Comparative evaluation of a bioluminescent bacterial assay in terrestrial ecotoxicity testing
D.
Trott
*a,
J. J. C.
Dawson
a,
K. S.
Killham
ab,
Md. R. U.
Miah
a,
M. J.
Wilson
a and
G. I.
Paton
ab
aSchool of Biological Sciences, Cruickshank Building, St. Machar Drive, University of Aberdeen, Aberdeen, UK AB24 3UU. E-mail: d.trott@abdn.ac.uk; Fax: 44 (0)1224 272703; Tel: 44 (0)1224 272269
bRemedios Limited, Balgownie Technology Centre, Aberdeen Science and Technology Park, Balgownie Drive, Aberdeen, UK AB22 8GW
First published on 22nd November 2006
Abstract
Despite the widespread and successful use of luminescence-based bioassays in water testing, their applications to soils and sediments is less proven. In part this is because such bioassays have mainly been carried out in an aqueous-based medium and, as such, favour contaminants that are readily water-soluble. In this study, aqueous solutions and soils contaminated with heavy metals (HM), polar organic contaminants and hydrophobic organic contaminants (HOCs) were tested using a range of luminescence-based bioassays (Vibrio fischeri, Escherichia coli HB101 pUCD607 and Pseudomonas fluorescens 10586r pUCD607). For the first two chemical groups, the assays were highly reproducible when optimised extraction procedures were employed but for HOCs the bioassay response was poor. Quantitative structure–activity relationships (QSARs ) obtained from aqueous solutions had a linear response although correlation for the chemicals tested using bacterial bioassays was significantly less sensitive than that of sublethal tests for Tetrahymena pyriformis. Bacterial and Dendrobaena venetabioassay responses to extracts from HM amended soils showed that a clear relationship between trophic levels could be obtained. There is no doubt that the wide range of bioluminescent-based bioassays offers complementary applications to traditional testing techniques but there is a significant need to justify and optimise the extraction protocol prior to application.
Introduction
The use of the naturally bioluminescent marine bacterium Vibrio fischeri to assess toxicity was developed in the 1970s and made commercially available as the Microtox® assay.1 However, there have been concerns about the applicability of this bioassay to terrestrial environmental samples as assays making use of Vibrio based bioluminescence sensors require that the test be performed under saline conditions. This may alter the bioavailability of pollutants when compared with field conditions. This problem may be overcome by the use of ecologically relevant bacteria that have been genetically modified to include bioluminescent properties.Since their initial conception, these tests have been widely used in determining the toxicity of inorganic, polar organic and hydrophobic organic chemicals in waters, soils and sediments. In general, the test is a low cost, high throughput technique for environmental sample analysis.2 Many authors have reported that the bacterial bioassay can be interpreted as an indication of the bioavailable fraction of a given contaminant in a selected matrix. Ecotoxicological research has been conducted on other organisms such as nematodes, earthworms and fish.3–5 However, the crucial factor is whether or not there is relationship between these bioassays and their environmental relevance.
Cronin and Schultz proposed the use of QSARs for ecotoxicity tests on a range of organic compounds.6 Compounds with the same mode of action create a biological response related to physico-chemical differences of the molecules. This strategy is widely adopted to develop and validate new assays and to compare between assays and across trophic levels. While QSARs may be applicable in aqueous systems, this may not be the case with other environmental matrices.
Many different extraction techniques have been developed as researchers attempt to obtain samples that are chemically and environmentally relevant. For unsaturated soils, two water-based techniques are commonly used, centrifugation and porous membrane extraction.
Pore water can be removed quickly and easily from most soils by centrifugation, although some soil types can be more problematical and the method may not be suitable for in situ extractions.7–11 Porous membrane techniques can remove water from large volumes of soil where centrifugation is unsuitable.10,11 Filamentous porous membrane samplers (Rhizons) have also been used to extract samples under a given matric suction from re-packed soils in pots.12 Tiensing et al. compared centrifugation and Rhizon sampler approaches and acknowledged that both were suitable for metal application.13
Water is often an ineffective extraction solvent for organic compounds. To this end co-solvency with either an alcohol or dimethylsulfoxide (DMSO) has been proposed, as this increases the pollutant recovery, yet is miscible in water and compatible with luminescence based bacterial bioassays .14,15 This approach has been shown to work for a wide range of contaminants including chlorophenols, herbicides, organotins, naphthalene and pesticides.16–21 Non-exhaustive extraction techniques (NEETs) have been used to extract PAHs from soil with mixed success.19,21
The aim of this paper was to compare the response of bioluminescent bacterial assays to those of higher organisms in order to evaluate their relative merits in terrestrial ecotoxicity testing.
Materials and methods
Bioassay methodology
Ecotoxicity testing of heavy metals (HM), polar and hydrophobic organic compounds was performed using three different bioluminescent bacteria; a naturally luminescent marine organism, Vibrio fischeri and 2 genetically modified lux-based organisms Escherichia coli HB101 pUCD607 and Pseudomonas fluorescens 10586r pUCD607.22,23 All bioassays were performed either in water or in environmental extracts from soils. The luminescence emitted by each of the bacteria was measured at a wavelength of 550 nm using a Bio-Jade luminometer (Labtech International, UK).Cultures of the three bacteria were freeze-dried using laboratory protocols and stored at –20 °C.24 Freeze-dried V. fischeri were defrosted at room temperature for 10 min, 1 mL of artificial seawater was added to each vial and the contents incubated at 22 °C for 10 min at 200 rpm on an orbital incubator shaker, prior to use in the bioassay . Freeze dried cells of E. coli and P. fluorescens were resuscitated by adding 10 mL sterile 0.1 M KCl and placed on an orbital incubator shaker set at 25 °C and 200 rpm for 1 h or 20 min, respectively, prior to use.
In the V. fischeribioassay , a 100 µL aliquot of the resuscitated cells was added to 900 µL of aqueous or soil extract test solutions and 100 µL of a 22% NaCl solution, at 20 s intervals in a 4 mL luminometer cuvette. After an exposure time of 10 min, luminescence was measured. For the E. coli and P. fluorescensbioassay , 100 µL aliquots of the resuscitated cells were added to 900 µL of aqueous or soil extract test solutions at 15 s intervals and luminescence of the samples measured after 15 min exposure time. Three independent replicates of each assay were performed for each aqueous or soil extract and data were expressed as percentage of a control luminescence (non-contaminated).
Heavy metal and organic compounds in aqueous solution
V. fischeri was used to determine toxicity of a range of HM and organic compounds in water extracts. For HM the metal salt was made up in water. For organic compounds in aqueous test solutions, methanol was used as a cosolvent .14QSARs were produced based on the light inhibition of V. fischeri (biological activity) to log Kow (physico-chemical property). The octanol–water partitioning constant (log Kow) of a chemical is a measure of the equilibrium of that chemical between octanol and water. The values used were obtained from Sangster Research Laboratories (http://logKow.cisti.nrc.ca/logKow).Heavy metal and organic compounds in soil extracts
Extracts from three soils were selected for determining ecotoxicity responses by all three biosensors. Insch—a freely draining sandy loam from improved grassland of the Insch Association with pH 6.32, 3.8% organic matter and 11.5% clay content; (ii) Cruden Bay—an imperfectly drained clay loam, agricultural soil of the Tipperty Association with pH 6.05, 7.4% organic matter and 24.3% clay content and (iii) Boyndie—a freely drained loamy sand agricultural soil of the Boyndie Association with pH 5.50, 2.5% organic matter and 6.5% clay content. The soils were sieved to 2 mm removing stones and visible plant debris and stored at 4 °C prior to amendment with the chemical of concern.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg Zn kg–1) and copper (Cu(NO3)2·3H2O to achieve final soil concentrations of 100, 200, 400 and 1000 mg Cu kg–1). The earthworm assays were performed as Spurgeon and Hopkin but using 40–60 mm Dendrobaena veneta.4 After 28 days exposure, the earthworms were removed from the soil, rinsed with deionised water and allowed to depuriate on filter paper for 2 days before being killed by freezing. The dead earthworms were then oven-dried at 60 °C for 24 h, weighed and ground immediately after removal from the oven; digested using perchloric/sulfuric acid; diluted to 2% acid and analysed using graphite furnace atomic absorption spectroscopy (PerkinElmer AAnalyst 300). Rhizon soil moisture samplers (Rhizosphere Research Products, Holland) were used to extract the interstitial soil pore-waters used for the bioassays .13,25
000 mg Zn kg–1) and copper (Cu(NO3)2·3H2O to achieve final soil concentrations of 100, 200, 400 and 1000 mg Cu kg–1). The earthworm assays were performed as Spurgeon and Hopkin but using 40–60 mm Dendrobaena veneta.4 After 28 days exposure, the earthworms were removed from the soil, rinsed with deionised water and allowed to depuriate on filter paper for 2 days before being killed by freezing. The dead earthworms were then oven-dried at 60 °C for 24 h, weighed and ground immediately after removal from the oven; digested using perchloric/sulfuric acid; diluted to 2% acid and analysed using graphite furnace atomic absorption spectroscopy (PerkinElmer AAnalyst 300). Rhizon soil moisture samplers (Rhizosphere Research Products, Holland) were used to extract the interstitial soil pore-waters used for the bioassays .13,25
The soil was allowed to equilibrate for 24 h following amendments, after which 25 g of each sample were placed in replicate Universal bottles. The soil samples, maintained at 80% water holding capacity were left in the dark at 15 °C for 1 week prior to extraction. Samples were extracted by centrifugation in either water or 100% methanol, which was subsequently diluted with deionised water for the bioassay .26 Confirmatory chemical analysis was performed by GC -MS (organotins); HPLC (PAHs, chlorophenol and herbicides) or GC -FID (refined hydrocarbons ).13,18,25,27,28 Dose response curves were developed against the measured total concentration in the soil prior to extraction. EC20 values (20% decline in luminescence) were derived for each compound.
Environmental assessment of mixed contaminants in soils
The three bioassays were used to assess toxicity of extracts from soils historically contaminated with a range of pollutants from a former coal tar works site. One hundred and ninety-two samples were collected in glass jars, sealed and transported to the laboratory and stored at 5 °C before use. Soils were extracted by weighing 5 g of sample into Wheaton vials; 25 mL of 100% methanol was added; vials were shaken for 16 h and the contents left to settle before pipetting the methanol extract into a clean Wheaton vial. For the bioassay , an aliquot of the methanol extract was diluted to 0.5%. This concentration was found to be most suitable for determining an optimised response between the three bioassays .A hexane extraction was undertaken on each methanol extract solution to investigate if the hydrocarbon (HC) concentration could be related to the bioassay responses. For this, 4.9 mL of the methanol extract were removed and placed in a clean Wheaton vial; 0.1 mL of extraction standard (40 g L–1 of decane in methanol) and 10 mL of hexane added and the samples shaken for 10 min. After partitioning, 4.9 mL of the hexane fraction was taken, 0.1 mL of internal standard added (40 g L–1 of squalane in hexane) for analytical calibration and total hydrocarbon determined by GC -FID.28
Statistical analysis
Statistical analyses were carried out using Minitab. Using Sigmaplot, EC20 and EC50 values were estimated fitting data to sigmoidal curves and QSARs by linear regression. Tests of significance were carried out with ANOVA and Fisher’s least significant difference (LSD) among the means. For all statistical tests, differences were regarded as significant at p ≤ 0.05.Results and discussion
Heavy metal and organic compounds in aqueous solution
The ecotoxicity of V. fischeri (EC50 values) to Zn and Cu in aqueous solutions were 1.9 and 11.0 mg L–1, respectively (7 doses tested, n = 3). Comparative data in the literature gave EC50 values for E. coli of 0.40 (Zn) and 0.36 (Cu) mg L–1, and P. fluorescens values of 0.09 (Zn) and 0.09 (Cu) mg L–1.29 The data obtained using V. fischeri showed that this bioassay was less sensitive to Zn and Cu than the other assays. Variation in sensitivity to contaminants between bacterial biosensors is common and may be due to factors such as metal speciation and membrane permeability.30The relationship between the V. fischeribioassay and the non-polar organic compounds may be explained by QSARs .6 The QSAR in this work was derived by comparing the EC50 values of a range of organic compounds to the physical constant Kow, the mode of action being non-polar narcosis. The compounds used were toluene (log Kow = 2.73), benzene (log Kow = 2.13), m-xylene (log Kow = 3.2), 1-pentanol (log Kow = 1.51), 2-pentanone (log Kow = 0.84) and 2-butanone (log Kow = 0.29). When plotted (Fig. 1), a graph of 1/(log EC50 mol) against log Kow produced a strong correlation (R2 = 0.87) for a range of non-polar narcotic compounds (alcohols, single aromatic and ketones ) as opposed to a single group (ketones ), as described in Cronin and Schultz.6
 | ||
| Fig. 1 Quantitative structure–activity relationships between log (1/EC50) against log Kow using V. fischeri (□) compared with T. pyriformis (■) Schultz (1997). | ||
Fig. 1 also presents the V. fischeribioassay data together with previously published toxicity data on a range of non-polar narcotic compounds developed from a freshwater ciliate (Tetrahymena pyriformis).5 The compounds selected were heptanol (log Kow = 2.62), naphthalene (log Kow = 3.35), 1-pentanol (log Kow = 1.51), 2-pentanone (log Kow = 0.84), 2-butanone (log Kow = 0.29) and 2-tridecanol (log Kow = 5.82). The responses between these two organisms were different due to many interacting factors including the test procedure and the physiology of the organisms used. T. pyriformisbioassays were conducted in freshwater, while V. fischeri was conducted in a saline solution. However, the results demonstrated that the use of bacteria in toxicity assessment could be justified as an indicator of physico-chemical parameters and related to the response of another biological assay.
Heavy metal and organic compounds in soil extracts
The measurement of body burden of metals in earthworm is a genuine assessment of bioavailability of HM in soils. The effect of Zn followed a logarithmic dose response to the earthworm; the dose required was much greater than for Cu. Heavy metal partitioning in the three soils could be explained by the binding affinity of the soil for the Zn and Cu amendments.29,31 The Cu also had a greater impact in terms of earthworm body burden than the Zn and to some extent, a lesser impact on the bacterial bioassay . The actual response, the lethality index of the earthworm and levels of accumulated Zn and Cu in this work were similar to those reported by Spurgeon and Hopkin.4
In this part of the study, the objective was to relate the response of the bacterial bioassay to earthworm bioaccumulation. When the body burden of assimilated metal was plotted against the bioassay luminescence, a clear non-linear correlation was found for both Zn (Fig. 2a) and Cu (Fig. 2b). To some extent this work served to validate the efficacy of the Rhizon sampling technique. The procedure of pore-water extraction mimics the mechanistic transfer of soil water pollutants to the earthworm. This in turn meant that the accumulated HM that partitions into the earthworm was correlated with the aqueous fraction that caused the change in bioluminescence response. Although Tandy et al. and Paton et al. have used the concept of bioassay surrogacy by relating the response with other measurable soil biological parameters, these results actually correlated the bacterial bioassay with a standard higher soil organism ecological assay.32,33 While luminescence based bioassays are widely used in soil HM analyses, this is the first direct correlation between these methods and a higher organism assay.
 | ||
| Fig. 2 The percentage of luminescence response of E. coli against accumulation of (a) Zn and (b) Cu in the body tissue of earthworms for Cruden Bay (△) Insch (○) and Boyndie (●) soils. | ||
| Aqueous extract | Methanol extract | |||||
|---|---|---|---|---|---|---|
| E. coli | P. fluorescens | V. fischeri | E. coli | P. fluorescens | V. fischeri | |
| Letter suffixes indicate significant differences (p ≤ 0.05) within each group and across extraction techniques. ANOVA was not carried out between groups of compounds. | ||||||
| (1) Chlorophenols | ||||||
| 2,4-DCP | 1.76a | 1.54a | 5.14b | 0.53c | 0.47c | 3.22d |
| 2,3,5-TCP | 3.71d | 3.25d | 4.11e | 0.74c | 0.41c | 1.68a |
| PCP | 1.47a | 1.55a | 3.41d | 0.47c | 0.39c | 1.78a |
| (2) Herbicides | ||||||
| Atrazine | 68.1a | 59.7a | 71.4a | 2.14b | 1.95b | 1.89b |
| Diuron | Nr | Nr | Nr | Nr | Nr | Nr |
| Mecoprop | 65.1a | 63.7a | — | 2.98b | 3.08b | — |
| Paraquat | Nr | Nr | Nr | 21.4c | 23.49c | 58.9a |
| (3) Organotins | ||||||
| TBT | — | — | — | 1.45a | 1.29a | 0.14b |
| TPT | — | — | — | 5.47c | 6.02d | 0.37b |
| (4) PAHs | ||||||
| Naphthalene | 121a | 132a | 185b | 34.1c | 28.7c | 33.7c |
| Pyrene | Nr | Nr | Nr | 187b | Nr | 175b |
| Phenanthrene | Nr | Nr | Nr | 202b | Nr | 275c |
| Benzo[a]pyrene | Nr | Nr | Nr | Nr | Nr | Nr |
| (5) Refined HC | ||||||
| Diesel | Nr | Nr | Nr | 12.132a | 11.185a | Nr |
| Motor oil | Nr | Nr | Nr | 8.434b | 7.137b | Nr |
| Lubricating oil | Nr | Nr | Nr | Nr | Nr | Nr |
| Polyaromatic olephins | Nr | Nr | Nr | 6.182b | 3.155b | 8.748b |
| Aqueous extract | Methanol extract | |||||
|---|---|---|---|---|---|---|
| E. coli | P. fluorescens | V. fischeri | E. coli | P. fluorescens | V. fischeri | |
| Letter suffixes indicate significant differences (p ≤ 0.05) within each group and across extraction techniques. ANOVA was not carried out between groups of compounds. | ||||||
| (1) Chlorophenols | ||||||
| 2,4-DCP | 3.22a | 2.81a | 4.57b | 0.87c | 0.65c | 1.12d |
| 2,3,5-TCP | 4.11b | 3.97ab | 5.72e | 0.98c | 0.84c | 1.24d |
| PCP | 2.91a | 3.12a | 4.17ab | 0.76c | 0.54c | 0.89cd |
| (2) Herbicides | ||||||
| Atrazine | 74.2a | 68.4a | 112b | 1.21c | 2.01c | 17.4d |
| Diuron | Nr | Nr | Nr | Nr | Nr | Nr |
| Mecoprop | 112a | 114.8a | — | 12.3e | 14.7e | — |
| Paraquat | Nr | Nr | Nr | 47.5f | 54.2f | 61.5a |
| (3) Organotins | ||||||
| TBT | 11.2a | 21.5b | 9.41a | 2.77c | 3.81c | 0.32d |
| TPT | 24.8e | 41.2f | 11.5a | 6.84g | 8.54ag | 1.12d |
| (4) PAHs | ||||||
| Naphthalene | 145a | 197b | 113c | 61.2d | 82.6cd | 64.2d |
| Pyrene | Nr | Nr | Nr | 192a | Nr | 189b |
| Phenanthrene | Nr | Nr | Nr | 288a | Nr | 245c |
| Benzo[a]pyrene | Nr | Nr | Nr | Nr | Nr | Nr |
| (5) Refined HC | ||||||
| Diesel | Nr | Nr | Nr | 18.147a | 17.164b | Nr |
| Motor oil | Nr | Nr | Nr | 12.457c | 13.174d | Nr |
| Lubricating oil | Nr | Nr | Nr | Nr | Nr | Nr |
| Polyaromatic olephins | Nr | Nr | Nr | 9.145e | 6.142f | 8.321e |
In the case of the chlorophenols, E. coli and P. fluorescens responses were similar regardless of the soil type and both bioassays were more sensitive (lower EC20) than the V. fischeribioassay . Tiensing et al. and Sinclair et al. reported a similar response of a range of bacterial bioassays to a suite of chlorophenols.16,20 In general, the response of the three bioassays to herbicides was related to each particular chemical. V. fischeri was significantly less sensitive to paraquat; atrazine sensitivity was reduced in Insch soil but not in Boyndie and an EC20 was not reached for Diuron in any assay. By contrast, V. fischeri was significantly more sensitive to the organotin compounds; results being similar to those reported by Paton et al.18 The EC20 values for PAHs (not including naphthalene) and refined hydrocarbons were not reached for the aqueous extracts.
Semple et al. reviewed the parameters that determine the bioavailability of hydrophobic compounds in soils and it is likely that the results reflected extraction protocols.34 This was due to a combination of compound hydrophobicity, reducing the effectiveness of an aqueous extract, and a lack of sensitivity of the bioassay to the extracted organic compound. However, in the methanol extracts for the refined oils, V. fisheri was again less sensitive. For the aqueous extraction, the EC20 values for naphthalene were lower for V. fischeri than the other two bioassays using Insch soil but higher in Boyndie.
Organotin compounds have agrochemical value and as such may be found in soil, but they are also associated with antifouling paints and as such are common in harbour environments. Their chemical analysis can be complex and costly, hence a biological assay may be a suitable, high throughput and cost effective alternative.18 Unfortunately these bioassays were too insensitive for regulatory needs (by nearly three orders of magnitude), but they did demonstrate responsiveness to the chemicals of concern. It is likely that V. fisheri was physiologically more receptive to these groups of compounds, as physical aspects associated with the more saline conditions of the assay are unlikely to alter the mobility and bioavailability of these chemicals.
The assays were not very responsive to the PAHs tested other than naphthalene. Reid et al. reported the lack of sensitivity of these assays for such hydrophobic compounds in aqueous samples and as a consequence these soil results reinforced this finding.21 When these PAHs are interpreted in the context of the QSAR, it is hardly surprising that naphthalene was the only compound causing a clear response, as the high Kow values for the other compounds demonstrated their capacity for being bound to the soil. However, there are an increasing number of researchers using these bioassays for the study of refined hydrocarbons .26,35
In the case of the organic pollutants tested, the extraction technique used was significant. Chlorophenols and certain herbicides gave consistent toxicity responses in either aqueous or methanol extractions. The same was evident for the organotin compounds, where the methanol extraction yielded greater sensitivity, however, the sensitivity was inadequate in the context of regulatory requirements. The bioassays were poor in determining toxicity of PAHs or refined hydrocarbons ; the issues of bioavailability and partitioning of these molecules between solid and liquid phases explained the responses.
Environmental assessment of mixed contaminants in soils
Historically, contaminated sites commonly contain a mixture of unknown pollutants but the bioassays gave an ecologically integrated response to those chemicals extracted. This was dependent on a number of criteria, including the physical properties of the soil/sediment, pollutants present and extractant used.Fig. 3 shows the luminescence of each bacterium (as a percentage of the control) plotted against the weight per gram of total hydrocarbons from each of the 192 samples extracted using the procedure described. The V. fischeri and P. fluorescens responses shown in Fig. 3 indicated a similar range of sensitivities, while E. coli showed an increased detrimental response to the mixture of hydrocarbons . However, there was no direct correlation between bioassay response and hydrocarbon concentration showing that measured toxicity may be due to chemicals that were not transferred into the hexane phase but remained in the methanol phase. This confirms findings from other investigations and indicates that comparing biological toxicity and chemical data can be complex.36,37
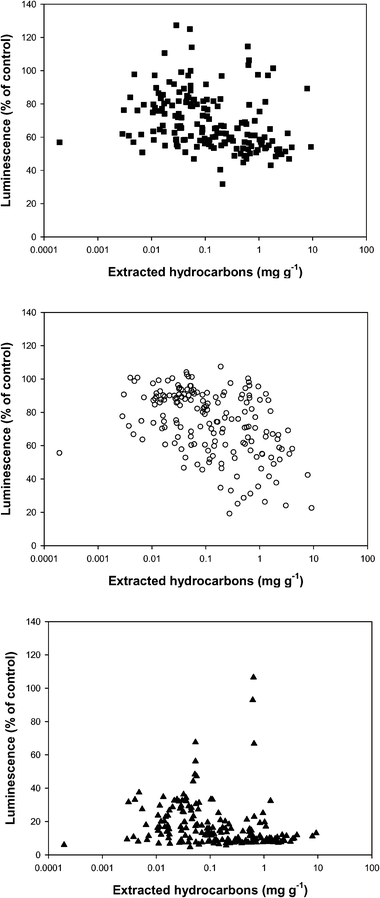 | ||
| Fig. 3 Luminescence as percentage of control (measured in 0.5% methanol extract) against total extractable hydrocarbons (measured in hexane fraction) for V. fischeri (○), P. fluorescens (■) and E. coli (▲) bioassays . | ||
Industrial sites, such as coal tar works, may be contaminated with a wide range of individual compounds, not only those initially present on the site but also their breakdown products. These metabolites may alter toxicity and the interaction of chemicals can be synergistic or additive.38 PAHs are often the major group of contaminants at such sites, although many of the chemicals making up this group exhibit limited toxicity to V. fischeri; it may be that toxicity is attributable to PAHs containing functional groups, which have been found at similar industrial sites.39 The presence of functional groups would increase the polarity of the compound and may result in preferential solution in the methanol phase, so explaining why the total hydrocarbon measurements on the hexane phase did not correlate with toxicity.
Conclusion
The bioassays responded effectively to a wide range of metal doses and to chlorophenols in aqueous solutions and extracts from soils. Co-solvency was essential for testing hydrophobic compounds, but bioassay results using the more hydrophobic compounds, such as PAHs, was still poor. QSARs demonstrated a linear response at the trophic levels investigated. The bacterial and earthworm bioassay responses to extracts from HM amended soils showed that a clear relationship between trophic levels could be obtained. Luminescent-based bacterial bioassays are rapid and easy to apply but without validation and biologically relevant extraction methods they may have poor correlation with some soil-based assays, including those for higher trophic organisms.Acknowledgements
The authors wish to acknowledge Remedios Ltd. and the Engineering and Physical Sciences Research Council for funding the studentship of D. Trott.References
- A. A. Bulich and D. L. Isenberg, Adv. Instrum., 1980, 35, 35–40 Search PubMed.
- S. Parvez, C. Venkataraman and S. Mukherji, Environ. Int., 2006, 32, 265–268 CrossRef CAS.
- G. W. Korthals, M. Bongers, A. Fokkema, T. A. Dueck and T. M. Lexmond, Ecotoxicology, 2000, 9, 219–228 CrossRef CAS.
- D. J. Spurgeon and S. P. Hopkin, Appl. Soil Ecol., 1999, 11, 227–243 Search PubMed.
- T. W. Schultz, Toxicol. Methods, 1997, 7, 289–309 CrossRef CAS.
- M. T. D. Cronin and T. W. Schultz, Ecotoxicol. Environ. Saf., 1998, 39, 65–69 CrossRef CAS.
- E. A. Elkhatib, J. L. Hern and T. E. Staley, Soil Sci. Soc. Am. J., 1987, 51, 578–583 CAS.
- M. Eriksson, A. Swartling and G. Dalhammar, Appl. Microbiol. Biotechnol., 1998, 50, 129–134 CrossRef CAS.
- D. H. Thibault and M. I. Sheppard, Commun. Soil Sci. Plant Anal., 1992, 23, 1629–1641 CrossRef.
- B. Sigfusson, S. R. Gislason and G. I. Paton, J. Geochem. Explor., 2006, 88, 321–324 CrossRef CAS.
- J. Wolt and J. G. Graveel, Soil Sci. Soc. Am. J., 1986, 50, 602–605.
- R. Vulkan, F. J. Zhao, V. Barbosa-Jefferson, S. Preston, G. I. Paton and E. Tipping, Environ. Sci. Technol., 2000, 34, 5115–5121 CrossRef CAS.
- T. Tiensing, S. Presto, N. Strachan and G. I. Paton, J. Environ. Monit., 2001, 3, 91–96 RSC.
- J. G. Bundy, H. Maciel, M. T. D. Cronin and G. I. Paton, Bull. Environ. Contam. Toxicol., 2003, 70, 1–8 CrossRef CAS.
- K. T. Y. Ho and J. G. Quinn, Environ. Toxicol. Chem., 1993, 12, 615–625 CrossRef CAS.
- T. Tiensing, N. Strachan and G. I. Paton, J. Environ. Monit., 2002, 4, 482–489 RSC.
- G. Strachan, S. Preston, H. Maciel, A. J. R. Porter and G. I. Paton, Water Res., 2001, 35, 3490–3495 CrossRef CAS.
- G. I. Paton, W. Cheewasedtham, I. L. Marr and J. J. C. Dawson, Environ. Pollut., 2006, 144, 746–751 CrossRef CAS.
- C. J. Patterson, K. T. Semple and G. I. Paton, FEMS Microbiol. Lett., 2004, 241, 215–220 CrossRef CAS.
- G. M. Sinclair, G. I. Paton, A. A. Meharg and K. Killham, FEMS Microbiol. Lett., 1999, 174, 273–278 CrossRef CAS.
- B. J. Reid, K. T. Semple, C. J. Macleod, H. J. Weitz and G. I. Paton, FEMS Microbiol. Lett., 1998, 169, 227–233 CrossRef CAS.
- E. A. S. Rattray, J. I. Prosser, K. Killham and L. A. Glover, Appl. Environ. Microbiol., 1990, 56, 3368–3374 CAS.
- S. Amin-Hanjani, PhD thesis, University of Aberdeen, 1992.
- S. Sousa, C. Duffy, H. Weitz, L. A. Glover, E. Bär, R. Henkler and K. Killham, Environ. Toxicol. Chem., 1998, 17, 1039–1045 CAS.
- J. J. C. Dawson, H. Maciel, K. T. Semple and G. I. Paton, in Soil and Environmental Analysis: Modern Instrumental Techniques, ed. K. A. Smith and M. S. Cresser, Marcel Dekker, New York, 3rd edn, 2004, ch. 12, pp. 515–542 Search PubMed.
- J. G. Bundy, C. D. Campbell and G. I. Paton, J. Environ. Monit., 2001, 3, 404–419 RSC.
- G. Strachan, S. Capel, H. Maciel, A. J. R. Porter and G. I. Paton, Eur. J. Soil Sci., 2002, 53, 37–44 CrossRef CAS.
- G. I. Paton, C. O. Iroegbu and J. J. C. Dawson, Mar. Pollut. Bull., 2003, 46, 903–906 CrossRef CAS.
- H. Nikaido and M. Vaara, Microbiol. Rev., 1985, 49, 1–32 CAS.
- J. J. C. Dawson, C. D. Campbell, W. Towers, C. M. Cameron and G. I. Paton, Environ. Pollut., 2006, 142, 493–500 CrossRef CAS.
- S. Sauvé, W. H. Hendershot and H. E. Allen, Environ. Sci. Technol., 2000, 34, 1125–1131 CrossRef CAS.
- S. Tandy, V. Barbosa, A. Tye, S. Preston, H. Zhang and S. McGrath, Environ. Toxicol. Chem., 2005, 24, 530–536 CrossRef CAS.
- G. I. Paton, E. R. Viventsova, J. Kumpene, M. J. Wilson, H. Weitz and J. J. C. Dawson, Sci. Total Environ., 2006, 355, 106–117 CrossRef CAS.
- K. T. Semple, A. W. J. Morriss and G. I. Paton, Bioavailability of hydrophobic organic contaminants in soils: fundamental concepts and techniques for analysis, Eur. J. Soil Sci., 2003, 54, 809–818 CrossRef CAS.
- R. Juvonen, E. Martikainen, E. Schultx, A. Joutti, J. Ahtainen and M. Lehtokaris, Ecotoxicol. Environ. Saf., 2000, 47, 156–166 CrossRef CAS.
- G. A. Harkey and T. M. Young, Environ. Toxicol. Chem., 2000, 19, 276–282 CrossRef CAS.
- M. W. Jacobs, J. A. Coates, J. J. Delfino, G. Bitton, W. M. Davis and K. L. Garcia, Arch. Environ. Contam. Toxicol., 1993, 24, 461–468 CrossRef CAS.
- J. E. Bauer and D. G. Capone, Appl. Environ. Microbiol., 1988, 54, 1649–1655 CAS.
- S. Lundstedt, P. Haglund and L. Oberg, Environ. Toxicol. Chem., 2003, 22, 1413–1420 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2007 |
