Switching the light emission of (4-biphenylyl)phenyldibenzofulvene by morphological modulation: crystallization-induced emission enhancement†
Yongqiang
Dong
ab,
Jacky W. Y.
Lam
a,
Anjun
Qin
a,
Zhen
Li
a,
Jingzhi
Sun
b,
Herman H.-Y.
Sung
a,
Ian D.
Williams
a and
Ben Zhong
Tang
*ab
aDepartment of Chemistry, The Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk; Fax: +852-2358-1594; Tel: +852-2358-7375
bDepartment of Polymer Science and Engineering, Zhejiang University, Hangzhou, 310027, China
First published on 21st November 2006
Abstract
(4-Biphenylyl)phenyldibenzofulvene is weakly luminescent in the amorphous phase but becomes highly emissive upon crystallization; this unusual crystallization-induced emission enhancement effect allows its emission to be repeatedly switched between dark and bright states by fuming-heating and heating-cooling processes.
Much effort has been devoted to the creation of new luminophoric molecules owing to their potential applications in electronics and optics.1 Of particular interest are those molecules whose emissions can be repeatedly switched between two different, e.g., dark/bright or on/off, states, by external stimuli in the solid state, though many dyes have been found to show such properties in the solution state. A problem associated with the dye emission in the solid state is the aggregation-caused quenching (ACQ): during film formation, dye molecules aggregate, which often quenches their light emissions.2 This notorious ACQ problem must be solved, because luminophores are commonly used as solid thin films in their practical applications. We have recently discovered a phenomenon exactly opposite to the ACQ effect, that is, aggregation-induced emission (AIE): a series of propeller-shaped molecules that are nonemissive in the solution state are induced to emit efficiently when aggregated in the solid state.3–5 This novel AIE process is of obviously scientific value and technological implication.
Closely related to the ACQ effect is the crystallization of dye molecules in the solid state, which normally shifts the luminescence spectra of the dyes to the red region and weakens their emissions, hence lowering their fluorescence quantum yields (ΦF). Scientists have worked hard to fabricate amorphous films in the construction of light-emitting diodes, although crystalline films are known to exhibit higher charge mobilities.5,6 During our study on the AIE phenomenon, we have observed that many AIE-active dyes emit bluer lights in higher efficiencies in the crystalline phase than in the amorphous phase. Amorphous hexaphenylsilole, for instance, emits an intense bluish-green light of 500 nm, whereas its crystal emits a blue light at 462 nm in a ∼2.5-fold higher intensity.5d In this communication, we report an AIE dye with a large crystallization-induced emission enhancement (CIEE) effect: (4-biphenylyl)phenyldibenzofulvene (BpPDBF; 1) emits faintly in the amorphous phase but strongly in the crystalline phase, with a difference in ΦF as high as 32 times. This remarkable CIEE effect enabled ready modulation of the light emission of 1 between dark and bright states by simple external stimuli such as fuming, heating and cooling processes.
BpPDBF was prepared according to the synthetic route given in Scheme S1 (ESI†). Coupling of fluorene with 4-benzoylbiphenyl afforded 1 in 52% yield. The product was spectroscopically and crystallographically characterized,‡ and satisfactory analysis data were obtained (ESI†). During the product purification process, we noted that neither the wet spots of 1 nor its dried spots on TLC plates could be clearly visualized with UV lamp, although the dried spots were expected to emit intensely under UV illumination. To understand this intriguing visual observation, we studied emission behaviours of 1 by fluorescence spectroscopy.
The emission spectrum of a dilute solution of 1 in acetonitrile is shown in Fig. 1(A), which is almost a flat line parallel to the abscissa. Its ΦF value is ∼0.03%, indicating that the dye is virtually nonluminescent when molecularly dissolved. When a large amount of water is added into its solution, its emission spectrum intensifies. Water is a nonsolvent of 1 and the dye molecules thus must have aggregated in the mixtures with high water contents. Apparently, the light emission of 1 is induced by aggregate formation; in other words, 1 is AIE-active.
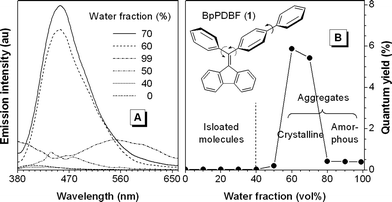 | ||
| Fig. 1 (A) Emission spectra of 1 in acetonitrile and acetonitrile–water mixtures and (B) quantum yield vs. composition of the acetonitrile–water mixture. Dye concentration: 10 µM; excitation wavelength (λex): 350 nm. | ||
Closer inspection of the spectra of 1 in the aqueous mixtures reveals that its ΦF is decreased dramatically, accompanying with a red-shift in the emission maximum, when the water content of the mixture is increased from 70 to 99%. In the mixture with a “low” water content of 70%, molecules of 1 may steadily cluster together to form ordered, crystalline aggregates. On the other hand, in the mixture with a very high water fraction of 99%, the dye molecules may abruptly agglomerate to form random, amorphous aggregates. This is indeed the case: as can be seen from the TEM images and ED patterns shown in Fig. S2 (ESI†), the aggregates formed in the mixtures with water contents of 70 and 99% are crystalline and amorphous in nature, respectively.
To confirm that the crystalline aggregates of 1 are more efficient emitters than its amorphous counterparts, crystals of 1 were grown from an ethanol–THF mixture and its amorphous powders were prepared by rapidly quenching its melt with liquid nitrogen.7 The crystalline and amorphous nature of the samples is verified by their X-ray diffractograms: the former exhibits many sharp reflection peaks, while the latter gives only a weak, broad, diffuse halo (Fig. 2(A)). Upon photoexcitation, the crystals emit an intense blue light of ∼450 nm in a ΦF of 16%, but the amorphous powders emit a faint yellow light of ∼550 nm in a ΦF of 0.5% (Fig. 2(B)), hence showing a distinct CIEE effect. The dye molecules deposited on the TLC plate may be well dispersed in the silica-gel matrix or segregated by the fine gel particles, which prevents them from forming crystals. This is why the light emitted from the dried spot of 1 on the TLC plate was hardly discernable with the UV illumination.
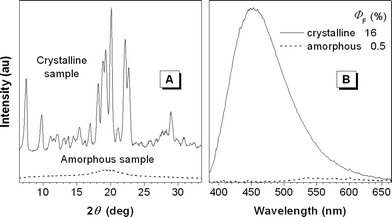 | ||
| Fig. 2 (A) Powder X-ray diffractograms and (B) emission spectra of the crystalline and amorphous samples of 1. λex = 350 nm. | ||
It now becomes crystal clear that crystallization can dramatically enhance the light emission of 1. We then explored the possibility of modulating its emission by a simple engineering process of solvent fuming. This exploration is of practical implications because there is an urgent call for the development of new chemosensors that can sensitively detect volatile organic compounds (VOCs) of potential health hazards.8 A thin film of 1 was coated on the inner wall of a quartz cell, which was then placed in a small container saturated with acetone vapours. Interestingly, the emission spectrum of 1 is gradually intensified with exposure time, with a swift increase in the emission intensity at the beginning of the fuming process (Fig. 3). The emission of the film fumed for ∼0.5 h is ∼35-fold stronger than that of the unexposed parent film. Similar effect is observed when the film is exposed to other toxic VOCs such as chloroform and dichloromethane (Fig. 3(B) and S3, ESI†). Evidently, 1 can be used as a sensitive chemosensor for detecting VOCs.
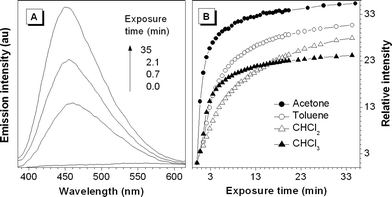 | ||
| Fig. 3 (A) Effect of acetone vapour on the emission spectrum of a thin film of 1 coated on a quartz plate. (B) Change in the emission intensity with the time of exposure to solvent vapour. λex = 350 nm. | ||
The emission enhancement suggests that the dye molecules have been induced to crystallize by the vapour fuming process.9 To prove this speculation, we examined the morphology of the films before and after exposure to the solvent vapours by electron microscopy. The TEM images and ED patterns affirm that the unexposed and exposed films are amorphous and crystalline, respectively (Fig. S4, ESI†). Thus, like in the fuming processes of many other dyes,9 the solvent vapours have activated a dynamic recrystallization process of the molecules of 1, hence resulting in the large CIEE effect.
The amorphous and crystalline films of 1 emit weak and strong lights, respectively. Can its light emission be reversibly switched between dark and bright states? The answer to this question should be yes, provided the fumed film can be amorphized. DSC analysis reveals that the crystals of 1 melt at ∼211 °C (Fig. S5, ESI†). We thus heated the fumed film of 1 to 215 °C and then quickly cooled it to room temperature. The thus amorphized film now emits only weak light, returning to the original dark state. This fuming–heating process changes the morphology of the film between crystalline and amorphous phases, resulting in the emission switching between bright and dark states (Fig. 4(A)). This switching can be reliably repeated many times because of the nondestructive nature of the fuming–heating process.
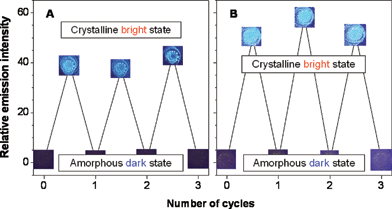 | ||
| Fig. 4 Repeated switching between dark and bright states of the emission of the thin solid films of 1 coated on quartz plates by (A) fuming–heating and (B) heating–cooling cycles. The photos were taken under illumination of a UV lamp. | ||
Heat-mode phase-change technology has been widely used in the rewritable optical media.10 It would be nice if the emission of 1 can be switched by a pure thermal process. DSC thermogram of an amorphous sample of 1 indicates that the dye molecules undergo crystallization and amorphization transitions at 115.6 and 206.6 °C, respectively (Fig. S5, ESI;† noting that the crystallization window is as wide as 91 °C!). This suggests that the emission of 1 can be manipulated by a heating–cooling process. As described above, the film of 1 coated on a quartz cell is almost nonluminescent. After annealing at 160 °C for ∼1 min, the dye molecules crystallizes and the film becomes emissive (Fig. 4(B)). Further heating to 215 °C followed by quick cooling to room temperature amorphizes the film and switches its emission back to the dark state. This switching can be repeated without fatigue because of the excellent thermal stability of 1. The change in reflectivity has been commonly used as an output in rewritable optical media. A luminescence-based process is of advantage because it offers higher sensitivity, lower background noises and potential for two-dimensional imaging.11 Dye 1 is thus a promising candidate for innovative applications in optical information storage systems.
Crystallization usually red-shifts emission spectra and decreases emission intensity, but why does 1 show the opposite CIEE effect? Both absorption and emission spectra of the amorphous aggregates of 1 are red-shifted from those of its crystalline counterparts. This implies the formation of such detrimental species as excimers in the amorphous aggregates via π–π stacking of the biphenyl and fulvene rings, which weakens the emission of the aggregates.12,13 On the other hand, X-ray analysis data tell that there is little such π–π interaction in the crystals of 1 (Fig. S6, ESI†), which may account for their bluer emission. The molecular conformations in the dye crystals are locked/stabilized by multiple C–H⋯π hydrogen bonds, which hampers the aromatic rings from undergoing conformational changes caused by such movements as intramolecular rotations.13 This structural rigidification may have made the crystals stronger emitters.3–5,14
In summary, in this work, we have found a striking CIEE effect in dye 1. Crystallization has induced significant enhancements in its fluorescence efficiency: ∼180- and 32-fold increases in ΦF when compared to those of molecular solutions and amorphous powders, respectively. By modulating the morphology of the thin film of 1 between amorphous and crystalline phases, its emission is switched between dark and bright states repeatedly by simple fuming–heating and heating–cooling cycles. This attribute may enable the CIEE dye to find an array of novel applications in chemosensors, environmental protection, optical displays, and rewritable optical media.
This project was partially supported by the Research Grants Council of Hong Kong (602706, HKU2/05C, 603505, 603304 and 664903), the Ministry of Science & Technology (2002CB613401) and the National Science Foundation of China (50603021 and 20634020). B. Z. T. thanks the Cao Guangbiao Foundation of Zhejiang University.
Notes and references
- (a) Z. Xie, B. Yang, F. Li, G. Cheng, L. Liu, G. Yang, H. Xu, L. Ye, M. Hanif, S. Liu, D. Ma and Y. Ma, J. Am. Chem. Soc., 2005, 127, 14152 CrossRef CAS; (b) C. P.-Y. Chan, M. Haeussler, B. Z. Tang, Y. Q. Dong, K. Sin, W.-C. Mak, D. Trau, M. Seydack and R. Renneberg, J. Immunol. Methods, 2004, 295, 111 CrossRef CAS; (c) S. J. Toal, K. A. Jones, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2005, 127, 11661 CrossRef CAS; (d) C. J. Bhongale and C.-S. Hsu, Angew. Chem., Int. Ed., 2006, 45, 1404 CrossRef CAS.
- (a) B.-K. An, S.-K. Kwon, S.-D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410 CrossRef CAS; (b) S. Jayanty and T. P. Radhakrishnan, Chem. Eur. J., 2004, 10, 791 CrossRef CAS; (c) H. J. Tracy, J. L. Mullin, W. T. Klooster, J. A. Martin, J. Haug, S. Wallace, I. Rudloe and K. Watts, Inorg. Chem., 2005, 44, 2003 CrossRef CAS.
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC; (b) G. Yu, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng, Z. Shuai, B. Z. Tang, D. Zhu, W. Fang and Y. Luo, J. Am. Chem. Soc., 2005, 127, 6335 CrossRef CAS; (c) J. Chen, C. W. Law, J. W. Y. Lam, Y. Dong, S. M. F. Lo, I. D. Williams, D. Zhu and B. Z. Tang, Chem. Mater., 2003, 15, 1535 CrossRef CAS; (d) H. Tong, Y. Q. Dong, M. Häußler, Y. Hong, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, H. S. Kwok and B. Z. Tang, Chem. Phys. Lett., 2006, 428, 326 CrossRef CAS.
- (a) H. Tong, M. Häußler, Y. Q. Dong, Z. Li, B. Mi, H. S. Kwok and B. Z. Tang, J. Chinese Chem. Soc. (Taiwan), 2006, 53, 243 Search PubMed; (b) H. Tong, Y. Hong, Y. Q. Dong, M. Häußler, J. W. Y. Lam, Z. Li, Z. f. Guo, Z. H. Guo and B. Z. Tang, Chem. Commun., 2006, 3705 RSC.
- (a) H. Tong, Y. Dong, M. Häußler, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Commun., 2006, 1133 RSC; (b) H. Tong, Y. Q. Dong, M. Häußler and B. Z. Tang, Mol. Cryst. Liq. Cryst., 2006, 446, 183 CrossRef CAS; (c) Z. Li, Y. Q. Dong, Y. H. Tang, M. Häußler, H. Tong, Y. P. Dong, J. W. Y. Lam, K. S. Wong, P. Gao, I. D. Williams, H. S. Kwok and B. Z. Tang, J. Phys. Chem. B, 2005, 109, 10061 CrossRef CAS; (d) Y. Q. Dong, J. W. Y. Lam, Z. Li, H. Tong, Y. P. Dong and B. Z. Tang, J. Inorg. Organomet. Polym. Mater., 2005, 15, 287 CrossRef CAS.
- (a) C.-C. Wu, Y.-T. Lin, K.-T. Wong, R.-T. Chen and Y. Y. Chien, Adv. Mater., 2004, 16, 61 CrossRef CAS; (b) Y. Kan, L. Wang, L. Duan, Y. L. Hu, G. Wu and Y. Qiu, Appl. Phys. Lett., 2004, 84, 1513 CrossRef.
- (a) J. W. Y. Lam and B. Z. Tang, Acc. Chem. Res., 2005, 38, 745 CrossRef CAS; (b) B. Z. Tang, X. Kong, X. Wan, H. Peng, W. Y. Lam, X.-D. Feng and H. S. Kwok, Macromolecules, 1998, 31, 2419 CrossRef CAS.
- (a) E. S. Snow, F. K. Perkins, E. J. Houser, S. C. Badescu and T. L. Reinecke, Science, 2005, 307, 1942 CrossRef CAS; (b) J. Janata and M. Josowicz, Nat. Mater., 2003, 2, 19 CrossRef CAS.
- B. Z. Tang, H. Z. Chen, R. S. Xu, J. W. Y. Lam, K. K. L. Cheuk, H. N. C. Wong and M. Wang, Chem. Mater., 2000, 12, 213 CrossRef CAS.
- A. V. Kolobov, P. Fons, A. I. Frenkel, A. Ankudinov, J. Tominaga and T. Uruga, Nat. Mater., 2004, 3, 703 CrossRef CAS.
- (a) T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685 CrossRef CAS; (b) G. Y. Jiang, S. Wang, W. Yuan, L. Jiang, Y. L. Song, H. Tian and D. B. Zhu, Chem. Mater., 2006, 18, 235 CrossRef CAS; (c) H. Tian, J. A. Gan, K. C. Chen, J. He, Q. L. Song and X. Y. Hou, J. Mater. Chem., 2002, 12, 1262 RSC.
- A more detailed picture is as follows: In the amorphous aggregates, intramolecular rotations are restricted, which enhances light emission (AIE).3 The formation of excimer species, however, diminishes light emission (ACQ).13 The net effect of these two antagonistic processes is the limited increase in the ΦF of the aggregates (∼0.4%) from that of the parent solution (∼0.03%).
- L. D. Lu, R. Helgeson, R. M. Jones, D. McBranch and D. Whitten, J. Am. Chem. Soc., 2002, 124, 483 CrossRef CAS.
- K. S. Wong, H. Wang and G. Lanzani, Chem. Phys. Lett., 1998, 288, 59 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation and characterization data of 1; UV spectra of its solution and aggregates; TEM images and ED patterns of its aggregates and films in amorphous and crystalline phases; effects of different solvent vapors on its emission spectra; DSC thermograms of its amorphous and crystalline samples; and its crystal structures with multiple C–H⋯π hydrogen bonds marked. See DOI: 10.1039/b613157c |
| ‡ Crystal data for 1: C34H26Cl2, M = 505.45, monoclinic, P21/c, a = 13.8275(7), b = 9.9939(5), c = 19.6086(9) Å, β = 108.0360(10)°, V = 2576.6(2) Å3, Z = 4, Dc = 1.303 Mg m−3, μ = 0.274 mm−1, F(000) = 1056, T = 100(2) K, 2θmax = 52.00°, 15935 measured reflections, 4723 independent reflections (Rint = 0.0234), R1 = 0.0572, wR2 = 0.1136 (all data), Δρ = 0.586 and −0.411 e Å−3. CCDC 620483. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b613157c |
| This journal is © The Royal Society of Chemistry 2007 |
