Near IR absorbing planar aromatic [34]octaphyrins(1.1.0.1.1.0.0.0) containing a quaterthiophene subunit†‡
Rajeev
Kumar
a,
Rajneesh
Misra
a,
Tavarekere K.
Chandrashekar
*ab and
Eringathodi
Suresh
c
aDepartment of Chemistry, Indian Institute of Technology, Kanpur, 208016, India. E-mail: tkc@iitk.ac.in
bDirector, Regional Research Laboratory, Trivandrum, 695019, Kerala, India
cCentral Salt and Marine Chemicals Research Institute, Bhavnagar, Gujarat -, 364 002, India
First published on 14th November 2006
Abstract
The synthesis and structural characterization of the first examples of planar aromatic core modified [34]octaphyrins(1.1.0.1.1.0.0.0) with three different heteroatoms containing a quaterthiophene subunit are reported.
Expanded porphyrins continue to attract the attention of chemists and material scientists because of their diverse applications such as PDT, MRI contrasting agents, neutral substrate binding, anion recognition, and as NLO materials.1,2,9 Octaphyrins are a class of expanded porphyrins in which eight pyrrole/heterocyclic rings are connected to each other through meso carbon bridges as in 1–5,3–7 or through direct pyrrole–pyrrole link as in 6 (Fig. 1).8 Octaphyrins exhibit different conformations such as figure-eight, twisted or planar depending on the nature of linkage, number of meso carbon bridges as well as the steric bulk of the meso aryl substituents. Adaptation of twisted or figure eight conformations leads to loss of aromaticity. Thus development of methods to synthesize planar, aromatic octaphyrin is still a synthetic challenge. Recently we were successful in synthesizing aromatic, planar octaphyrins6 by: (a) replacing the few pyrrole rings by heavier heterocyclic rings such as thiophene or selenophene in the core; and (b) by increasing steric bulk on the meso aryl substituents to avoid the twisting of the macrocycle. These core modified octaphyrins exhibit ring inversions, where one or more pyrrole/heterocyclic rings are inverted and this ring inversion depends on the nature of the meso substituents and nature of heteroatom. In continuation of this strategy of synthesizing aromatic, planar octaphyrins, in this communication we wish to examine the effect of changes in the steric bulk of the meso substituents and the increase in the number of heteroatoms on the ring inversions and the conformation of the octaphyrins.
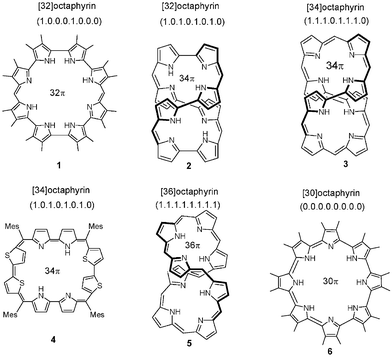 | ||
| Fig. 1 Octaphyrins known in literature. | ||
Our synthetic strategy involved preparation of a novel quaterthiophene fragment; 5,5‴-bis(mesitylhydroxymethyl)-2,5′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2′,2″
2′,2″![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5″,2‴-quaterthiophene, 7 as one of the precursors and the modified tetrapyrranes (8–11) in which meso substituents were varied from bulkier mesityl group to less bulkier anisal and phenyl groups, as the other precursor. Further, X can be either S or Se, leading to increase in the number of the heteroatoms. Thus, reaction of 7 with 8–11, under acid-catalyzed condensation with p-toluenesulfonic acid (PTSA) as catalyst, followed by chloranil oxidation leads to formation of the desired octaphyrins 12–15 (Scheme 1). Reduction in the steric bulk in the precursor 8–11, from mesityl to anisal and phenyl did not change the conformation of the octaphyrins, suggesting the two meso mesityl substituents are good enough to prevent twisting at the meso position, therefore avoiding the formation of figure-eight conformation. The advantage of this methodology is the formation of a single product in good yields (18–20%), thus, avoiding tedious chromatographic separations.
5″,2‴-quaterthiophene, 7 as one of the precursors and the modified tetrapyrranes (8–11) in which meso substituents were varied from bulkier mesityl group to less bulkier anisal and phenyl groups, as the other precursor. Further, X can be either S or Se, leading to increase in the number of the heteroatoms. Thus, reaction of 7 with 8–11, under acid-catalyzed condensation with p-toluenesulfonic acid (PTSA) as catalyst, followed by chloranil oxidation leads to formation of the desired octaphyrins 12–15 (Scheme 1). Reduction in the steric bulk in the precursor 8–11, from mesityl to anisal and phenyl did not change the conformation of the octaphyrins, suggesting the two meso mesityl substituents are good enough to prevent twisting at the meso position, therefore avoiding the formation of figure-eight conformation. The advantage of this methodology is the formation of a single product in good yields (18–20%), thus, avoiding tedious chromatographic separations.
![Synthesis of octaphyrins by [4 + 4] acid catalyzed condensation.](/image/article/2007/CC/b614290g/b614290g-s1.gif) | ||
| Scheme 1 Synthesis of octaphyrins by [4 + 4] acid catalyzed condensation. | ||
The proposed structure of the macrocycle comes from the various spectroscopic analysis and the single-crystal X-ray structure obtained for 12 (see ESI‡). The FAB mass spectra show peak at m/z = 1238.70 for 12, and m/z = 1144.25 for 13, confirming the free base composition of these macrocycles. The UV/Vis absorption spectrum of 12 in dry methylene chloride is characterized by the presence of an intense Soret-band [578 nm (log ε = 4.98)] and Q-bands [766 nm (4.44) and 842 nm (4.70)] into the near IR region and interesting results were obtained on careful protonation with TFA. At less acid concentration (1 eq.), there were three distinct bands at 650 nm (log ε = 4.02), 757 nm (2.35), and 1090 nm (2.13). This absorption spectrum represents the diprotonated form of 12 (Fig. 2). Further addition of TFA results in gradual increase in absorption at 650 and 1090 nm at the expense of absorption at 757 nm. This changes are typical of binding of anion to the macrocycle. A similar behaviour was observed for octaphyrins 12–15, suggesting that these macrocycles bind trifluoroacetate anion in the diprotonated state.
![UV/Vis- near IR absorption spectra of 12 (- - - -) [7.73 × 10−6] and the protonated derivative (—) in CH2Cl2.](/image/article/2007/CC/b614290g/b614290g-f2.gif) | ||
| Fig. 2 UV/Vis- near IR absorption spectra of 12 (- - - -) [7.73 × 10−6] and the protonated derivative (—) in CH2Cl2. | ||
Detailed 1H and 2D NMR studies provide important information about the conformation and aromaticity of these octaphyrins. Specifically, the 1H NMR spectrum of 12 (Fig. 3) taken in CDCl3 revealed eight sets of doublets between 8.75 and 4.05 ppm, integrating to two protons each, observed for β-CH protons, which suggests it has a C2 symmetric conformation in CDCl3. Both the selenophene rings opposite to the tetrathiophene unit are inverted in free base as well as in protonated forms. Very interesting results were obtained when trifluoroacetic acid (TFA) was added stepwise to a solution of free base octaphyrin 12 in CDCl3. The degree of inversion of the selenophene rings was found to be dependent on TFA concentration. In the freebase form the β-CH protons of these inverted rings resonate at 5.57 and 4.05 ppm. On addition of 10 µl TFA solution, (10% TFA in CDCl3) these β-CH protons resonate at 0.56 and 0.28 ppm and this upfield shift increases further as more TFA was added. Finally at excess TFA concentration (50 µl) these β-CH protons resonate at −1.73 and −2.54 ppm and the NH protons resonate at −4.11 ppm (Fig. 3). These observations suggest that after adding TFA, biselenophene unit comes into plane of the macrocycle and macrocycle becomes more palnar. Δδ value of 16.10 ppm supports a strong diatropic ring current, inferring their aromatic nature.
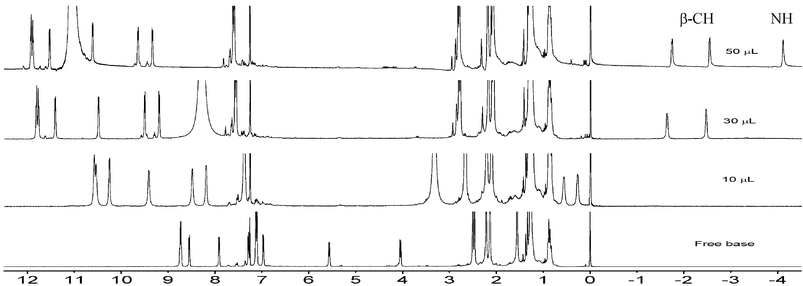 | ||
| Fig. 3 TFA concentration dependent 1H NMR spectra of 12 in CDCl3. Amounts of TFA added, are shown in spectra. | ||
Additional evidence for the aromatic character comes from the cyclic voltammetric studies. Octaphyrins 12–15 exhibit two reversible reductions and two reversible oxidations (see ESI‡). An estimated HOMO–LUMO gap of 1.09 V for 14 indicates a significant reduction relative to meso aryl sapphyrin (1.88 V), rubyrin (1.64 V), and heptaphyrin (1.38 V), thus explaining large red shifts observed in the electronic spectrum. A linear correlation obtained (Fig. 4) when the energy of the Soret maximum and HOMO–LUMO gap (Δredox) were plotted versus increase in π electrons in the delocalization pathway, gives a strong evidence of the aromatic nature of 12–15.
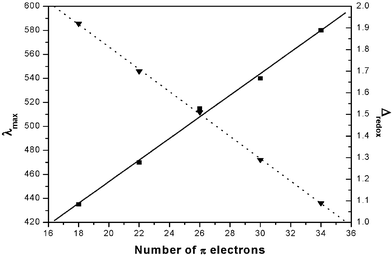 | ||
| Fig. 4 Variation of energy of the Soret band(■) and HOMO–LUMO gap (▼) with increasing number of π electrons. | ||
The confirmation of planar structure for 12 came from the single-crystal X-ray analysis10§ (CCDC-622228). The structure shown in Fig. 5 confirms the inversion of both the selenophene rings. There are two independent molecules in the asymmetric unit and the inverted biselenophene unit, having different dihedral angles (9.52 and 22.6°) with respect to the mean plane defined by four meso carbon atoms. The molecules deviate slightly from planarity as seen in side view (Fig. 5b). NMR studies also support such an observation where the degree of inversion of the biselenophene unit depends on the amount of TFA added and at excess TFA concentration, the biselenophene unit comes into the plane as defined by the four meso carbons. And the macrocycle adopts a planar conformation.
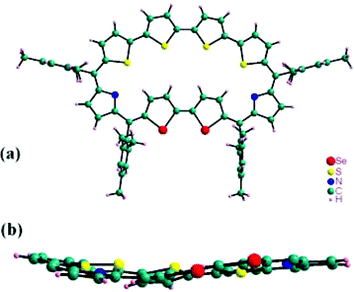 | ||
| Fig. 5 Single crystal X-ray structure of 12, top view (a) and its side view (b). Meso substituents in side view are removed for clarity and only one of the two independent molecules in the asymmetric unit of 12 is shown. | ||
In summary, it has been shown that: (a) use of rigid quaterthiophene subunit as one of the precursors restricts the ring inversion only on the tertapyrrane subunit unlike in 4, where both the subunits are involved in ring inversions; (b) variation of X in the tetrapyrrane moiety has no bearing on the ring inversions; (c) reduction in the steric bulk of the meso substituents in the tetrapyrrane moiety did not result in the twisting of the macrocycle. These subtle variations in the structure can play a major role in the resulting conformation of the octaphyrin leading to change in the electronic structure and aromaticity. Systematic studies of these variations on the structure property corelations are underway.
Notes and references
- J. L. Sessler, A. Gebauer and S. J. Weghorn, The Porphyrin Handbook, Vol. 2, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 1999, ch. 9 Search PubMed; A. Jasat and D. Dolphin, Chem. Rev., 1997, 97, 2267 Search PubMed; E. Vogel, J. Heterocycl. Chem., 1996, 33, 1461 CrossRef CAS; T. D. Lash, Angew. Chem., 2000, 112, 1833 CrossRef CAS; T. D. Lash, Angew. Chem., Int. Ed., 2000, 39, 1763 CrossRef.
- J. L. Sessler, M. J. Cyr, V. Lynch, E. MaGhee and J. A. Ibers, J. Am. Chem. Soc., 1990, 112, 2810 CrossRef CAS; J. L. Sessler, T. Morishima and V. Lynch, Angew. Chem., Int. Ed. Engl., 1991, 30, 977 CrossRef; J. L. Sessler, S. J. Weghorn, V. Lynch and M. R. Johnson, Angew. Chem., Int. Ed. Engl., 1994, 33, 1509 CrossRef; E. Vogel, M. Bröring, J. Fink, D. Rosen, H. Schmickler, J. Lex, K. W. K. Chan, Y.-D. Wu, D. A. Plattner, M. Nendel and K. N. Houk, Angew. Chem., 1995, 107, 2705 CrossRef; Richard A. Stevens, , Casey C. Raymond and Peter K. Dorhout, Angew. Chem., Int. Ed. Engl., 1995, 34, 2511 CAS; J. Setsune and S. Maeda, J. Am. Chem. Soc., 2000, 122, 12405 CrossRef CAS; N. Sprutta and L. Latos-Grazynski, Chem.–Eur. J., 2001, 7, 5099 CrossRef CAS; D. Siedel, V. Lynch and J. L. Sessler, Angew. Chem., Int. Ed., 2002, 41, 1422 CrossRef CAS.
- J. L. Sessler, D. Seidel and V. Lynch, J. Am. Chem. Soc., 1999, 121, 11257 CrossRef CAS.
- M. Bröring, J. Jendrny, L. Zander, H. Schmickler, J. Lex, Y.-D. Wu, M. Nendel, J. G. Cheng, D. A. Plattner, K. N. Houk and E. Vogel, Angew. Chem., 1995, 107, 2709 CrossRef; Martin Bröring, Jörg Jendrny, Lars Zander, Hans Schmickler, Johann Lex, Yun-Dong Wu, Maja Nendel, Jiangang Chen, Dietmar A. Plattner, Kendall N. Houk and Emanuel Vogel, Angew. Chem., Int. Ed. Engl., 1995, 34, 2515 CrossRef.
- E. Vogel, M. Bröring, J. Fink, D. Rosen, H. Schmickler, J. Lex, K. W. K. Chan, Y.-D. Wu, D. A. Plattner, M. Nendel and K. N. Houk, Angew. Chem., 1995, 107, 2705 CrossRef; Emanuel Vogel, Martin Bröring, Jürgen Fink, Daniel Rosen, Hans Schmickler, Johann Lex, Kyle W. K. Chan, Yun-Dong Wu, Dietmar A. Plattner, Maja Nendel and Kendall N. Houk, Angew. Chem., Int. Ed. Engl., 1995, 34, 2511 CAS; H. Rath, J. Sankar, V. Prabhuraja, T. K. Chandrashekar, B. S. Joshi and R. Ray, Chem. Commun., 2005, 3343 RSC.
- V. G. Anand, S. K. Pushpan, S. Venkatraman, A. Dey, T. K. Chandrashekar, B. S. Joshi, R. Roy, W. Teng and K. R. Senge, J. Am. Chem. Soc., 2001, 123, 8620 CrossRef CAS; R. Misra, V. G. Anand, H. Rath and T. K. Chandrashekar, J. Chem. Sci., 2005, 117, 99 Search PubMed.
- J.-Y. Shin, H. Furuta, K. Yoza, S. Igarashi and A. Osuka, J. Am. Chem. Soc., 2001, 123, 7190 CrossRef CAS; N. Sprutta and L. Grazynski, Chem.–Eur. J., 2001, 7, 5099 CrossRef CAS.
- D. Seidel, V. Lynch and J. L. Sessler, Angew. Chem., 2002, 114, 1480 CrossRef; Daniel Seidel,, Vincent Lynch and Jonathan L. Sessler, Angew. Chem., Int. Ed., 2002, 41, 1422 CrossRef CAS.
- T. K. Ahn, J. H. Kwon, D. Y. Kim, D. W. Cho, D. H. Jeong, S. K. Kim, M. Suzuki, S. Shimizu, A. Osuka and D. Kim, J. Am. Chem. Soc., 2005, 127, 12856 CrossRef CAS; H. Rath, J. Sankar, V. PrabhuRaja, T. K. Chandrashekar, A. Nag and D. Goswami, J. Am. Chem. Soc., 2005, 127, 11608 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, University of Göttingen, 1997.
Footnotes |
| † The HTML version of this article has been additionally enhanced with colour images. |
| ‡ Electronic supplementary information (ESI) available: Details of experimental procedures, characterization of compounds 7 and 12–15. See DOI: 10.1039/b614290g |
| § Crystallographic summary for 12: C72H60N2S4Se2, M = 1239.42. Crystals were grown by slowly diffusing dry n-hexane over a chloroform solution of 12. Blue rectangular, triclinic, P-1, μ = 1.134 mm−1, Z = 4 in a cell of dimensions: a = 20.276(4) Å, b = 21.240(4) Å, c = 21.313(4) Å; α = 81.06(3)°, β = 62.88(3)°, γ = 66.62(3)°, V = 7495(3)Å3, ρcal = 1.098 Mg m−3, F(000) = 2552. A total of 26051 independent reflections were measured on a CCD area detector using graphite monochromatized Mo Kα radiation (λ = 0.71073 Å) at −173 °C. The structure was refined on F2 to an Rw = 0.1411, with a conventional R = 0.0614, and a goodness of fit = 0.920 for 1465 parameters using the SHELXTL10 package. CCDC 622228. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b614290g. |
| This journal is © The Royal Society of Chemistry 2007 |
