Ni2(R*COO)4(H2O)(4,4′-bipy)2—a robust homochiral quartz-like network with large chiral channels†
Zhen-Feng
Chen
*a,
Shu-Feng
Zhang
a,
Hai-Sheng
Luo
a,
Brendan F.
Abrahams
*b and
Hong
Liang
*a
aThe Key Laboratory of Medicinal Chemical Resources and Molecular Engineering (Ministry of Education), School of Chemistry and Chemical Engineering, Guangxi Normal University, Guilin, 541004, P. R. China. E-mail: chenzfubc@yahoo.com
bSchool of Chemistry, University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: bfa@unimelb.edu.au
First published on 14th November 2006
Abstract
The generation of a chiral network of composition, Ni2(R*COO)4(H2O)(4,4′-bipy)2 (R*COO− = dehydroabietate), was achieved through the deliberate use of enantiomerically pure carboxylic acid, dehydroabietic acid, under hydrothermal conditions.
Chirality has long held a position of central importance within the general spheres of chemistry, molecular biology, pharmacology and materials science.1 In recent times, chiral coordination polymers have been a very active interdisciplinary research area of increasing interest due to their intriguing potential applications in enantioselective synthesis, asymmetric catalysis and their possible function as porous materials, nonlinear optical materials, and magnetic materials.2 In general, the deliberate synthesis of coordination polymers as enantiomerically pure products may be accomplished by two routes: (i) the generation of enantiopure hosts around a resolved template; and (ii) from the assembly of homochiral building blocks.3 When achiral or racemic ligands are employed to form coordination polymers, bulk racemates typically result.4,5 The use of enantiopure ligands may be expected to lead to the generation of a single enantiomeric product. This synthetic strategy has been successfully employed in the generation of homochiral coordination polymers where the bulk product is a pure enantiomer.2a,6
Pine resin is an abundant renewable material mainly composed of diterpenic resin acids of the general formula C19H29COOH. It has a wide range of industrial uses and is also a source of fine chemicals. Dehydroabietic acid is a natural enantiopure organic ligand, and can be easily obtained by catalytic dehydrogenation of abietic-type resin acids. It is widely used in the synthesis of bioactive compounds,7 but to the best of our knowledge, no metal complexes of the anion have been reported. In our efforts to generate a pure enantiomeric coordination polymer, we have attempted to incorporate the chiral carboxylate anion, dehydroabietate, into a coordination network in which metal centres would be bridged by the commonly employed 4,4′-bipyridine (4,4′-bipy) ligand.
Single-crystals of composition, Ni2(R*COO)4(H2O)(4,4′-bipy)2, 1, were obtained in 62% yield by hydrothermal treatment with dehydroabietic acid, 4,4′-bipyridine, Ni(NO3)2·6H2O, and H2O at 160°C for 7 d (Scheme 1).‡ A single-crystal X-ray diffraction study of 1 reveals the formation of an enantiopure homochiral coordination network constructed from dehydroabietic acid, 4,4′-bipyridine and Ni(NO3)2.§ Compound 1 crystallizes in the chiral trigonal space group P3121. The structure of 1 consists of pairs of Ni(II) centres coordinated by enantiopure chiral dehydroabietate (R*COO−) anions, a water molecule and bridging 4,4′-bipy ligands. Each pair of Ni centres is bound to four R*COO− ligands, two of which span the Ni centres while the other two coordinate in monodentate mode to a single Ni centre as shown in Fig. 1. In addition to the bidentate R*COO− ligands, a bridging water molecule links the metal centres. This binuclear unit is further supported by a pair of hydrogen bonding interactions involving the non-coordinated oxygen atoms of the two monodentate R*COO ligands and the bridging water molecule. Four bridging 4,4′-bipy ligands link this binuclear unit to equivalent units within a 3-D polymeric structure. The coordination environment of the nickel(II) centres may be described as distorted octahedral. The Ni–O (carboxylic and water oxygen) distances fall within the range 2.004(3)–2.086(3) Å. The geometric parameters of dehydroabietate are comparable to those of dehydroabietic acid derivatives.8
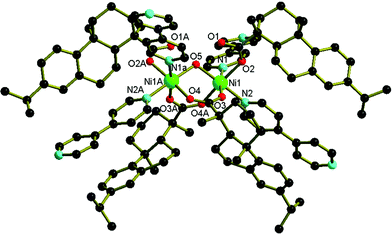 | ||
| Fig. 1 The coordination environment of the binuclear Ni units within Ni2(R*COO)4(H2O)(4,4′-bipy)2. The 4,4′-bipy ligands bridge to Ni atoms belonging to symmetry related binuclear units. The hydrogen atoms are omitted for clarity. Selected bond distances (Å) and angles (°): Ni(1)–O(2) 2.086(3), Ni(1)–O(3) 2.004(3), Ni(1)–O(4A) 2.043(4), Ni(1)–O(5) 2.069(3), Ni(1)–N(1) 2.101(4), Ni(1)–N(2) 2.095(4); O(2)–Ni(1)–O(3) 87.49(15), O(2)–Ni(1)–O(4A) 171.98(13), O(2)–Ni(1)–O(5) 91.26(12), O(3)–Ni(1)–N(1) 172.80(15), O(2)–Ni(1)–N(2) 88.12(15), O(3)–Ni(1)–O(5) 90.71(12), O(4A)–Ni(1)–O(5) 94.86(13), N(1)–Ni(1)–N(2) 90.89(16). (Symmetry code: A x − y, −y, −z + 5/3). | ||
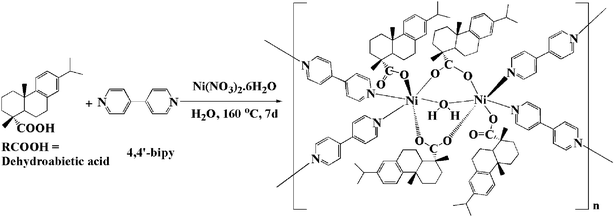 | ||
| Scheme 1 Formation reaction of 1. | ||
From a topological perspective the binuclear unit may be considered as a 4-connecting node with the 4,4′-bipy ligands acting as bridges to equivalent nodes. The resulting network has the topology of the quartz net (see Fig. 2a). Characteristic features of this net include 3-fold axes parallel to the c-axis, the unique axis in a trigonal system. Double 6-fold helices are also apparent in the structure, which are also parallel with the c-axis. While all 3-fold axes are of the same orientation, the 6-fold double helices are of the opposite orientation to the 3-fold axes. The hydrocarbon groups of the carboxylate ligands tend to aggregate around the axes of the 3-fold helices as indicated in Fig. 2b. In contrast, large channels with an almost triangular cross section are associated with the axes of the 6-fold double helices of the ideal net.
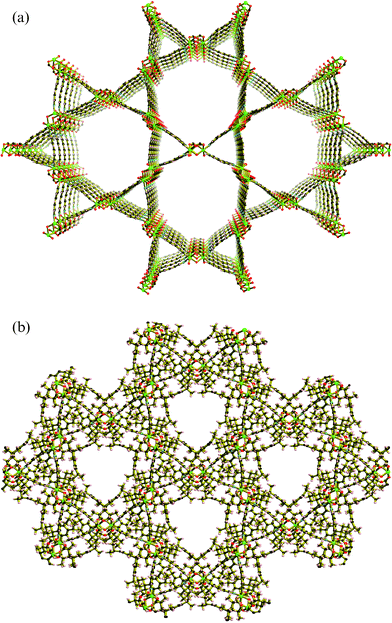 | ||
| Fig. 2 (a) The extended quartz-like network of Ni2(R*COO)4(H2O)(4,4′-bipy)2 with R* groups of the carboxylates and H atoms omitted for clarity. (b) The extended quartz-like network of Ni2(R*COO)4(H2O)(4,4′-bipy)2 with R* groups from the carboxylate included. | ||
Analysis of difference maps reveals that the trigonal channels are effectively empty, with the highest residual peak of electron density less than 0.40 e Å−3. Further analysis of the intraframework voids using the SQUEEZE routine within PLATON9 reveal that a mere 11 electrons occupy a volume of over 2191 Å3 in each unit cell. This represents approximately 26% of the unit cell volume.
Thermogravimetric analysis of 1 (see below) indicates that there is only a small quantity of solvent retained in the crystal, certainly much less than one would expect if the channels were filled with water. Furthermore, the loss of this small amount of solvent is underway at the starting temperature of the thermal analysis. On this basis we believe that solvent loss from the channels commences immediately upon removal of the crystals from solution and continues under ambient conditions until the channels are effectively empty. The loss of water from the channels is certainly not surprising given that the interior surface of the channel is formed from hydrophobic groups. The crystal used in the diffraction study was part of a sample that had been exposed to the atmosphere and so, remarkably, this solvent loss is not accompanied by loss of the single crystal character. Thus the crystals represent an example of a robust homochiral material with large channels.
The quartz net occupies a special position within structural chemistry. Unlike the commonly occurring diamond net, the quartz net is intrinsically chiral, a consequence of the parallel helices which are a central feature of this beautiful, highly symmetric net. Examples of quartz nets often involve a short bridge between two tetrahedral centers as in GaPO4,10 BeF2,11 Co[Au(CN)2]2,12 and stuffed structures such as eukryptite, LiAlSiO4.13 Up to now, there are only limited reports of coordination networks with a quartz-like topology.14–17 In general, these networks have arisen from the use of achiral building blocks and while individual crystals may be chiral, the bulk product is a racemic mixture. In this particular case the use of the chiral carboxylate anion has led to the formation of a single enantiomeric product with the double helices being left-handed. Presumably, the generation of the enatiomeric form with right-handed double helices would require the use of the enantiomeric partner of dehydroabietic acid.
Thermogravimetric analysis shows that 1 undergoes three weight losses upon heating. The first is a 1.7% weight loss by 180 °C, corresponding to loss of water. The second is a 24.8% weight loss completed by 300 °C and corresponding to the loss of two 4,4′-bipy molecules and two –CH(CH3)2 groups of the dehydroabietate ligands (expected 24.2%). Further heating led to a 67.5% weight loss by 400 °C, corresponding to the loss of remaining dehydroabietate ligands and decomposed (expected 67.0%). Finally, the remaining mass of 6.4%, seems likely to correspond to Ni2O3. The formulation of 1 is supported by elemental microanalysis. The IR spectrum of 1 exhibits strong signals at ∼1600 and 1390 cm−1 attributed to the coordinating carboxylate groups. A middle strong peak at ∼3434 cm−1 is consistent with the presence of coordinated water.
Financial support by the National Natural Science Foundation of China (No. 20361002, 30460153), the Natural Science Foundation of Guangxi Province of China (No.0575046, 0575049) and the Program for New Century Excellent Talents in University of the Ministry of Education China (NCET-04-0836) is gratefully acknowledged.
Notes and references
- (a) L. J. Prins, J. Huskens, F. D. Jong, P. Timmermanand and D. N. Reinhoudt, Nature, 1999, 398, 498 CrossRef CAS; (b) J. Chin, S. S. Lee, K. J. Lee, S. Park and D. H. Kim, Nature, 1999, 401, 254 CrossRef CAS; (c) R. Ueshima and T. Asami, Nature, 2003, 425, 679 CrossRef CAS.
- (a) J. S. Seo, D. Whang, H. Lee, S. Jun, J. Ok, Y. Jin and K. Kim, Nature, 2000, 404, 982 CrossRef CAS; (b) R.-G. Xiong, X.-Z. You, B. F. Abrahams, Z. Xue and C.-M. Che, Angew. Chem., Int. Ed., 2001, 40, 4422 CrossRef CAS; (c) Y. Cui, S. J. Lee and W. Lin, J. Am. Chem. Soc., 2003, 125, 6014 CrossRef CAS; (d) S. J. Lee and W. Lin, J. Am. Chem. Soc., 2002, 124, 4554 CrossRef CAS; (e) K. Inoue, K. Kikuchi, M. Ohba and H. Okawa, Angew. Chem., Int. Ed., 2003, 42, 4810 CrossRef CAS; (f) E. Coronado, J. R. Galán-Mascarós, C. J. Gómez-García and J. M. Martínez-Agudo, Inorg. Chem., 2001, 40, 113 CrossRef CAS.
- (a) T. J. Prior and M. J. Rosseinsky, Inorg. Chem., 2003, 42, 1564 CrossRef CAS; (b) Y. Cui, H. L. Ngo and W. Lin, Chem. Commun., 2003, 1388 RSC.
- (a) C. Kaes, M. W. Hosseini, C. E. F. Rikard, B. W. Skelton and A. H. White, Angew. Chem., Int. Ed., 1998, 37, 920 CrossRef CAS; (b) S. Sailaja and M. V. Rajasekharan, Inorg. Chem., 2000, 39, 4586 CrossRef CAS; (c) D. Whang, J. Heo, C.-A. Kim and K. Kim, Chem. Commun., 1997, 2361 RSC; (d) Q. Ye, X.-S. Wang, H. Zhao and R.-G. Xiong, Tetrahedron: Asymmetry, 2005, 16, 1595 CrossRef CAS.
- (a) M. Kondo, M. Miyazawa, Y. Irie, R. Shinagawa, T. Horiba, A. Nakamura, T. Naito, K. Maeda, S. Utsuno and F. Uchida, Chem. Commun., 2002, 2156 RSC; (b) A. Erxleben, Inorg. Chem., 2001, 40, 412 CrossRef CAS; (c) A. Erxleben, Inorg. Chem., 2001, 40, 2928 CrossRef CAS; (d) Q. Sun, Y. Bai, G. He, C. Duan, Z. Lin and Q. Meng, Chem. Commun., 2006, 2777 RSC.
- (a) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483 CrossRef CAS; (b) B. Kesanl and W. Lin, Coord. Chem. Rev., 2003, 246, 305 CrossRef CAS.
- A. J. D. Silvestre, S. M. C. Monsteiro, A. M. S. Silva, J. A. S. Cavaleiro, V. M. S. Félix, P. Ferreira and M. G. B. Drew, Monatsh. Chem., 1998, 129, 1183 CrossRef CAS.
- (a) Y.-M. Pan, Y. Zhang, H.-S. Wang, B.-H. Tong, Z.-F. Chen and Y. Zhang, Acta Crystallogr., Sect. E: Struct. Rep. Online , 2005, 61, o3003 CrossRef; (b) F.-Y. Li, Y.-M. Pan, H.-S. Wang, Z.-F. Chen and Y. Zhang, Acta Crystallogr., Sect. E: Struct. Rep. Online , 2006, 62, o1895 CrossRef.
- PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, A. L. Spek, 1998.
- O. Baumgartner, A. Preisinger, P. W. Krempi and H. Mang, Z. Kristallogr., 1984, 168, 83 CAS.
- A. F. Wright, A. N. Fitch and A. C. Wright, J. Solid State Chem., 1988, 73, 298 CrossRef CAS.
- S. C. Abrahams, L. E. Zyontz and J. L. Bernstein, J. Chem. Phys., 1982, 76, 5458 CrossRef CAS.
- H. G. F. Winkler, Acta Crystallogr., 1948, 1, 27 CrossRef.
- B. F. Hoskins, R. Robson and N. V. Y. Scarlett, Angew. Chem., Int. Ed. Engl., 1995, 34, 1203 CrossRef CAS.
- J. Sun, L. Weng, Y. Zhou, J. Chen, Z. Chen, Z. Liu and D. Zhao, Angew. Chem., Int. Ed., 2002, 41, 4471 CrossRef CAS.
- S. Hu and M.-L. Tong, Dalton Trans., 2005, 1165 RSC.
- A. Wiesch and K. Bluhm, Z. Naturforsch., B: Chem. Sci., 1998, 53, 157 CAS.
- Programs for crystal structure analysis (Release 97-2) G. M. Sheldrick, Institut fur Anorganische Chemie der Universitat, Tammanstrasse 4, D-3400 Gottingen, Germany.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental details. See DOI: 10.1039/b613047j |
| ‡ Synthesis of 1: Dehydroabietic acid (0.600 g, 2 mmol), 4,4′-bipyridine (0.156 g, 1 mmol) and Ni(NO3)2·6H2O (0.290 g, 1 mmol), 15 mL of H2O were placed in a Teflon-lined stainless steel vessel (25 mL) which was sealed and heated at 160°C for 7 d to form green block crystals with only one phase in 62% yield based on 4,4′-bipyridine. Elemental analysis for 1: C100H126N4Ni2O9,calc.: C 72.99%, H 7.72%, N 3.40%, found: C 73.07%, H 7.63%, N 3.32%. IR data (KBr,cm−1): 3434m, 2959w, 2928w, 1604s, 1535w, 1488w, 1390s, 1362w, 1221m, 1069w, 809s, 629m. |
| § Crystal data for1: C100H126N4Ni2O9, M = 1645.47, blocks, 0.30 × 0.28 × 0.25 mm, trigonal, P3121, a = 25.531(5), c = 14.907(5) Å, V = 8415(5) Å3, Z = 3, Dc = 0.973 g cm−3, F000 = 2646, Mo Kα radiation, λ = 0.71073 Å, μ = 0.383 mm−1, T = 193(2) K, 2θmax = 50.68°, 58691 reflections, 10246 unique (Rint = 0.0778), 8843 with Io > 2σ(Io), absorption corrections Tmax and Tmin = 0.909 and 0.774. Full-matrix least-squares on F2, 546 parameters, 12 restraints, GoF = 1.165, R1 = 0.0769, wR2 = 0.1892 (all reflections), −0.303 < Δρ < 0.393 e Å−3, Flack parameter χ = 0.05(2). Crystal structure solved and refined using SHELX-97,18 CCDC reference number 619677. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b613047j |
| This journal is © The Royal Society of Chemistry 2007 |
