A triptycene-based polymer of intrinsic microposity that displays enhanced surface area and hydrogen adsorption
Bader S.
Ghanem
a,
Kadhum J.
Msayib
a,
Neil B.
McKeown
*a,
Kenneth D. M.
Harris
a,
Zhigang
Pan
a,
Peter M.
Budd
*b,
Anna
Butler
b,
James
Selbie
b,
David
Book
c and
Allan
Walton
c
aSchool of Chemistry, Cardiff University, Cardiff, UK CF10 3AT. E-mail: mckeownnb@cardiff.ac.uk; Tel: + 44 (0)2920 875851
bOrganic Materials Innovation Centre, School of Chemistry, University of Manchester, Manchester, UK M13 9PL. E-mail: peter.budd@manchester.ac.uk; Tel: +44 (0)161 275 4711
cDepartment of Metallurgy and Materials, School of Engineering, University of Birmingham, Birmingham, UK B15 2TT
First published on 15th November 2006
Abstract
A novel triptycene-based polymer of intrinsic microporosity (Trip-PIM) displays enhanced surface area (1065 m2 g−1) and reversibly adsorbs 1.65% hydrogen by mass at 1 bar/77 K and 2.71% at 10 bar/77 K.
Polymers of intrinsic microporosity (PIMs) are rigid and contorted macromolecules, wholly composed of fused-ring components, which form microporous organic materials due to their inability to pack space efficiently.1 PIMs combine high internal surface areas with the synthetic diversity of polymers and can be used for a wide range of applications including heterogeneous catalysis,2 membrane separations3 and the adsorption of organic compounds.4 Of interest is the possibility of using such materials for the low-pressure adsorption of molecular hydrogen (H2) to address the ‘grand challenge’5 of finding a safe and efficient storage material for vehicular use.6 To date, many types of microporous materials (i.e. zeolites, carbons)7 have been examined for H2 physisorption with the best results being obtained from carbons of very high surface area (>2500 m2 g−1).8,9 Inorganic coordination polymers, especially the highly porous metal-organic-frameworks (MOFs), have also demonstrated real potential for H2 storage.10,11 However, purely organic polymers have only very recently been considered as candidate materials for H2 storage but are attractive because they are composed only of relatively light elements (e.g. C, H, N, O).12
Recently, we reported the H2 adsorption properties at 77 K of three PIMs (PIM-1; HATN-network and CTC-network; Fig. 1), all of which display moderate Brunauer–Emmett–Teller (BET) surface areas in the range 750–850 m2 g−1.6 Hydrogen uptake for these materials was encouraging with, at best, 1.4% by mass adsorbed at 1 bar and 1.7% by mass at 10 bar. Subsequently, Cooper and co-workers described the H2 adsorption properties of a ‘Davankov-type’ hypercrosslinked resin, prepared by the Friedel–Crafts alkylation of chloromethylated polystyrene beads.13 This material, of BET surface area = 1466 m2 g−1, adsorbs a comparable amount of H2 to the PIMs at 1 bar (1.27% by mass) but a significantly greater amount at 10 bar (2.75% by mass). Almost concurrently, the group of Svec and Fréchet published the H2 adsorption characteristics of both a range of commercially available hypercrosslinked resins (surface areas up to 1200 m2 g−1) and a specially prepared hypercrosslinked polystyrene of very high surface area (1930 m2 g−1), with the latter displaying a H2 uptake of 1.4% by mass at 1 bar/77 K.14 A comparison of the micropore size distributions derived from low pressure nitrogen adsorption data confirms that the three PIM materials have a greater predominance of ultramicropores (i.e. <0.7 nm) which accounts for their similar adsorption of H2 at low pressure (1 bar) despite possessing much lower BET surface areas than the hypercrosslinked polymers. However, in order to approach the US Department of Energy's much stated 2010 target of a storage system containing 6% H2 by mass, it will be necessary to obtain PIMs with much greater porosity, as demonstrated by larger surface areas and pore volumes, whilst retaining their ultramicroporous structure.
As part of a programme to establish the structure–property relationships for PIMs, triptycene was identified as an interesting structural building block due to its rigid, fused-ring skeleton and three-fold symmetry. Indeed, the unique shape of triptycene results in what has been termed ‘internal molecular free volume’15 and Swager's group has used this concept to introduce and explain some impressive mechanical and electronic properties of triptycene-containing materials.16
PIM synthesis is carried out using dibenzodioxane formation (a double aromatic nucleophilic substitution) between catechol- and o-difluorobenzene-containing monomers to form the required fused-ring linkage. Therefore, a suitable triptycene derivative for incorporation into a PIM network is 9,10-diethyl-2,3,6,7,12,13-hexahydroxytriptycene 1, which is readily prepared from the Diels–Alder reaction between 9,10-diethyl-2,3,6,7-tetramethoxyanthracene and 4,5-dimethoxybenzyne (prepared in-situ from 4,5-dimethoxyanthralinic acid), followed by demethylation of the triptycene product using BBr3.17 Polymerisation was achieved by the reaction between 1 and commercially available 2,3,5,6-tetrafluoroterephthalonitrile (Scheme 1) to give, after washing with a variety of solvents and drying in vacuo, the triptycene network PIM (Trip-PIM) in quantitative yield. The insoluble yellow powder was characterised by IR spectroscopy and elemental analysis, which from the lack of residual fluorine (ca. 0.8%), indicates an efficient network formation. Powder X ray diffraction confirmed that the material is non-crystalline.
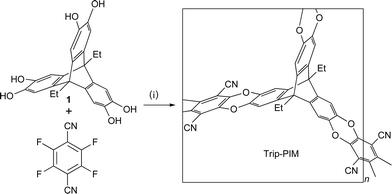 | ||
| Scheme 1 Synthesis of the triptycene-based PIM (Trip-PIM). Reagents and conditions: (i) K2CO3, DMF, 80 °C. | ||
Nitrogen adsorption measurements at 77 K (Fig. 1) indicate that the BET surface area for Trip-PIM is 1064 m2 g−1 – higher than for any previously prepared PIM.18 The adsorption isotherm displays remarkably distinct hysteresis extending to low partial pressures between the adsorption and desorption cycles. This indicates that there is activated adsorption arising either from a swelling of the polymer matrix or from the restricted access of nitrogen molecules to pores due to blocking at narrow openings. Analysis of the low-pressure nitrogen adsorption data by the Horvath–Kawazoe method19 indicates that the pore size distribution within Trip-PIM is strongly biased towards sub-nanometre pores (Fig. 2).
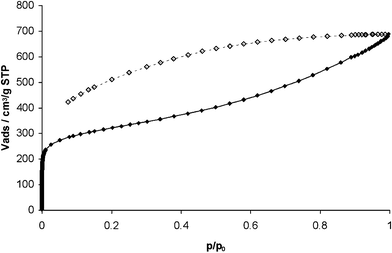 | ||
| Fig. 1 N2 adsorption (◆) and desorption (◇) isotherms at 77 K for Trip-PIM. | ||
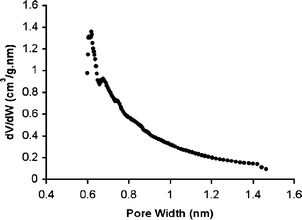 | ||
| Fig. 2 Pore size distribution for Trip-PIM derived from low-pressure N2 adsorption showing the predominance of sub-nanometre pores. | ||
Consideration of a molecular model of a fragment of Trip-PIM (Fig. 3) reveals a possible explanation for the enhanced porosity. The triptycene component clearly provides the necessary non-linearity required for microporosity, a role performed by the spiro-centre in PIM-1 and the HATN-PIM, or the bowl-shaped subunit in the CTC-network (linear polymers composed of fused rings generally assemble into densely packed, non-porous solids). However, it also encourages the growth of the polymer within the same plane with the exposed faces of the ribbon-like struts, between the bifurcating triptycenes, oriented perpendicular to the plane of the polymer. The effective blocking of close, face-to-face association between these fused-ring struts may help to further frustrate space-efficient packing of the macromolecules leading to greater microporosity.
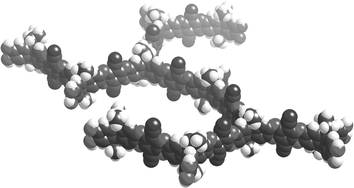 | ||
| Fig. 3 Space-filling model of a fragment of Trip-PIM to illustrate its planar, bifurcated ribbon-like structure. | ||
Complementary H2 adsorption measurements were performed at 77 K for Trip-PIM using both gravimetric analysis (Hiden IGA-1), over the pressure range 0 to 15 bar, and volumetric analysis (Micromeritics ASAP 2020), over the range 0 to 1 bar.20 For the gravimetric analysis, the buoyancy correction required to take into account the mass of hydrogen displaced by the sample at a given pressure was calculated using a density of 1.4 g ml−1, which was based upon helium pyconometry measurements. Highly consistent results were obtained for both types of measurement as shown in Fig. 4. In addition, the adsorption is completely reversible which is consistent behaviour with that expected for the physisorption of H2 on a microporous material.
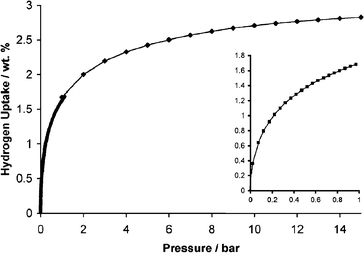 | ||
| Fig. 4 H2 adsorption isotherms of Trip-PIM at 77 K obtained using two separate runs (0–1 bar and 0–15 bar) for gravimetric analysis. The inset shows data from volumetric analysis (0–1 bar). There is no significant hysteresis observed during desorption cycles (not shown). | ||
The H2 uptake at 1 bar/77 K is 1.65% by mass, which is significantly higher relative to that of all previously reported organic polymers (Table 1). It is particularly noteworthy that, under these conditions, Trip-PIM adsorbs more H2 than a hypercrosslinked polymer with a BET surface area of 1930 m2 g−1.14 This reflects the large proportion of sub-nanometre pores accessible to H2 (Fig. 2). However, H2 adsorption at 10 bar/77 K (2.71% by mass) is very similar to that found for the hypercrosslinked styrene of BET surface area 1466 m2 g−1 and at high pressures uptake is greater on this hypercrosslinked material (ca. 3% at 15 bar/77 K).13 Even at low pressures, the values of H2 uptake for Trip-PIM fall short in comparison with those of the best performing carbons (e.g. ca. 3% by mass at 1 bar)8 or MOFs (ca. 2.5% by mass at 1 bar)11,21 but they perform well in comparison to any type of microporous material of a similar surface area. Hence the challenge is to further increase the accessible surface area and pore volume of PIMs without losing their micropore size distribution profile, which is so beneficial to low-pressure H2 adsorption. Work is in progress to achieve these aims via the incorporation of appropriate structural components and by exploiting polymer processing techniques.
We thank the EPSRC (grants EP/D074312/1 and GR/T24289) for funding.
Notes and references
- For recent reviews of PIMs, see: N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 Search PubMed; N. B. McKeown, P. M. Budd, K. J. Msayib, B. S. Ghanem, H. J. Kingston, C. E. Tattershall, S. Makhseed, K. J. Reynolds and D. Fritsch, Chem.–Eur. J., 2005, 11, 2610–2620 RSC; P. M. Budd, N. B. McKeown and D. Fritsch, J. Mater. Chem., 2005, 15, 1977–1986 CrossRef CAS.
- P. M. Budd, B. Ghanem, K. Msayib, N. B. McKeown and C. Tattershall, J. Mater. Chem., 2003, 13, 2721–2726 RSC; N. B. McKeown, S. Makhseed and P. M. Budd, Chem. Commun., 2002, 2780–2781 RSC.
- P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Adv. Mater., 2004, 16, 456–458 CrossRef CAS; P. M. Budd, K. J. Msayib, C. E. Tattershall, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263–269 CrossRef CAS; P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231 RSC.
- A. V. Maffei, P. M. Budd and N. B. McKeown, Langmuir, 2006, 22, 4225–4229 CrossRef CAS.
- L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353–358 CrossRef.
- N. B. McKeown, B. Ghanem, K. J. Msayib, P. M. Budd, C. E. Tattershall, K. Mahmood, S. Tan, D. Book, H. W. Langmi and A. Walton, Angew. Chem., Int. Ed., 2006, 45, 1804–1807 CrossRef.
- M. G. Nijkamp, J. Raaymakers, A. J. van Dillen and K. P. de Jong, Appl. Phys. A: Mater. Sci. Process., 2001, 72, 619–623 CrossRef CAS.
- N. Texier-Mandoki, J. Dentzer, T. Piquero, S. Saadallah, P. David and C. Vix-Guterl, Carbon, 2004, 42, 2744–2747 CrossRef CAS.
- H. G. Schimmel, G. J. Kearley, M. G. Nijkamp, C. T. Visserl, K. P. de Jong and F. M. Mulder, Chem.–Eur. J., 2003, 9, 4764–4770 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670–4679 CrossRef CAS; A. G. Wong-Foy, A. J. Matzger and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 3494–3495 CrossRef CAS; N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127–1129 CrossRef CAS.
- B. L. Chen, N. W. Ockwig, A. R. Millward, D. S. Contreras and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4745–4749 CrossRef CAS.
- Metal-free analogues of the MOFs termed ‘covalent organic frameworks’ (COFs) have been suggested as hydrogen storage materials but data have yet to be published, see: A. P. Coté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 Search PubMed.
- J. Y. Lee, C. D. Wood, D. Bradshaw, M. J. Rosseinsky and A. I. Cooper, Chem. Commun., 2006, 2670–2672 RSC.
- J. Germain, J. Hradil, J. Fréchet and F. Svec, Chem. Mater., 2006, 18, 4430–4435 CrossRef CAS.
- N. T. Tsui, A. J. Paraskos, L. Torun, T. M. Swager and E. L. Thomas, Macromolecules, 2006, 39, 3350–3358 CrossRef CAS.
- S. Rifai, C. A. Breen, D. J. Solis and T. M. Swager, Chem. Mater., 2006, 18, 21–25 CrossRef CAS; Z. H. Chen, J. P. Amara, S. W. Thomas and T. M. Swager, Macromolecules, 2006, 39, 3202–3209 CrossRef CAS; T. M. Long and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 14113–14119 CrossRef CAS.
- X. Z. Zhu and C. F. Chen, J. Am. Chem. Soc., 2005, 127, 13158–13159 CrossRef CAS.
- Previously, the highest surface area (ca. 1000 m2 g−1) measured for a PIM was for a porphyrin-based network, see: N. B. McKeown, S. Hanif, K. Msayib, C. E. Tattershall and P. M. Budd, Chem. Commun., 2002, 2782–2783 Search PubMed . The hydrogen uptake at 77 K for this material is 1.25% at 1 bar and 1.95% at 10 bar.
- G. Horvath and K. Kawazoe, J. Chem. Eng. Jpn., 1983, 16, 470–475 Search PubMed.
- The measurement of H2 adsorption is technically demanding and can be prone to errors. The classical volumetric method can give false high values due to H2 leaks, whereas gravimetric analysis may exaggerate H2 adsorption greatly due to contamination with molecules of greater mass (e.g. H2O). Hence our use of both methods to avoid error, see: A. Anson, M. Benham, J. Jagiello, M. A. Callejas, A. M. Benito, W. K. Maser, A. Zuttel, P. Sudan and M. T. Martinez, Nanotechnology, 2004, 15, 1503–1508 Search PubMed.
- X. Lin, J. Jia, X. Zhao, K. M. Thomas, A. J. Blake, G. S. Walker, N. R. Champness, P. Hubberstey and M. Schroder, Angew. Chem., Int. Ed., 2006, 45, 7358–7364 CrossRef.
| This journal is © The Royal Society of Chemistry 2007 |
