Lactic acid production from waste sugarcane bagasse derived cellulose
Mukund G.
Adsul
a,
Anjani J.
Varma
b and
Digambar V.
Gokhale
*a
aScientist In-Charge, NCIM, National Chemical laboratory, Pune, 411 008, Maharashtra, India. E-mail: dv.gokhale@ncl.res.in; Tel: +91-20-25902670; Fax: +91-20-25902671
bPolymer Science and Engineering Division, National Chemical Laboratory, Dr Homi Bhabha Road, Pune, 411 008, Maharashtra, India
First published on 17th October 2006
Abstract
Production of L(+)lactic acid from sugarcane bagasse cellulose, one of the abundant biomass materials available in India, was studied. The bagasse was chemically treated to obtain a purified bagasse cellulose sample, which is much more amenable to cellulase enzyme attack than bagasse itself. This sample, at high concentration (10%), was hydrolyzed by cellulase enzyme preparations (10 FPU g–1 cellulose) derived from mutants generated in our own laboratory. We obtained maximum hydrolysis (72%), yielding glucose and cellobiose as the main end products. Lactic acid was produced from this bagasse cellulose sample by simultaneous saccharification and fermentation (SSF) in a media containing a cellulase enzyme preparation derived from Penicillium janthinellum mutant EU1 and cellobiose utilizing Lactobacillus delbrueckii mutant Uc-3. A maximum lactic acid concentration of 67 g l–1 was produced from a concentration of 80 g l–1 of bagasse cellulose, the highest productivity and yield being 0.93 g l–1 h–1 and 0.83 g g–1, respectively. The mutant Uc-3 was found to utilize high concentrations of cellobiose (50 g l–1) and convert it into lactic acid in a homo-fermentative way. Considering that bagasse is a waste material available in abundance, we propose to valorize this biomass to produce cellulose and then sugars, which can be fermented to products such as ethanol and lactic acid.
Introduction
Lactic acid and its derivatives have been widely used in food, pharmaceutical, cosmetic and industrial applications.1 It has also been receiving great attention as a feedstock for manufacture of polylactic acid (PLA), a biodegradable polymer used as environmentally friendly biodegradable plastic. Lactic acid is produced commercially either by chemical synthesis or by microbial fermentation. Approximately 90% of the total lactic acid produced worldwide is made by bacterial fermentation and the rest is produced synthetically by the hydrolysis of lactonitrile. The chemical synthesis of lactic acid always results in a racemic mixture of lactic acid, which is a major disadvantage. Fermentative production of lactic acid offers the advantages in both utilization of renewable carbohydrates and production of optically pure L- or D-lactic acid, depending on the strain selected.Lignocellulosic substances are abundantly available resources, which can be utilized as a feedstock for producing a number of bulk chemicals like ethanol or lactic acid through fermentation processes. The possible lignocellulosic substances include sugarcane bagasse, waste paper and agricultural wastes. These resources are seen as an interesting energy sources for several reasons. The application of lignocellulosic residues in bioprocesses not only provides alternative substrates but also helps solve their disposal problems, and with the advent of biotechnological innovations, mainly in the area of enzyme and fermentation technology, many new avenues have opened up for their proper utilization as value added products. Several chemical or physical pretreatments followed by enzymatic hydrolysis of pretreated lignocellulosic materials are necessary to produce fermentable sugars, which can be diverted to ethanol or lactic acid. Currently, optically pure lactic acid is produced mainly from cornstarch. However, utilization of lignocellulosic agricultural waste by-products for lactic acid production appears to be more attractive because of their low cost; further, they do not impact the food chain for humans. In fact, an attempt to produce lactic acid from cellulosic materials was first reported by Wang et al.2 Since then, several reports appeared on the production of lactic acid from cellulosic materials.3–9 Recently, Patel et al.10 reported Bacillus sp. Strain 36D1 capable of converting lignocellulosic biomass into lactate with high product yield.
India is one of the largest sugar cane growing countries, producing approximately 200 million tons per year, which generate about 45 million tons of bagasse on dry weight basis. We have generated several bagasse samples with decreasing content of lignin, which were used to produce cellulase with high productivities.11 These bagasse samples were also evaluated as a source for the production of sugars (glucose, xylose, arabinose) using enzymes that were produced by treating delignified bagasse samples with a mutant of Penicillium janthinellum NCIM 1171 obtained in our own laboratory,12,13 thus completing the full cycle of enzyme generation and monosaccharide generation from the same bagasse cellulose broth. This strategy gives the most suitable enzyme for hydrolysis of the bagasse cellulose. In this paper, production of lactic acid by a mutant of Lactobacillus delbrueckii NCIM 2365, isolated in our laboratory, was investigated with bagasse derived cellulose sample as a carbon source.
Experimental
Chemicals
Cellulose powder-123 (CP-123) was obtained from Carl Schleicher and Schull Co., Dassel, FRG. p-Nitrophenyl β-D-glucopyranoside (pNPG), carboxymethylcellulose (CMC) 3,5-dinitrosalysilic acid were obtained from Sigma–Aldrich Co., St Louis, MO, USA. Sodium azide was obtained from S.D.Fine-Chem (India). Avicel PH-101 was obtained from Fluka Chemie GmbH. The α-cellulose with 0.18% lignin and 98% cellulose was prepared from sugarcane bagasse in our laboratory.Preparation of sugarcane bagasse cellulose
Sugarcane bagasse was obtained from Tamil Nadu Pulp and Paper Mills, Chennai, India. This bagasse contains about 43% cellulose, 30% xylan, and 20% lignin, in addition to some silica and other constituents. It was cut into small shreds of 1–3 mm size and then pre-treated with steam and alkali by a proprietary process (under patenting) to remove the xylan, lignin, and other impurities. The final product consisted of 93.5% α-cellulose, 5.3% β-cellulose (low molecular weight cellulose and traces of hemicellulose), 1.02% γ-cellulose, and 0.18% lignin.Strain information and cellulase production
Penicillium janthinellum NCIM 1171 was obtained from NCIM Resource Center, Pune, India. Mutants (EMS-UV-8, EU1, EU2D21) of P. janthinellum were generated by exposing conidia of the parent strain to UV-irradiation. The mutant EMS-UV-8 is selected on the basis of hydrolysis of phosphoric acid swollen cellulose. EU1 mutant was selected on the basis of Avicel hydrolysis and EU2D-21 is selected on the basis of phosphoric acid swollen cellulose in presence of 2-deoxyglucose (1.5%). The procedure of generation of these mutants and their crude enzyme mixtures have already been reported earlier.13 These cultures were maintained on potato dextrose agar (PDA) and sub-cultured once every three months. PDA contained (per litre) extract from 200 g of potatoes, glucose (20.0 g), yeast extract (1.0 g), and agar (20.0 g). Enzyme production was carried out in a 250 ml Erlenmeyer flask, with 70 ml of production medium containing 1% (w/v) cellulose-123 powder and 2.5% wheat bran.13Lactobacillus delbrueckii mutant Uc-3 producing L(+)lactic acid with high productivity was isolated as described earlier.14 It was maintained in liquid MRS medium supplemented with 0.1% CaCO3.Enzymatic hydrolysis of Avicel and sugarcane bagasse cellulose
The saccharification experiments were carried out in a 50 ml conical flask with 25 ml citrate buffer (pH 4.5, 50 mM), 1.25 g or 2.5 g of either bagasse cellulose or Avicel, 2.5 mg sodium azide and crude enzyme preparations from P. janthinellum NCIM 1171 or its mutants (EMS-UV-8, EU1, EU2D21). This mixture was incubated at 50 °C with shaking at 150 rpm. The samples were analyzed for the reducing sugars after suitable time intervals.Simultaneous saccharification and fermentation (SSF)
SSF was carried out in a 250 ml screw cap conical flask with the production medium consisting of bagasse sample (10.0 g), CaCO3 (5.0 g), yeast extract (1.0 g) in 125 ml citrate buffer (pH 4.5, 50 mM). The production medium was sterilized at 121 °C for 20 min, the crude enzyme preparation was added, and Lactobacillus delbrueckii Uc-3 mutant cells (5%) grown in sucrose based medium14 were inoculated. The flasks were incubated at 42 °C with shaking at 150 rpm. All the SSF experiments were performed for 72 h in media containing 10 filter paper units (FPU) g–1 of substrate. The initial pH of the fermentation medium was 6.0. The samples harvested at various time intervals were centrifuged at 5000 rpm for 20 min to separate the cells. The supernatant was acidified by adding an equal volume of 1 N HCl, where free acid is liberated and analyzed by HPLC for lactic acid.Analytical methods
The reducing sugar content was estimated as the glucose equivalent by the dinitrosalicylic acid (DNS) method.15Cell growth was monitored by visible spectral peak absorbances (UV–VIS Spectrometer–117, Systronics, Mumbai, India) at a wavelength of 660 nm. The glucose, cellobiose and lactic acid in the samples were determined using a high performance liquid chromatography (HPLC ) system (Dionex India Limited) equipped with UV- or RI-detectors. An ion exclusion column (Aminex HPX-87H, Biorad, Hercules, CA) was used at a temperature of 30 °C, with 0.008 N H2SO4 as a mobile phase at a flow rate of 0.6 ml min–1. The injection volume of the sample was 50 µl.Results and discussions
Initially, experiments were carried out to evaluate the potential of cellulase enzyme preparations derived from mutants EMS-UV-8, EU1 and EU2D21 towards the hydrolysis of sugarcane bagasse cellulose (lignin content 0.18) and Avicel. Hydrolysis was carried out at two different substrate concentrations (5% and 10%) using 5 FPU g–1 and 10 FPU g–1 of the cellulose substrate. It was found that all mutant enzyme preparations gave higher bagasse cellulose/Avicel hydrolysis than that obtained with parent enzyme preparation (Table 1). However, hydrolysis of the most crystalline substrate, Avicel, was always lower than that of bagasse sample irrespective of enzyme preparations used. The mutant enzyme preparations derived from EU1 and EU2D-21 yielded maximum hydrolysis at both 5% and 10% bagasse sample. With the same preparations, the Avicel hydrolysis (at 10% concentration) was only 38%, which was better than that obtained with the parental strain (21%). The rate of hydrolysis of both Avicel and bagasse cellulose sample with the mutant enzyme was faster than with the parental enzyme in spite of very low amounts (10 times less) of β-glucosidase in the mutant enzyme preparation compared to the parent enzyme (Fig. 1). The lower hydrolysis (40%) of Avicel could be due to its microcrystalline structure, which prevents an easy access to enzymes. We got approximately 84% hydrolysis (at 5% bagasse cellulose concentration) and 72% hydrolysis (at 10% bagasse cellulose concentration) with EU1 enzyme preparation (10 FPU g–1 bagasse).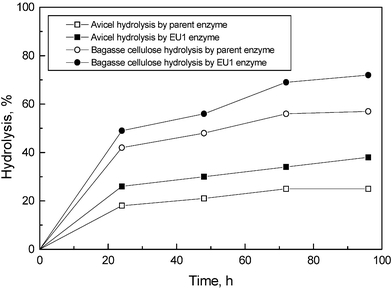 | ||
| Fig. 1 Profile of hydrolysis of Avicel and bagasse cellulose by parent and mutant (EU1) enzyme preparations. The hydrolysis was carried out using Avicel and sugarcane bagasse cellulose at 10% with parent and mutant enzyme preparations (10 FPU g–1). | ||
| Strains | Enzyme activities/g of substrate | % Hydrolysis after 96 h | |||||
|---|---|---|---|---|---|---|---|
| FPUa | β-glucosidase | CMCase b | Avicel (5%) | Avicel (10%) | BSc (5%) | BSc (10%) | |
| a FPU—filter paper cellulase units. b CMCase—carboxymethylcellulase activity.c BS—sugarcane bagasse cellulose sample. | |||||||
| Parent | 5.0 | 45.0 | 210 | 21 | 21 | 46 | 39 |
| EMS-UV-8 | 5.0 | 6.5 | 140 | 32 | 33 | 48 | 45 |
| EU-1 | 5.0 | 9.0 | 175 | 41 | 41 | 63 | 62 |
| EU2D-21 | 5.0 | 3.8 | 170 | 39 | 38 | 52 | 51 |
| Parent | 10.0 | 90.0 | 420 | 26 | 25 | 64 | 57 |
| EMS-UV-8 | 10.0 | 13.0 | 280 | 39 | 32 | 70 | 56 |
| EU-1 | 10.0 | 18.0 | 350 | 46 | 36 | 84 | 72 |
| EU2D-21 | 10.0 | 7.6 | 340 | 44 | 37 | 80 | 60 |
The hydrolysis of bagasse cellulose sample with parent enzyme preparation resulted in production of glucose as the only end product, probably due to the presence of very high amounts of β-glucosidase. On the other hand, the hydrolysis broth derived from the treatment of bagasse cellulose with mutant enzyme preparations contained both glucose and cellobiose as end products (Table 2). The amount of xylose detected was insignificant, indicating a much lower amount of hemicellulose present in sugarcane bagasse cellulose. The mutants produced high levels of glucose because they are selected on the basis of hydrolysis in the presence of 2-deoxyglucose. The presence of cellobiose in the mutant hydrolysate is due to a lower amount of β-glucosidase in the crude enzyme mixture (Table 1). The presence of both glucose and cellobiose in the broth may hinder the further hydrolysis to glucose because they are strong inhibitors of cellulases. However, this drawback can be overcome by SSF methodology to produce lactic acid from bagasse cellulose sample using cellobiose utilizing microbes. Considering the inexpensive nature of bagasse samples, there is no doubt about their high potential as substrates for commercial production of glucose and further fermentation of glucose to other platform chemicals by SSF.
| Substrates | Enzyme | Enzyme activity used | |||
|---|---|---|---|---|---|
| 5 FPU g–1 substrate | 10 FPU g–1 substrate | ||||
| Cellobiose/mg | Glucose/mg | Cellobiose/mg | Glucose/mg | ||
| a ND not detected. | |||||
| Avicel (2.5 g) | Parent | 3.0 | 520.0 | 6.1 | 610.0 |
| EMS-UV-8 | 14.0 | 805.0 | 33.0 | 780.0 | |
| EU1 | 33.0 | 1000.0 | 20.0 | 870.0 | |
| EU2D-21 | 31.0 | 940.0 | 20.0 | 900.0 | |
| Sugarcane bagasse cellulose (2.5 g) | Parent | NDa | 970.0 | NDa | 1420.0 |
| EMS-UV-8 | 35.0 | 1090.0 | 47.6 | 1345.0 | |
| EU1 | 84.0 | 1460.0 | 48.5 | 1740.0 | |
| EU2D-21 | 93.0 | 1200.0 | 65.0 | 1420.0 | |
SSF experiments were carried out under the selected conditions (42 °C and pH 6.5) because the organism used in this fermentation is a mutant of L. delbrueckii (Uc-3) and cannot grow at temperatures more than 42 °C or at pH less than 5.5. We carried out the SSF at pH 6.5, where the cellulases used were active, with retention of 50% activity. The mutant (Uc-3) used in this study produces lactic acid with very high productivity.14 SSF experiments were performed in production media containing cellulases (10 FPU g–1 of substrate). The pH of the fermentation broth also dropped to 5.3 within 24 h (Fig. 2), which is the pH at which the enzymes are most active. There was no cellobiose accumulation during the fermentation at any time. Cellobiose was either converted to glucose by β-glucosidase present in the cellulase preparations or utilized by the mutant strain to produce lactic acid. The presence of higher cellobiose concentration could result in significant inhibition , which could be removed by supplementation of the media with additional cellobiase, leading to a remarkable improvement in lactic acid production in fed batch SSF.6 However, in simple batch operations with cellulase from T. reseei and L. delbrueckii, supplementation of media with fresh cellobiase did not improve the overall process.16 We obtained 67 g l–1 of lactic acid from 80 g l–1 of bagasse cellulose sample when we used EU1 enzyme preparations for hydrolysis . The yield (g g–1) and productivity (g l–1 h–1) of lactic acid were 0.83 and 0.93, respectively. In comparison to other reports in the literature (Table 3), this is the highest yield and productivity of lactic acid reported so far in spite of using less cellulase enzyme (10 FPU g–1 of the substrate) with low amounts of β-glucosidase in batch SSF. There is one report on maximum lactic acid production (108 g l–1), which was achieved by combining multiple substrate addition, supplementation with fresh nutrients and enzymes and removal of lactic acid.6
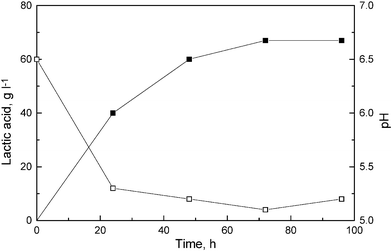 | ||
| Fig. 2 Course of lactic acid production (■) and pH (□) during SSF of sugarcane bagasse cellulose (80 g l–1) using mutant enzyme preparation (EU1, 10FPU g–1). | ||
| Substrate/g l–1 | Microorganism | Enzyme/FPU g–1 | F. T.a | Cmaxb | Yp/sc | Qpd | Reference |
|---|---|---|---|---|---|---|---|
| a F.T.—Fermentation time (h). b Cmax—Maximum lactic acid concentration (g l–1). c Yp/s—% Product yield (g g–1). d Qp—Lactic acid productivity (g l–1 h–1). | |||||||
| α-cellulose | L. delbrueckii NRRL-B445 | — | 75 | 62 | 0.34 | 19 | |
| Defatted rice bran (100) | L. delbrueckii IFO 3202 | Cellulase-Y-NC | 36 | 28 | 28 | 0.77 | 9 |
| Filter paper (33) | L. coryniformi ATCC 25600 | Celluclast and Novozyme (28) | 48 | 25 | 75 | 0.5 | 17 |
| Solka Floc (60) | Bacillus sp. Strain 36D1 | Spezyme (10) | 192 | 40 | 65 | 0.22 | 10 |
| Solka Floc (20) | B. coagulans strain 36D1 | Genecore International GC220 (10) | 24 | 13.5 | 67 | 0.63 | 20 |
| Preated cardboard (41) | L. coryniformi ATCC 25600 | Celluclast & Novozyme (22.8) | 48 | 23 | 56 | 0.49 | 8 |
| Paper mill sludge (23.4) | L. paracasei | Meicelase MCB8-6 (46) | 72 | 17 | 72 | 0.23 | 18 |
| Sugarcane bagasse cellulose (80) | L. delbrueckii Uc-3 | P.janthinellum EU1 (10) | 72 | 67 | 83 | 0.93 | This work |
Since there was no cellobiose detected during SSF, we wanted to know the potentiality of the mutant L. delbrueckii Uc-3 to utilize cellobiose and convert it into lactic acid. The fermentation experiments were carried out in production medium (initial pH 6.5) containing 50 g l–1 of cellobiose. The mutant produced 42 g l–1 of lactic acid within 48 h with 84% yield and 1.0 g l–1 h–1 productivity (Fig. 3). The results suggested that mutant is capable of utilizing cellobiose at concentrations as high as 50.0 g l–1.
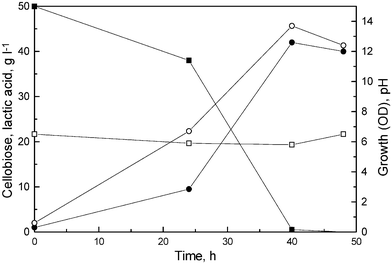 | ||
| Fig. 3 Profile of growth (○), pH (□), lactic acid production (●) and cellobiose utilization (■) during fermentation by L. delbrueckii mutant, Uc-3. The fermentation was carried out in a medium containing cellobiose (50 g l–1). | ||
Conclusion
Batch experiments were conducted for conversion of bagasse sample to lactic acid by simultaneous saccharification and fermentation using a cellulase preparation derived from a mutant of P. janthinellum (EU1) and L. delbrueckii mutant Uc-3. Saccharification experiments were also carried out using mutant enzymes to characterize the cellulose degradation. L. delbrueckii mutant Uc-3 was capable of utilizing high concentrations of cellobiose, producing lactic acid with high yield (0.9 g lactic acid per g cellobiose). Batch SSF yielded 67 g l–1 of lactic acid from 80 g l–1 of bagasse sample with yield and productivity of 0.83 g g–1 of cellulose substrate and 0.93 g l–1 h–1. Applying SSF to lactic acid production has more advantage than SS since we could operate the SSF at conditions suitable and optimum for both cellulose hydrolysis and growth of L. delbrueckii mutant. The further improvements in batch SSF to make it cost effective are necessary, as this work indicates great advantages from the industrial viewpoint. The work on utilization of other biomass materials with proper pretreatment and proper integrated saccharification and fermentation processes may lead to bio-recycling of biomass to produce value added chemicals. We are currently working on improving the techno-economic efficiency of the sugarcane bagasse derived cellulose process, so as to obtain an optimum and inexpensive bagasse constitution (cellulose, xylan, and lignin) more amenable to enzyme/microbial attack and further fermentation to lactic acid and other feedstock chemicals that can take place in a facile manner.Acknowledgements
The authors gratefully acknowledge the financial support from Department of Biotechnology (DBT), New Delhi and TNBD (NMITLI) Division, CSIR, New Delhi.References
- T. B. VickRoy, Comprehensive Biotechnology, ed. M. Moo-Young, Pergamon Press, New York, 1985, vol 3, pp. 761–776 Search PubMed.
- D. I. C. Wang, C. L. Cooney, A. L. Demain, R. F. Gomez and A. J. Sinskey, Springfield: National Information Service Report, COO-4198-4, 1977, pp. 106–123 Search PubMed.
- S. Abe and M. Takagi, Biotechnol. Bioeng., 1991, 37, 93–96 CrossRef CAS.
- K. V. Venkatesh, Bioresour. Technol., 1997, 62, 91–98 CrossRef CAS.
- S. Thomas, Appl. Biochem. Biotechnol., 2000, 84–86, 455–468 CrossRef CAS.
- A. B. Moldes, J. L. Alonso and J. C. Parajo, J. Chem. Technol. Biotechnol., 2001, 76, 279–284 CrossRef CAS.
- G. Bustos, A. B. Moldes, J. M. Cruz and J. M. Dominguez, Biotechnol. Prog., 2005, 21, 793–798 CrossRef CAS.
- R. Yanez, J. L. Alonso and J. C. Parajo, J. Chem. Technol. Biotechnol., 2005, 80, 76–84 CrossRef CAS.
- T. Tanaka, M. Hoshina, S. Tanabe, K. Sakai, S. Ohtsubo and M. Taniguchi, Bioresour. Technol., 2006, 97, 211–217 CrossRef CAS.
- M. A. Patel, M. S. Ou, L. O. Ingram and K. T. Shanmugam, Biotechnol. Prog., 2005, 21, 1453–1460 CrossRef CAS.
- M. G. Adsul, J. E. Ghule, R. Singh, H. Shaikh, K. B. Bastawde, D. V. Gokhale and A. J. Varma, Carbohydr. Polym., 2004, 54, 67–72 CrossRef.
- M. G. Adsul, J. E. Ghule, R. Singh, H. Shaikh, K. B. Bastawde, D. V. Gokhale and A. J. Varma, Carbohydr. Polym., 2005, 62, 6–10 CrossRef CAS.
- M. G. Adsul, K. B. Bastawde, A. J. Varma and D. V. Gokhale, Bioresour. Technol., 2006 DOI:10.1016/j.biortech.2006.02.036.
- S. R. Kadam, S. S. Patil, K. B. Bastawde, J. M. Khire and D. V. Gokhale, Process Biochem., 2006, 41, 120–126 CrossRef CAS.
- E. H. Fischer and E. A. Stein, Biochem. Prep., 1961, 8, 27–32 Search PubMed.
- J. C. Parajo, J. L. Alonso and A. B. Moldes, Food Biotechnol., 1997, 11, 45–58 Search PubMed.
- R. Yanez, A. B. Moldes, J. L. Alonso and J. C. Parajo, Biotechnol. Lett., 2003, 25, 1161–1164 CrossRef CAS.
- K. Nakasaki, N. Akakura, T. Adachi and T. Akiyama, Environ. Sci. Technol., 1999, 33, 198–200 CrossRef CAS.
- V. P. Iyer and Y. Y. Lee, Biotechnol. Lett., 1999, 21, 371–373 CrossRef.
- M. A. Patel, M. S. Ou, R. Harbrucker, H. C. Aldrich, M. L. Buszko, L. O. Ingram and K. T. Shanmugam, Appl. Environ. Microbiol., 2006, 72, 3228–3235 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2007 |
