Metal release from stainless steel particles in vitro—influence of particle size
K.
Midander
*,
J.
Pan
,
I.
Odnevall Wallinder
and
C.
Leygraf
Division of Corrosion Science, Department of Materials Science and Engineering, School of Industrial Engineering and Management, Royal Institute of Technology, Drottning Kristinas väg 51, SE-100 44 Stockholm, Sweden. E-mail: klarami@kth.se; Fax: +46 8 20 82 84; Tel: +46 8 790 68 78
First published on 28th November 2006
Abstract
Human inhalation of airborne metallic particles is important for health risk assessment. To study interactions between metallic particles and the human body, metal release measurements of stainless steel powder particles were performed in two synthetic biological media simulating lung-like environments. Particle size and media strongly influence the metal release process. The release rate of Fe is enhanced compared with Cr and Ni. In artificial lysosomal fluid (ALF, pH 4.5), the accumulated amounts of released metal per particle loading increase drastically with decreasing particle size. The release rate of Fe per unit surface area increases with decreasing particle size. Compared with massive sheet metal, fine powder particles (<4 μm) show similar release rates of Cr and Ni, but a higher release rate of Fe. Release rates in Gamble’s solution (pH 7.4), for all powders investigated, are significantly lower compared to ALF. No clear trend is seen related to particle size in Gamble’s solution.
Introduction
In urban environments, humans are often exposed to airborne particulate matter that contains metallic particles originating from, for example, different traffic sources and building/construction sites. Besides, professionals in certain occupations are more exposed to metallic particles due to fabrication and use of metallic materials. Airborne particles are associated with a wide range of adverse health effects including exacerbation of asthma and chronic obstructive pulmonary diseases, as well as lung cancer and cardiovascular diseases.1–3 Studies on toxic aspects indicate the ability of, for example, soluble transition metal components (such as Fe, Cr, Ni) of particles to induce oxidative stress and, hence, potentially damage DNA.4–7 Owing to an increasing awareness of adverse effects on human health, the exposure to airborne particles has become an important issue in environmental health risk assessment, development of health policies and establishment of regulations.Particles less than 100 μm in size are considered inhalable: smaller particles, less than 5 μm, are respirable and can reach the alveolar region of the deep lung, possibly causing an inflammatory response.3,8,9 Airborne particulate matter has been measured and characterized with respect to particle size range, composition and morphology,10–13 and epidemiological and toxicological aspects are assessed in various studies.14–19 A metallic particle in contact with lung tissue/cells involves the release of metal ions into the biological system. It is important to clarify whether released ions, or the particle itself, cause any adverse effect on health. Usually, released elemental concentrations of collected particles on filters are measured after extraction with water or diluted acid,5,20,21 and speciation of particulate metals in sediment, for example, is often assessed by chemical sequential extractions.22 Such an approach may be quite different from the real situation. The mechanism of metal release induced by corrosion, and/or chemical dissolution, depends on the material and the surrounding environment. It is therefore of importance to perform metal release studies in relevant test environments. The selection of a synthetic medium is crucial for a metal release study since different species present in body fluids may either enhance or inhibit metal release. Synthetic biological media that mimic the lung compartment have previously been used to investigate such things as the solubility of mineral fibres, bioavailability of cobalt-containing materials and metal release from massive stainless steel.23–26 It should be mentioned that, despite the complexity in composition, these test media only model the body condition to some extent since they do not contain proteins. Interactions between inhaled particles and cells/tissues of the lung compartment are complex processes that likely depend on the composition, size and shape of the particles. From a materials scientific point of view, the behaviour of the particulate material involved in these interactions is relatively undefined and unknown.
Scarce data exist on the metal release process from metallic particles. An experimental method has recently been elaborated which enables such measurements.27 The technique has so far been applied to μm-sized particles of stainless steel, nickel and copper.27,28 Studies of stainless steel are of general importance since the material spontaneously forms a passive surface film, which reduces metal release rates by several orders of magnitude,29,30 and its main alloy constituents are chromium and nickel. Both metals are considered to potentially cause adverse effects on human health, e.g., the chromium(VI) ion and insoluble nickel compounds are carcinogenic via inhalation.31,32 Nickel is also known to cause contact dermatitis. Contrary to stainless steel massive sheet, large variations in composition and thickness of the chromium and iron-rich surface film occur on particles of stainless steel. This is a result of the formation process at high temperatures. Moreover, below a certain particle size (a few μm), the oxide thickness decreases and impurity elements like S may be segregated on the surface, which may alter the physical/chemical/mechanical properties, i.e., the corrosion resistance, of the surface oxide on the particle significantly.33 This size-dependence most likely has an influence on the metal release process. However, no literature is available that assesses the metal release process from particles of stainless steels.
The aim of this study is to examine the importance of size of stainless steel 316L powder particles on the metal release process in synthetic lung fluids simulating an inhalation scenario. Two different fluids were investigated, artificial lysosomal fluid (ALF, pH 4.5), which simulates conditions occurring in conjunction with phagocytosis by cells, similar to an inflammation, and Gamble’s solution (pH 7.4), which mimics the interstitial fluid deep within the lung at normal health conditions. Since inhalation of particles might correspond to a consecutive exposure to Gamble’s solution followed by ALF, this scenario was also examined. Knowledge of the metal release process from stainless steel particles in artificial body fluids may contribute to a general understanding of potentially harmful effects on respiratory health.
Experimental
Stainless steel powder particles
All powders of stainless steel grade 316L were manufactured by gas-atomization and were commercially available: one fine-sized, trade name “ultrafine” from Sandvik Osprey Limited, and four coarser powders, denoted I–IV, Table 1. Powders I and II were supplied by Eurofer, Belgium, powder III was obtained from Carpenter Powder Products, Sweden, and powder IV from ARCAM AB, Sweden. Powders III and IV are relatively more coarse compared with the other powders. These types of stainless steel powders are mainly used for powder metallurgy applications such as the manufacturing of components, e.g., within the automotive industry, by injection of powder into a mould, producing components very close to the defined dimensions.| Powder | Grade | C | Si | Mn | P | S | Cr | Ni | Al | Mo |
|---|---|---|---|---|---|---|---|---|---|---|
| a Below detection limit (alt. not measured) | ||||||||||
| Ultrafine | 316L | 0.048 | 0.65 | 1.4 | 0.027 | 0.008 | 18.5 | 11.6 | — | 2.3 |
| I | 316L | 0.017 | 0.71 | 1.6 | —a | — | 16.7 | 13.2 | — | 2.7 |
| II | 316L | 0.005 | 0.71 | 1.2 | — | — | 16.7 | 11.8 | 0.008 | 2.7 |
| III | 316L | 0.024 | 0.61 | 1.2 | 0.026 | 0.006 | 16.7 | 10.3 | — | 2.1 |
| IV | 316L | 0.030 | 0.49 | 1.4 | 0.025 | 0.010 | 16.8 | 10.3 | — | 2.1 |
Table 2 provides information on the particle size distribution of the ultrafine powder, and powders I and II. All size fractions of powder particles, from sieve analysis, are presented in terms of particle diameter, where d10 is the largest particle diameter representative for 10 wt% of the particles. 50 wt% and 90 wt% of the particles have diameters less than d50 and d90, respectively. Table 3 presents the size fractions from sieve analyses for the two more coarse powders, III and IV, respectively. All information on particle size of the powders was provided by the supplier/producers.
| Powder diameter | Ultrafine | I | II |
|---|---|---|---|
| d 10 | 1.1 μm | 1.9 μm | 5.4 μm |
| d 50 | 1.8 μm | 3.5 μm | 8.5 μm |
| d 90 | 3.6 μm | 5.5 μm | 12.6 μm |
| Micron | 44 | 31 | 22 | 16 | 11 | 7.8 | 5.5 | 3.9 | 2.8 | 1.9 |
|---|---|---|---|---|---|---|---|---|---|---|
| III wt% < | — | 100 | 99.4 | 87.6 | 58.8 | 29.0 | 12.7 | 4.9 | 1.5 | 0.2 |
| IV wt% < | 86.6 | 60.5 | 31.5 | 15.3 | 6.1 | 3.3 | 0.7 | — | — | — |
For a given mass of particles, the specific surface area of particles increases with decreasing particle diameter. However, the specific surface area also depends on the particle size distribution. The specific surface areas of all the investigated powders, Table 4, were measured by BET (Brunauer–Emmett–Teller) analysis (adsorption of nitrogen in cryogenic conditions). This was performed at Kanthal AB using a Micromeritics FlowSorb II 2300. The specific surface areas were used to calculate metal release rates. It should be noted that the specific surface area of the ultrafine powder is considerably larger compared with the other powders.
| 316L powder | Ultrafine | I | II | III | IV |
|---|---|---|---|---|---|
| BET area/m2 g−1 | 0.700 | 0.294 | 0.114 | 0.105 | 0.069 |
In the metal release experiments, powder samples were prepared for exposures (168 h) in the test media at a loading of 0.2 g L−1. This loading was considered as relevant and able to provide reproducible results.27 The powders were weighed using a Mettler AT20 balance with a readability of 2 μg. For the study of metal release from the ultrafine 316L powder particles, 3 replicate samples were prepared for duplicate exposures in each test medium. Accordingly, triplicate powder samples I–IV were exposed in duplicate exposures in each test medium. Triplicate samples of the ultrafine powder and the coarse powder IV were prepared for the consecutive exposure to Gamble’ solution and ALF, and kinetic studies in ALF. All powders were exposed in as-received condition.
Test media
Two synthetic biological media, ALF and Gamble’s solution, were used to simulate different lung fluids, i.e., lysosomal fluid and interstitial fluid of the deep lung, respectively. The chemical composition and pH of the test media are given in Table 5. Citrate in Gamble’s solution replaces proteins, and acetate is added to represent organic acids.26,34,35 These solutions have been used in previous studies of metal release from massive sheets of stainless steel.24| Chemical | ALF | Gamble’s solution |
|---|---|---|
| MgCl2 | 0.0497 | 0.0953 |
| NaCl | 3.210 | 6.0193 |
| KCl | — | 0.2982 |
| Na2HPO4 | 0.071 | 0.126 |
| Na2SO4 | 0.039 | 0.063 |
| CaCl·2H2O | 0.128 | 0.3676 |
| C2H3O2Na·H2O (sodium acetate) | — | 0.7005 |
| NaHCO3 | — | 2.6043 |
| C6H5Na3O7·2H2O (sodium citrate) | — | 0.097 |
| NaOH | 6.000 | — |
| Citric acid | 20.80 | — |
| Glycine | 0.059 | — |
| C6H5Na3O7·2H2O (Na3 citrate·2H2O) | 0.077 | — |
| C4H4O6Na2·2H2O (Na2tartrate·2H2O) | 0.090 | — |
| C3H5NaO3 (Na lactate) | 0.085 | — |
| C3H5O3Na (Na pyruvate) | 0.086 | — |
| pH | 4.5–5 | 7.4 |
Each solution was prepared using ultra-pure water and chemicals of analytical grade. To avoid any risk of metal-containing contamination, all vessels and tools used for mixing were acid cleaned in 10% HNO3 for at least 24 hours, then rinsed four times in ultrapure water and dried in ambient air in the laboratory. The components of Gamble’s solution were added following the order in Table 5 to prevent precipitation of salts. The pH was adjusted using 50% NaOH and with 25% HCl for ALF and Gamble’ solution, respectively.
Exposure condition
The powder samples were exposed in acid-cleaned 15 mL Nalgene® polyethylene flasks (ALF) and 10 mL glass flasks. The vessels were closed and sealed with Parafilm to avoid any leakage. The samples were placed in darkness in a mini-incubator at 37 ± 2 °C, and gently shaken (bi-linearly, about 25 cycles per min) for 1, 4, 8 and 24 h, respectively. The pH of each sample was checked after the samples reached ambient room temperature (an increase in pH by 0.1 units for ALF and 0.4 units in Gamble’s solution was accepted) before separating the powder particles from the test solution by centrifugation for 10 min at 3000 rpm. The resulting supernatant solution could then simply be poured off into a storage flask (Kartell® LDPE). In some cases very fine and light particles were agglomerated and floated on the solution surface due to the relatively high surface tension of the test solution. These particles were carefully removed using acid-cleaned Pasteur pipettes before the solution was transferred into the storage flask. Prior to analysis, the supernatant solution was acidified to a pH less than 2 using 150 μL of 65% ultra-pure HNO3.For the consecutive study in Gamble’s solution and ALF, the powder samples were first exposed in Gamble’s solution for 8 h, and then separated from the test medium by centrifugation according to the procedures described above. The separated powder particles, and a small volume (<1 mL) of Gamble’s solution, which could not be easily removed, were transferred back to the exposure flask, and 10 mL of ALF was added for further exposure of 168 h. After exposure, the test medium was separated from the powder particles by centrifugation and the supernatant was acidified prior to analysis.
Solution analysis
Graphite furnace atomic absorption spectroscopy (GF-AAS), and/or flame absorption spectroscopy (AAS) using a PerkinElmer AAnalyst 800 instrument, were used to analyse released concentrations of Fe, Cr and Ni. The limits of detection were 9 μg L−1, 1.5 μg L−1 and 1.5 μg L−1, for Fe, Cr and Ni, respectively. Mean concentrations were based on three replicate readings of each sample and calibration curves were consecutively verified during analysis. For some samples, analyses were performed with inductively coupled plasma mass spectroscopy (ICP-MS) using a PerkinElmer 6100 DCR instrument at the Environmental Research Laboratory, Department of Forest Ecology, SLU, Umeå. The limits of detection were 60 μg L−1, 4.0 μg L−1 and 2.5 μg L−1 for Fe, Cr and Ni, respectively. However, due to high salt concentrations in the test solutions, mass interferences had to be taken into account. No significant deviations were observed between the two different techniques used. Background concentrations (media reference) were subtracted for each test solution. Metal release rates (expressed as μg cm−2 week−1) were calculated using the BET area. In general, the results are based on 6 replicate samples.Surface analysis
The morphology of the stainless steel powder particles was characterised by scanning electron microscopy (SEM), using a JEOL 840 instrument with a lateral resolution of approximately 20 nm.Results and discussion
Metal release from particles exposed to ALF and Gamble’s solution
Fig. 1 shows metal release rates per unit surface area (based on BET data) from the ultrafine (UF) powder particles exposed to ALF, in comparison with release rates for the coarser powder particles and massive sheet. | ||
| Fig. 1 Metal release rates of Fe, Cr, Ni per unit surface area for 316L powders of varying particle size and massive sheet24 exposed to ALF for one week. Note (1): 5 replicates. | ||
The ultrafine powder exhibits similar release rates of Cr and Ni to the massive sheet, but a significantly higher release rate of Fe. Compared with the more coarse particles (I–IV), the ultrafine powder exhibits somewhat higher release rates. One exception is the release rate of Cr from powder I, which also shows a significantly larger error. The release rate of Fe shows a decreasing trend as the specific surface area decreases (particle diameter increases), and is substantially higher for the ultrafine powder, approximately 2.3 μg cm−2 week−1, compared with the coarser powders and the massive sheet. Since the release rates are normalized to the surface area, the decreasing trend with increasing particle size indicates that also other factors influence the metal release process. One explanation may be that finer particles have a more “active” surface compared with larger particles as a result of a thinner surface oxide film and enhanced segregation of impurities. Moreover, the effective surface area, i.e., the exposed surface area actually exposed to the medium, is not identical to the area measured with BET. This may induce a certain error in the results.
For a quantitative view, steady-state release rates of Fe, Cr and Ni and corresponding concentrations after 1 week of exposure in ALF are compiled in Table 6 for all powders investigated.
| Powder | Ultrafine | I | II | III | IV |
|---|---|---|---|---|---|
| Fe/μg L−1 | 3701.8 ± 462.6 | 1024.3 ± 279.1 | 211.2 ± 56.2 | 174.3 ± 17.7 | 74.4 ± 13.4 |
| (μg cm−2 week−1) | 2.272 ± 0.303 | 1.450 ± 0.251 | 0.785 ± 0.135 | 0.733 ± 0.042 | 0.511 ± 0.083 |
| Cr/μg L−1 | 170.5 ± 17.7 | 80.3 ± 18.9 | 17.5 ± 4.5 | 16.6 ± 2.5 | 8.8 ± 2.3 |
| (μg cm−2 week−1) | 0.105 ± 0.011 | 0.118 ± 0.042 | 0.065 ± 0.007 | 0.069 ± 0.005 | 0.058 ± 0.019 |
| Ni/μg L−1 | 106.5 ± 52.1 | 28.0 ± 7.8 | 9.0 ± 1.7 | 7.8 ± 1.3 | 4.5 ± 1.8 |
| (μg cm−2 week−1) | 0.076 ± 0.033 | 0.039 ± 0.004 | 0.034 ± 0.007 | 0.033 ± 0.007 | 0.029 ± 0.009 |
Based on the amount of total released metal, assuming spherical geometry of the same size, the reduction of the particle size due to metal release was estimated. For an ultrafine powder particle of diameter 2 μm, the metal release results in a particle diameter reduction of ∼13 nm, after one week of exposure. This amount is considered to be negligible despite the highest release rate among the powders.
Release rates per unit area from the different powder particles can be used for comparison with rates from massive sheet of the same material. The concentrations, i.e., the accumulated amounts of the metal released, show a drastic increase with decreasing particle size, which implies that sub-micron particles may give a high amount of released metal per particle loading.
Metal release rates per unit surface area are presented in Fig. 2 for all powder particles exposed in Gamble’s solution. All rates are significantly lower in Gamble’s solution compared with ALF (Fig. 1). The highest release rate of Fe in Gamble’s solution is of similar magnitude to release rates of Cr and Ni in ALF. Measured metal concentrations were often close to or below the detection limits. No evident trend in metal release rate relative to the specific surface area can be observed. The release rate of Fe for the ultrafine powder is lower compared with massive sheet. Also, there is a tendency for the release rate of Fe to be lower compared with the more coarse powders, i.e., contradictory to the findings in ALF. Very low metal release rates in Gamble’s solution are most likely due to the neutral pH and the adsorption of calcium and/or phosphate ions from the solution, which might inhibit metal release from the surface. Precipitation of calcium phosphates has previously been observed on massive sheet, in particular at elevated temperatures (>38 °C) and pH (>8).24 The large difference in release rates between the exposures in ALF (2.5 μg cm−2 week−1) and Gamble’s solution (0.28 μg cm−2 week−1) indicates the importance of using a relevant test medium when assessing differences in metal release rates in different lung environments.
 | ||
| Fig. 2 Metal release rates of Fe, Cr, Ni per unit surface area for 316L powders of varying size and massive sheet24 exposed to Gamble’s solution for one week. Note (1): 5 replicates; (2): 4 replicates; and (3): duplicate samples. Results for the other samples were below the detection limit. | ||
Metal release from particles at a simulated inhalation scenario
With the aim of simulating an actual inhalation scenario (as far as practicable), ultrafine powder particles and coarse powder (IV) particles were consecutively exposed to Gamble’ solution for 8 h and ALF for 168 h. Fig. 3 illustrates the difference in weekly metal release rates in ALF after the 8-h pre-exposure in Gamble’s solution, compared with the corresponding release rates in ALF only.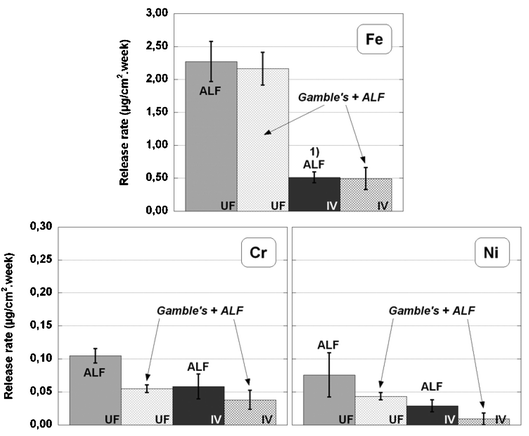 | ||
| Fig. 3 Metal release rates of Fe, Cr, Ni per unit surface area for ultrafine powder and the coarser powder (IV) exposed to Gamble’s solution and ALF in sequence (based on triplicate samples), compared with the release rates in ALF (based on 6 replicates). Note (1): 5 replicates. | ||
Metal release rates for particles consecutively exposed in Gamble’s solution and ALF follow a similar trend related to particle size, as observed for the exposure in ALF, i.e., decreasing rates with increasing particle size (increasing specific surface area). A general observation is that metal release rates are lower for powder particles exposed in Gamble’s solution prior to the exposure in ALF, compared with particles exposed in ALF only. This behaviour was also observed for 316L massive sheet samples.24 Similar to the findings in the previous section, a certain inhibiting effect of the pre-exposure to Gamble’s solution, probably due to adsorption of calcium and phosphate ions, is more pronounced when comparing the results from exposures in Gamble’s solution and ALF in sequence with the results of metal release in ALF. Moreover, the inhibiting effect is evident for Cr and Ni for all powders investigated. For Fe, on the other hand, no pronounced inhibiting effect is observed.
Metal release kinetics
To investigate the kinetic behaviour of the metal release process, exposures of ultrafine powder particles and coarse powder particles (IV) were performed in ALF, Fig. 4. In general, the release rates were initially high, which rapidly declined towards significantly lower steady-state rates. The magnitude of the initial high release rates was significantly higher for all metals released from the ultrafine powder compared with the coarse powder. As an example the release rate of Fe for the ultrafine powder was 96 μg cm−2 week−1 after the first hour of exposure, but decreased to a rate less than 20 μg cm−2 week−1 after 24 h and was approximately 3 μg cm−2 week−1 after one week of exposure. For the coarse powder, the release rate of Fe decreased from about 13 μg cm−2 week−1 after 1 h to 3 μg cm−2 week−1 after 24 h, and finally to a rate of 0.5 μg cm−2 week−1 after 168 h of exposure, i.e., approximately five times lower than the ultrafine powder. Moreover, it takes a longer time for the ultrafine powder to reach steady state release rates compared with the coarse powder. | ||
| Fig. 4 Metal release rates of Fe, Cr, Ni per unit surface area versus time of exposure for the ultrafine powder and the coarse powder (IV) exposed to ALF (triplicates). Note (1): duplicate samples for Cr and Ni results for the coarse powder after 8 h of exposure. | ||
Characteristics of particles
Secondary electron images of the ultrafine powder and powder III are shown in Fig. 5, revealing a substantial difference in particle size between the two powders. It is evident from the SEM images that the ultrafine powder contains a large number of very small (sub-micron) particles, which might not be obvious from results of sieve analysis, based on weight fractions. The surface area of fine powder particles, rather than the mass of the particles, plays an important role in surface-related phenomena and should therefore be considered in the discussion of the metal release process. According to BET measurements the specific surface area of the ultrafine powder is approximately 7 times larger compared with the specific area of powder III. The accumulated amount of released Fe, Cr and Ni from the ultrafine powder is approximately 10–20 times higher when compared with powder III in ALF. Hence, even the release rates per unit area are 2–3 times higher for ultrafine powder (Table 6). On the contrary, when exposed to Gamble’s solution, the metal release rates from powder III are higher compared with the ultrafine powder, as can be seen in Fig. 2. A more “active” surface of ultrafine particles (which leads to an enhanced metal release in ALF) may promote adsorption of inhibiting species such as calcium and/or phosphate ions, and hence reduce metal release in Gamble’s solution. | ||
| Fig. 5 Secondary electron images illustrating the difference in particle size of (a–b) ultrafine powder particles and (c–d) powder III at comparable magnification. | ||
Conclusions
Metal release measurements of stainless steel powder particles were performed in two synthetic lung fluids simulating an inhalation scenario. The results show clearly that the particle size and the test media influence the metal release process. In general, the release rate of Fe per unit surface area is significantly higher compared with Cr and Ni. When exposed to artificial lysosomal fluid (ALF, pH 4.5, simulates conditions at phagocytosis by cells similar to an inflammation), the accumulated amounts of released metal increase drastically with decreasing particle size. The release rate of Fe is the highest for the ultrafine 316L powder, and decreases with increasing particle size. The ultrafine powder shows about the same release rates of Cr and Ni as the massive sheet sample. In Gamble’s solution (pH 7.4, similar to normal health conditions in the lung compartment), metal release rates are significantly lower compared with ALF for all powders investigated. No clear trend related to the particle size was observed. It appears that the ultrafine particles are more active in terms of metal release and adsorption of inhibiting species in the medium. The inhibiting effect of Gamble’s solution was also evident for ultrafine and coarse powder particles exposed consecutively to Gamble’s solution and ALF. The effect was more pronounced for Cr and Ni. Moreover, the release rates are initially high and decline rapidly with exposure time towards a relatively lower steady-state level. For ultrafine powder particles, the metal release rates decline more slowly towards steady-state conditions compared with the coarser powder particles.Despite very simplified experimental conditions, which are far from a realistic inhalation scenario, the results indicate low metal release rates from stainless steel particles when inhaled at normal health conditions. At lung conditions similar to an inflammation, release rates are significantly higher and the worst-case scenario seems to happen for the smallest particles. However, a realistic inhalation scenario covers a broad spectrum of complex processes that most probably resembles a condition in between the two synthetic lung fluids studied.
Acknowledgements
Financial support from International Stainless Steel Forum (ISSF) is highly appreciated. The authors are grateful to Anders Magnusson at Kanthal AB, Sweden, for performing the BET analyses and Hans Bergqvist for the SEM. Thanks also to Gunilla Herting (KTH) for results on massive 316L, to Staffan Malm and Patricia Koundakjian (ISSF) and Tony Newson (Eurofer) for help and valuable discussions.References
- B. Brunekreef and B. Forsberg, Eur. Respir. J., 2005, 26(2), 309 CrossRef CAS.
- W. J. Gauderman, E. Avol, F. Gilliland, H. Vora, D. Thomas, K. Berhane, R. McConnell, N. Kuenzli, F. Lurmann, E. Rappaport, H. Margolis, D. Bates and J. Peters, N. Engl. J. Med., 2004, 351(11), 1057 CrossRef CAS.
- A. Seaton, W. MacNee, K. Donaldson and D. Godden, Lancet, 1995, 345, 176 CrossRef CAS.
- B. Dellinger, W. A. Pryor, R. Cueto, G. L. Squadrito, V. Hegde and W. A. Deutsch, Chem. Res. Toxicol., 2001, 14, 1371 CrossRef CAS.
- A. J. Ghio, J. Stonehuerner, L. Dailey and J. Carter, Inhalation Toxicol., 1999, 11(1), 37 Search PubMed.
- H. L. Karlsson, L. Nilsson and L. Möller, Chem. Res. Toxicol., 2005, 18(1), 19 CrossRef CAS.
- M. R. Riley, D. E. Boesewetter, R. A. Turner, A. M. Kim, J. M. Collier and A. Hamilton, Toxicol. in vitro, 2005, 19, 411 CrossRef CAS.
- T. L. Ogden, J. Aerosol Sci., 1992, 23, S445 CAS.
- ISO 7708:1995, Air quality—Particle size fraction definitions for health-related sampling.
- G.-C. Fang, Y.-S. Wu, P-C. Fu, C.-N. Chang, M.-H. Chen, T.-T. Ho, S.-H. Huang and J.-Y. Rau, Atmos. Res., 2005, 75, 1 CrossRef CAS.
- R. M. Harrison and J. Yin, Sci. Total Environ., 2000, 249, 85 CrossRef CAS.
- S. D. Machemer, Environ. Sci. Technol., 2004, 38(2), 381 CrossRef CAS.
- L. E. Murr, E. V. Esquivel and J. J. Bang, J. Mater. Sci.: Mater. Med., 2004, 15, 237 CrossRef CAS.
- I. Y. R. Adamson, H. Prieditis and R. Vincent, Toxicol. Appl. Pharmacol., 1999, 157, 43 CrossRef CAS.
- R. B. Hetland, F. R. Cassee, M. Låg, M. Refsnes, E. Dybing and P. E. Schwarze, Part. Fibre Toxicol., 2005, 2(4) Search PubMed , DOI: 10.1186/1743-8977-2-4.
- A. L. Lambert, W. Dong, M. J. K. Selgrade and M. I. Gilmour, Toxicol. Appl. Pharmacol., 2000, 165, 84 CrossRef CAS.
- J. D. McNeilly, M. R. Heal, I. J. Beverland, A. Howe, M. D. Gibson, L. R. Hibbs, W. MacNee and K. Donaldson, Toxicol. Appl. Pharmacol., 2004, 196, 95 CrossRef CAS.
- L. Merolla and R. J. Richards, Exp. Lung Res., 2005, 31(7), 671 CrossRef CAS.
- L. E. Pascal and D. M. Tessier, Toxicol. Lett., 2004, 147, 143 CrossRef CAS.
- A. J. Fernández Espinosa, M. Ternero Rodríguez, F. J. Barragán de la Rosa and J. C. Jinénez Sánchez, Atmos. Environ., 2002, 36, 773 CrossRef CAS.
- W. Roemer, G. Hoek, B. Brunekreef, J. Clench-Aas, B. Forsberg, J. Pekkanen and A. Schutz, Eur. Respir. J., 2000, 15(3), 553 CrossRef CAS.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51(7), 844 CrossRef CAS.
- V. R. Christensen, S. Lund Jensen, M. Guldberg and O. Kamstrup, Environ. Health Perspect., 1994, 102, 83 CAS.
- G. Herting, I. Odnevall Wallinder and C. Leygraf, Corros. Sci., 2006 Search PubMed , DOI: 10.1016/j.corsci.2006.05.008.
- S. M. Mattson, Environ. Health Perspect., 1994, 102, 87 CAS.
- W. Stopford, J. Turner, D. Cappelini and T. Brock, J. Environ. Monit., 2004, 5, 675 Search PubMed.
- K. Midander, J. Pan and C. Leygraf, Corros. Sci., 2006, 48, 2855 CrossRef CAS.
- K. Midander, I. Odnevall Wallinder and C. Leygraf, Environ. Pollut., 2007, 145, 51 CrossRef CAS.
- C. R. Clayton and I. Olefjord, in Corrosion Mechanisms in Theory and Practice, ed. P. Marcus, Marcel Dekker Inc., New York, 2nd edn, 2002, pp. 217–241 Search PubMed.
- B. MacDougall and M. J. Graham, in Corrosion Mechanisms in Theory and Practice, ed. P. Marcus, Marcel Dekker Inc., New York, 2nd edn, 2002, pp. 189–216 Search PubMed.
- E. Denkhaus and K. Salnikov, Crit. Rev. Oncol. Hemat., 2002, 42, 35 Search PubMed.
- M. Costa and C. B. Klein, Crit. Rev. Toxicol., 2006, 36(2), 155 Search PubMed.
- L. Nyborg, M. Norell and I. Olefjord, Surf. Interface Anal., 1992, 19, 607 CrossRef CAS.
- A. T. Kuhn and T. Rae, in The Use of Synthetic Environments for Corrosion, Testing ASTM STP, ed. P. E. Francis and T. S. Lee, American Society for Testing and Materials, Philadelphia, 1988, pp. 79–97 Search PubMed.
- O. R. Moss, Health Phys., 1979, 36, 447 CAS.
| This journal is © The Royal Society of Chemistry 2007 |
