerization as shown in Scheme 3. To determine the oxidizing power of peroxyacetic acid, it was used as an oxidant instead of H2O2. Fig. 5 shows the UV-vis spectra of poly(2,5-dimethoxyaniline) with different oxidizing agents. It is well known that H2O2 can inactivate the heme structure of horseradish peroxidase due to over oxidation. Therefore, the injection of H2O2 into an enzymatic reaction cannot be done in high concentrations due to the slow oxidation reaction of monomers. This can often be time consuming and difficult to control. In this case, the peroxyacetic acid may be mild enough to allow a more convenient and easier control of the peroxide addition.
 | ||
| Scheme 3 | ||
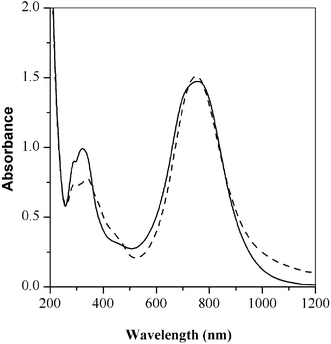 | ||
Fig. 5 The polaron transition of poly(2,5-dimethoxyaniline) with different oxidants; solid line (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ): with drop-wise addition of hydrogen peroxide, broken line ( ): with drop-wise addition of hydrogen peroxide, broken line (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ): immediate addition of peroxyacetic acid. ): immediate addition of peroxyacetic acid. | ||
The electrochemical behavior of the polyaniline derivatives were characterized in N,N-dimethylformamide with 0.1 M tetrabutylammonium hexafluorophosphate. Incorporation of an alkoxy group at the ortho position is known to decrease the oxidation potential of the aniline.26 Poly(2,5-dimethoxyaniline), poly(2-methyl-5-methoxyaniline) and poly(2-methoxy-5-methylaniline) showed one distinct quasi-reversible redox process at negative potential, which is similar to unsubstituted polyaniline.20 However, the second redox process is not so clear. Typically, two sets of redox peaks are observed with electrochemically grown and chemically prepared polyaniline.27 The absence of the second redox peak may be related to the exceptional resistance of the polyaniline derivatives to oxidation to the pernigraniline state.25 The shoulder in the cyclic voltammogram may be related to irregular couplings, degradation, and oligomer formations.28–30
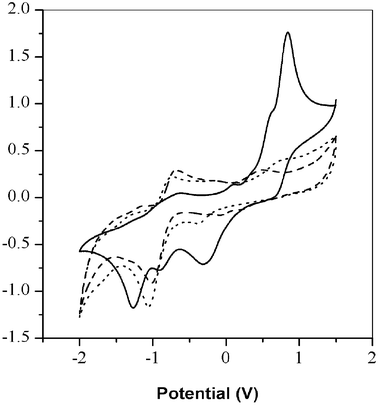 | ||
Fig. 6 Cyclovoltammograms of polyanilines; scan rate: (100 mV s–1); solid line (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ): poly(2,5-dimethoxyaniline), broken line ( ): poly(2,5-dimethoxyaniline), broken line (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ): poly(2-methyl-5-methoxyaniline), dashed line (⋯): poly(2-methoxy-5-methylaniline). ): poly(2-methyl-5-methoxyaniline), dashed line (⋯): poly(2-methoxy-5-methylaniline). | ||
To determine the oxidation state of doped and dedoped poly(2,5-dimethoxyaniline), FT-IR spectra of the poly(2,5-dimethoxyaniline) were monitored. Fig. 7 shows the FT-IR spectra of poly(2,5-dimethoxyaniline) with different doping states. Two primary NH stretching peaks merge to make a broad secondary NH stretching peaks at 3400 cm–1 after the polymerization of 2,5-dimethoxyaniline. The poly(2,5-dimethoxyaniline) dedoped with ammonium hydroxide has no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at 1670 cm–1, which suggests that the dedoped form of poly(2,5-dimethoxyaniline) is in the leucoemeraldine insulating form.31 Two correlated bands of –OCH3 appear at 1203 and 1042 cm–1 with medium intensity. The intensity of these two peaks decreases at the doped state of the emeraldine salt form, probably due to restricted asymmetric C–O–C deformation. As expected, the doped form of poly(2,5-dimethoxyaniline) shows the double bond character of C
N stretch at 1670 cm–1, which suggests that the dedoped form of poly(2,5-dimethoxyaniline) is in the leucoemeraldine insulating form.31 Two correlated bands of –OCH3 appear at 1203 and 1042 cm–1 with medium intensity. The intensity of these two peaks decreases at the doped state of the emeraldine salt form, probably due to restricted asymmetric C–O–C deformation. As expected, the doped form of poly(2,5-dimethoxyaniline) shows the double bond character of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch peak at 1669 cm–1 with the doping by HCl.
N stretch peak at 1669 cm–1 with the doping by HCl.
The polaron transition peak of poly(2-methyl-5-methoxyaniline) synthesized without templates was different from that obtained from template polymerization. Sulfonated polystyrene and sodium dodecylbenzene sulfonate can align 2-methyl-5-methoxyaniline along the negatively charged template surface, which guide a para linkage reaction for the polymerization of poly(2-methyl-5-methoxyaniline). The shoulder at 550 nm may be related with the branching during the polymerization. However, poly(2,5-dimethoxyaniline) shows only one sharp polaron transition at 800 nm, which demonstrates that two methoxy groups can prevent ortho coupling successfully without templates.
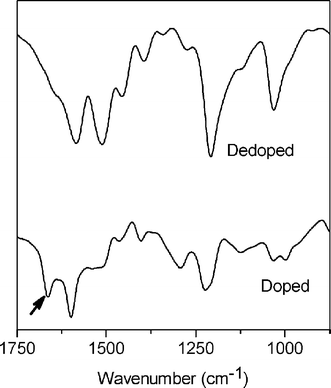 | ||
| Fig. 7 FT-IR spectra of poly(2,5-dimethoxyaniline) at different doping states. | ||
Conclusions
A new biological route for the synthesis of conducting polyaniline was presented. This approach has significant importance because conducting polyaniline was synthesized with horseradish peroxidase without any template by careful selection of aniline monomers. The ortho branching of polyaniline could be reduced significantly by introducing methyl or methoxy groups on the ortho position. The solubility of oligomers as well as the activity of horseradish peroxidase in a mixed solvent system had a significant role in the polymerization of substituted anilines. The synthesized polyanilines having methyl and methoxy groups had increased solubility in water and organic solvents such as DMF, ethanol and DMSO. These soluble homo-polyanilines may be further functionalized at the available ortho and meta position. Peroxyacetic acid, an oxidized form of a stabilizer found in 1,4-dioxane, proved to serve as a promising mild oxidant under certain reaction conditions and demonstrated a novel way to optimize enzymatic polymerizations. FT-IR spectra showed that the dedoped form of poly(2,5-dimethoxyaniline) is in the leucoemeraldine insulating state not in the emeraldine base form.Experimental
Horseradish peroxidase (EC 1.11.1.7) was purchased from Sigma Chemical Co. A stock solution of 10 mg mL–1 in pH 6.0, 50 mM phosphate buffer was prepared. Aniline monomers were obtained from Aldrich Chemical Co., Inc. All other chemicals and solvents used were also commercially available and used as received. The enzymatic polymerizations were typically carried out at room temperature in a 10 mL buffer solution of pH 3.0 which contained aniline monomer (concentration 0.1 mmol). To the solution, 0.1 mL of horseradish peroxidase stock solution was then added. The reaction was initiated by the addition of a stoichiometric amount of H2O2 or other peroxide, such as peroxyacetic acid, under vigorous stirring. To avoid the inhibition of horseradish peroxidase due to the excess amount of peroxide, diluted peroxide (0.3 wt%) was added drop wise.UV-Vis spectra were recorded on a Perkin–Elmer Lambda-9 UV/Vis/near-infrared spectrophotometer. FT-IR measurements were carried out on a Perkin–Elmer 1760X FT-IR spectrometer. The electrochemical characterization of polyaniline was carried out on an EG&G potentiostat/galvanostat model 263. Cyclic voltammograms were recorded by using a three-electrode cell in 1 M HCl. The conductivity of the synthesized polyaniline was measured by a typical four-probe method. The polyaniline was ground to fine powder and dried in a vacuum at 50 °C for 24 h prior to the measurements.
References
- A. G. MacDiarmid, Synth. Met., 1997, 84, 27 CrossRef CAS.
- A. G. MacDiarmid, J. C. Ciang, A. F. Richer and A. J. Epstein, Synth. Met., 1987, 18, 285 CrossRef CAS.
- Y. Cao, S. Li, Z. Xue and D. Guo, Synth. Met., 1986, 16, 305 CrossRef.
- R. Noufi, A. J. Nozik, J. White and L. F. Warren, J. Electrochem. Soc., 1982, 129, 2261 CAS.
- W. Westerweele, P. Smith and A. J. Heeger, Adv. Mater., 1995, 7, 788.
- A. F. Diaz and J. A. Logan, J. Electroanal. Chem., 1980, 111, 111 CrossRef CAS.
- A. Watanabe, K. Mori, A. Iwabuchi, Y. Iwasaki, Y. Nakamura and O. Ito, Macromolecules, 1989, 22, 3521 CrossRef CAS.
- W. W. Focke, G. E. Wnek and Y. Wei, J. Phys. Chem., 1987, 91, 5813 CrossRef CAS.
- C.-G. Wu and J.-Y. Chen, Chem. Mater., 1997, 9, 399 CrossRef CAS.
- G. Liu and M. S. Freund, Macromolecules, 1997, 30, 5660 CrossRef CAS.
- J. C. Chiang and A. G. MacDiarmid, Synth. Met., 1986, 13, 193 CrossRef CAS.
- M. T. Nguyen, P. Kasai, J. L. Miller and A. F. Diaz, Macromolecules, 1994, 27, 3625 CrossRef CAS.
- L. A. Samuelson, A. Anagnostopoulos, K. S. Alva, J. Kumar and S. K. Tripathy, Macromolecules, 1998, 31, 4376 CrossRef CAS.
- W. Liu, A. L. Cholli, R. Nagarajan, J. Kumar, S. Tripathy, F. F. Bruno and L. A. Samuelson, J. Am. Chem. Soc., 1999, 121, 11345 CrossRef CAS.
- W. Liu, J. Kumar, S. Tripathy, K. J. Senecal and L. A. Samuelson, J. Am. Chem. Soc., 1999, 121, 71 CrossRef CAS.
- W. Liu, J. Kumar, S. Tripathy and L. A. Samuelson, Langmuir, 2002, 18, 9696 CrossRef CAS.
- R. Nagarajan, W. Liu, J. Kumar and S. K. Tripathy, Macromolecules, 2001, 34, 3921 CrossRef CAS.
- B. C. Saunders, A. G. Holmes-Siedle and B. P. Stark, in Peroxidase, Butterworths, London, 1964 Search PubMed.
- P. Wang and J. S. Dordick, Macromolecules, 1998, 31, 941 CrossRef CAS.
- G. D'Aprano and M. Leclerc, Chem. Mater., 1995, 7, 33 CrossRef CAS.
- M. Thiyagrajan, L. A. Samuelson, J. Kumar and A. L. Cholli, J. Am. Chem. Soc., 2003, 125, 11502 CrossRef CAS.
- A. M. Klibanov, Trends Biotechnol., 1997, 15, 97 CrossRef CAS.
- H. B. Dunford, in Peroxidases in Chemistry and Biology, ed. J. Everse. K. E. Everse and M. B. Grisham, CRC Press, Boca Raton, FL, USA, 1991; vol. 2, pp. 1–24 Search PubMed.
- A. C. Maehly and B. Chance, The assay of catalases and peroxidases, in Methods of Biochemical Analysis, 1954, vol. 1, p. 357 Search PubMed.
- A. I. Vogel, A. R. Tatchell, B. S. Furnis, A. J. Hannaford and P. W. G. Smith, in Text Book of Practical Organic Chemistry, Prentice Hall, New York, 5th edn, 1989, p. 407 Search PubMed.
- E. M. Genies and C. Tsintavis, J. Electroanal. Chem., 1985, 195, 109 CrossRef CAS.
- Y. Wei, W. W. Focke, G. E.. Wnek, A. Ray and A. G. MacDiarmid, J. Phys. Chem., 1989, 93, 495 CrossRef CAS.
- L. T. Yu, M. S. Borredon, M. Jozefowicz, G. Belorgey and R. Buvet, J. Polym. Sci. Polym. Symp., 1967, 10, 2931 Search PubMed.
- R. L. Hand and R. F. Nelson, J. Am. Chem. Soc., 1974, 96, 850 CrossRef CAS.
- T. Kobayashi, H. Yneyma and H. Tamara, J. Electroanal. Chem., 1984, 177, 293 CrossRef CAS.
- M. G. Mikhael, A. B. Padias and H. K. Hall, Jr., J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 1673 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: UV-Vis spectra of poly(2-methyl-5-methoxyaniline) with and without templates. See DOI: 10.1039/b606839a |
| This journal is © The Royal Society of Chemistry 2007 |
