Fluorescence enhancements of benzene-cored luminophors by restricted intramolecular rotations: AIE and AIEE effects†
Qi
Zeng
a,
Zhen
Li
*a,
Yongqiang
Dong
b,
Chong'an
Di
c,
Anjun
Qin
b,
Yuning
Hong
b,
Li
Ji
a,
Zhichao
Zhu
a,
Cathy K. W.
Jim
b,
Gui
Yu
c,
Qianqian
Li
a,
Zhongan
Li
a,
Yunqi
Liu
c,
Jingui
Qin
*a and
Ben Zhong
Tang
*b
aHubei Key Laboratory on Organic and Polymeric Optoelectronic Materials, Department of Chemistry, Wuhan University, Wuhan, 430072, China. E-mail: lizhen@whu.edu.cn; jgqin@whu.edu.cn; Fax: +86-27-6875-6757; Tel: +86-27-6225-4108
bDepartment of Chemistry, The Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk; Fax: +852-2358-1594; Tel: +852-2358-7375
cOrganic Solids Laboratories, Institute of Chemistry, The Chinese Academy of Sciences, Beijing, 100080, China
First published on 21st November 2006
Abstract
Photoluminescence of simple arylbenzenes with ready synthetic accessibility is enhanced by two orders of magnitude through aggregate formation; viscosity and temperature effects indicate that the emission enhancement is due to the restriction of their intramolecular rotations in the solid state.
To find technological applications as biological probes, chemical sensors and organic light-emitting diodes, a luminophor must meet a prerequisite of exhibiting high fluorescence quantum yield (ΦF) in the solid state. The formation of delocalized excitons or excimers in the solid state, however, often quenches the emissions of organic luminophors.1–3 The best approach to this notorious problem is to develop new luminophoric materials whose aggregates can emit more efficiently than their solutions.4 Along this line of approach, two novel photoluminescence (PL) processes have been identified: one is the “aggregation-induced emission enhancement (AIEE)”5 and another is the “aggregation-induced emission (AIE)”.6–8 In the former, light emission of a chromophoric material is enhanced by aggregate formation, while in the latter, a nonemissive material is induced to emit by aggregation. An example of AIEE material is a cyclophane-decorated poly(p-phenyleneethynylene): it is emissive in the solution but becomes more emissive when aggregated, with a 3.5-fold increase in its ΦF.5a Hexaphenylsilole (Chart 1, structure A), on the other hand, is an AIE dye. Its solution is nonemissive but its aggregates are highly emissive: ΦF of the latter (23%) is two orders of magnitude higher than that of the former (0.2%).6b
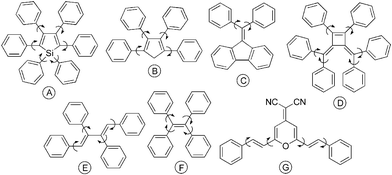 | ||
| Chart 1 Examples of AIE luminophors developed in our laboratories. | ||
The novel AIE(E) effects challenge our current understanding of PL processes, deciphering whose causes and mechanisms may help spawn new photophysical theories and technological innovations. We have been interested in studying structure-property relationship of AIE dyes. During our study on the silole-based AIE system, we have proposed that the restriction of intramolecular rotations (RIR) of the phenyl peripheries against the silacyclopentadiene core in the aggregation state is the main cause for the AIE effect.6–10 In an effort to verify this hypothesis and to develop more AIE systems, we have designed a series of propeller-like molecules consisting of mono- and polyene stators and multiple aryl rotors (Chart 1, B–G). As expected, all the dyes are AIE-active, emitting various colours including blue, green and red efficiently in the aggregation state.9 Among the AIE dyes, tetraphenylethene (TPE; Chart 1, F) and its derivatives are particularly noteworthy: they can be synthesized by simple reactions, emit highly efficiently in the solid state, and work as excellent biological probes for protein, DNA and RNA detection and quantitation.9e
The TPE system is simple, but can we develop even simpler AIE systems? For example, will the molecules with structures sketched in Chart 2 be AIE-active? These molecules are mono- (I), di- (II), and triarylated (III) benzenes with their aryl substituents directly attached to the benzene core without olefinic double-bond linkers, which are readily accessible by simple reactions. In this report, we show that 1–3 are AIE-active while 4–6 are AIEE-active. We prove that the RIR process is at work in both AIE and AIEE systems.
 | ||
| Chart 2 Chromophoric molecules comprising a benzene core (black oval) and one (I), two (II) and three (III) aryl substituents (grey oval). | ||
We first examined the PL behaviours of biphenyl, the simplest model of I, in solution and aggregation states. As can be seen from Fig. S1 (ESI†), its PL intensity is greatly enhanced by adding water into its DMF solution to induce aggregate formation. Encouraged by this result, we designed two groups of arylbenzenes with the aryl groups being phenyl (1–3) and carbazolyl (4–6; Chart 3). These molecules were readily prepared by the synthetic routes shown in Schemes S1 and S2 (ESI†). Their structures were characterized by spectroscopy methods, from which satisfactory analysis data were obtained (ESI†). The dyes are soluble in common organic solvents such as acetone, chloroform and THF but insoluble in water.
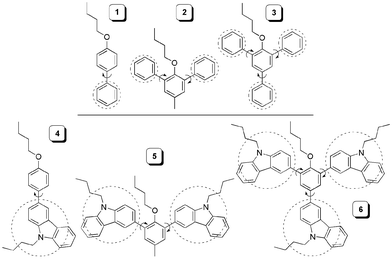 | ||
| Chart 3 Mono- (1 and 4), di- (2 and 5), and triarylated (3 and 6) benzene derivatives studied in this work. | ||
Dilute acetone solutions of 1–3 are practically nonluminescent. However, when large amounts of water were added to their acetone solutions (with final concentrations kept unchanged at 20 µM), the resultant mixtures gave intense PL spectra (Fig. 1(A)). Although the mixtures were macroscopically homogeneous, particle size analysis revealed that nanoscopic aggregates of the dyes were formed in the mixtures with high water contents. Evidently, the emissions of the dyes are induced by aggregation formation, or in other words, 1–3 are AIE-active.
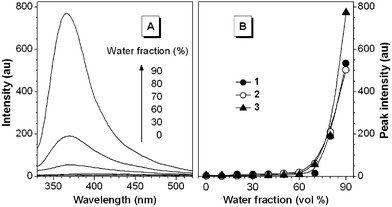 | ||
| Fig. 1 (A) PL spectra of the dilute solutions of 3 (20 µM) in water/acetone mixtures with different water contents. Excitation wavelength: 314 nm. (B) Changes in the PL peak intensities of 1–3 with different water fractions in the water/acetone mixtures. | ||
The ΦF values of 1–3 could not be accurately determined due to the involved technical difficulty. We thus plotted the changes in their PL peak intensities vs. water contents in the aqueous mixtures (Fig. 1(B)). The PL peak intensity remained almost unchanged when less than ∼60% water was added to the acetone solutions but started to swiftly increase afterwards because the solvating power of the mixture had been worsened to such an extent that the dyes began to aggregate. When the water fraction was increased to 90%, the PL peak intensities were increased by up to ∼200 times (Table 1). The similar results were obtained when water was added into the dye solutions in other organic solvents.
| λemb | Emission enhancement | |||||
|---|---|---|---|---|---|---|
| Dye | λ ab a | Solnc | Aggtd | Filme | I A/ISf | Φ F,A/ΦF,Sg |
| a Absorption maximum (nm). b Emission maximum (nm). c Solution (in acetone). d Aggregate. e With dye molecules dispersed in a PMMA matrix in ∼30 wt%. f Ratio of PL peak intensities of aggregate (IA) and solution (IS). g Ratio of fluorescence quantum yields of aggregate (ΦF,A) and solution (ΦF,S), given in the parentheses are the original ΦF values. h Not determined. i Aggregate in a water–acetone mixture with 90 vol% water. j Aggregate in a water–acetone mixture with 30 vol% water. | ||||||
| 1 | ndh | 343 | 343i | 335 | 163i | ndh |
| 2 | ndh | 368 | 370i | 358 | 199i | ndh |
| 3 | ndh | 365 | 368i | 359 | 155i | ndh |
| 4 | 323 | 396 | 396j | 400 | 1.15j | 1.43 (67/47)j |
| 5 | 323 | 380 | 381j | 382 | 1.43j | 1.37 (41/30)j |
| 6 | 323 | 394 | 394j | 401 | 1.47j | 1.88 (30/16)j |
The actual PL intensities of the dye aggregates should be higher than those shown in Fig. 1, because only have the dye molecules on the surfaces of the nanoparticles contributed to the PL spectra.11 We blended the dyes with poly(methyl methacrylate) (PMMA) and found that the PL peak intensities of the resultant films were much higher than those of the nanoaggregate suspensions (Fig. S2, ESI†). The dye molecules may have better miscibility with PMMA chains, leading to better dispersion of the dye molecules in the polymer matrix. This should result in the formation of smaller nanoparticles, hence the observed stronger emissions. Intriguingly, the λem values of 1–3 in the PMMA films are generally blue-shifted from those of their aggregates (Table 1), possibly due to the dye crystallization in the solid films.6e,12
It is known that increasing solvent viscosity and/or decreasing solution temperature can slow down intramolecular rotations13 or strengthen the RIR process. We thus checked how variations in the viscosity and temperature would affect the dye emissions. The PL intensities of 1–3 are increased by adding glycerol, a viscous liquid, into their ethanol solutions (Fig. 2(A)). In the mixtures with glycerol contents of <50%, the intensity increases are due to a pure viscosity effect because the dye molecules are soluble in these mixtures. In the mixtures with glycerol contents of >50%, the increases are due to the combined effects of viscosity and aggregation because the dye molecules become progressively less soluble in the mixtures with higher glycerol contents. As can be seen from Fig. 2(B) and Fig. S3(A) (ESI†), decreasing the solution temperatures leads to enhanced emissions. The viscosity and temperature effects prove that the RIR process is indeed involved in the AIE system.
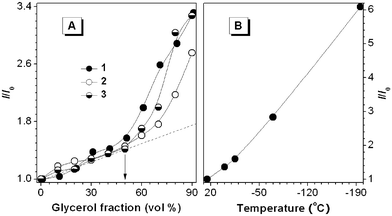 | ||
| Fig. 2 (A) Changes in the PL peak intensities (I) of the solutions of 1–3 (20 µM) with the glycerol contents in the ethanol/glycerol mixtures. I0 = intensity in pure ethanol solution. (B) Temperature effect on the PL peak intensity of 3. I0 = intensity at room temperature (25 °C). | ||
Different from 1–3, dyes 4–6 are emissive in the solution state. Their aggregates, however, are more emissive (Fig. S4, ESI†). The dyes are thus AIEE emitters. Their ΦF values continuously increase with increasing the water contents in the water/acetone mixtures and reach maximums at a water content of ∼30% (Fig. S5, ESI†). The ΦF values decrease with further increasing the water contents because of the poor solubilities of the dyes in the mixtures with higher water contents, as evidenced by the turbidity of the mixtures. Again, when the dyes were blended with PMMA, the resultant films emitted lights in very high intensities (Fig. S4, ESI†).
The PL intensities of THF solutions of 4–6 were enhanced by decreasing the solution temperatures (Fig. S3(B), ESI†), suggesting that the RIR process is also involved in the AIEE systems. It is interesting to note that their ΦF values increase in the order of 4 → 5 → 6 (Table 1), while the numbers of their carbazolyl substituents decrease in the order of 6 → 5 → 4. The ΦF value of 6 is the lowest, although it possesses the highest number of carbazolyl groups. This is easily understandable: the more the carbazolyl rotors, the higher the probability for intramolecular rotations, hence the lower the ΦF value.
Given that a carbozole solution is emissive,13–15 it is no wonder carbazolylbenzenes 4–6 are emissive in the dilute solutions. In the PMMA films, the carbazolyl blades may experience π–π stacking interaction, which red-shifts the dye emissions (Table 1). The π–π stacking should decrease the PL intensities of the dyes, but the RIR process in the solid state enhances light emission. The net outcome of these two antagonistic processes is the observed AIEE effect, indicating that the RIR process has played a predominant role in affecting the PL behaviours of the dye aggregates.
In summary, in this work, we have successfully developed a new series of AIE- (1–3) and AIEE-active (4–6) luminophors with very simple structures. All these dyes are “pure” aromatic compounds without olefinic functionality and thus should be very stable. With the tremendous synthetic flexibility offered by the rich reactions in aromatic chemistry, it is anticipated that many more AIE(E) dyes can be created with minimum efforts. Our results have proved that the RIR process plays a critical role in not only AIE but also AIEE systems. Further studies on the AIE(E) dyes with visible emissions are under way in our laboratories.
We acknowledge the financial support from the National Natural Science Foundation of China (Project Nos. 20402011, 20674059 and 90201002), the National Basic Research “973” Program, the Research Grants Council of Hong Kong (Project Nos. 602706, 603505 and HKU2/05C), and the Hubei Provincial Government.
Notes and references
- (a) H. Murata, Z. H. Kafafi and M. Uchida, Appl. Phys. Lett., 2002, 80, 189 CrossRef CAS; (b) S. J. Toal, K. A. Jones, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2005, 127, 11661 CrossRef CAS.
- (a) Z. Xie, B. Yang, W. Xie, L. Liu, F. Shen, H. Wang, X. Yang, Z. Wang, Y. Li, M. Hanif, G. Yang, L. Ye and Y. Ma, J. Phys. Chem. B, 2006, 110, 20993 CrossRef CAS; (b) C. J. Bhongale, C.-W. Chang, C.-S. Lee, E. W.-G. Diau and C.-S. Hsu, J. Phys. Chem. B, 2005, 109, 13472 CrossRef CAS; (c) S. A. Jenekhe and J. A. Osaheni, Science, 1994, 265, 765 CrossRef CAS.
- (a) J. Cornil, D. A. Dos Santos, X. Crispin, R. Silbey and J. L. Bredas, J. Am. Chem. Soc., 1998, 120, 1289 CrossRef CAS; (b) X. Liu, C. He, X. Hao, L. Tan, Y. Li and K. S. Ong, Macromolecules, 2004, 37, 5965 CrossRef CAS.
- (a) L. H. Chan, R. H. Lee, C. F. Hsieh, H. C. Yeh and C. T. Chen, J. Am. Chem. Soc., 2002, 124, 6469 CrossRef CAS; (b) S. Wang, W. J. Oldham, R. A. Hudack, Jr. and G. C. Bazan, Jr., J. Am. Chem. Soc., 2000, 122, 5695 CrossRef CAS; (c) Y. Li, J. Ding, M. Day, T. Tao, J. Lu and M. D'iorio, Chem. Mater., 2003, 15, 4936 CrossRef CAS; (d) F. Cherious and L. Guyard, Adv. Funct. Mater., 2003, 11, 305.
- (a) R. Deans, J. Kim, M. R. Machacek and T. M. Swager, J. Am. Chem. Soc., 2000, 122, 8565 CrossRef CAS; (b) B. K. An, S. K. Kwon, S. D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410 CrossRef CAS.
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC; (b) J. Chen, C. C. W. Law, J. W. Y. Lam, Y. Dong, S. M. F. Lo, I. D. Williams, D. Zhu and B. Z. Tang, Chem. Mater., 2003, 15, 1535 CrossRef CAS; (c) G. Yu, S. Yin, Y. Liu, J. Chen, X. Xu, X. Sun, D. Ma, X. Zhan, Q. Peng, Z. Shuai, B. Z. Tang, D. Zhu, W. Fang and Y. Luo, J. Am. Chem. Soc., 2005, 127, 6335 CrossRef CAS; (d) H. Chen, W. Y. Lam, J. Luo, Y. Ho, B. Z. Tang, D. Zhu, M. Wong and H. S. Kwok, Appl. Phys. Lett., 2002, 81, 574 CrossRef CAS; (e) Y. Dong, J. W. Y. Lam, Z. Li, H. Tong, Y. Dong, X. Feng and B. Z. Tang, J. Inorg. Organomet. Polym. Mater., 2005, 15, 287 CrossRef CAS.
- M. Freemantle, Chem. Eng. News, 2001, 79(41), 29.
- (a) H. C. Yeh, S. J. Yeh and C. T. Chen, Chem. Commun., 2003, 2632 RSC; (b) C. J. Bhongale and C. S. Hsu, Angew. Chem., Int. Ed., 2006, 45, 1404 CrossRef CAS; (c) W. Holzer, A. Penzkofer, R. Stockmann, H. Meysel, H. Liebegott and H. H. Horhold, Polymer, 2001, 42, 3183 CrossRef CAS; (d) S. Jayanty and T. P. Radhakrishnan, Chem. Eur. J., 2004, 10, 791 CrossRef CAS; (e) Z. Wang, H. Shao, J. Ye, L. Tang and P. Lu, J. Phys. Chem. B, 2005, 109, 19627 CrossRef CAS; (f) K. Itami, Y. Ohashi and J. Yoshida, J. Org. Chem., 2005, 70, 2778 CrossRef CAS; (g) M. Han and M. Hara, J. Am. Chem. Soc., 2005, 127, 10951 CrossRef CAS; (h) H. J. Tracy, J. L. Mullin, W. T. Klooster, J. A. Martin, J. Haug, S. Wallace, I. Rudloe and K. Watts, Inorg. Chem., 2005, 44, 2003 CrossRef CAS; (i) K. Itami and J. Yoshida, Chem. Eur. J., 2006, 12, 3966 CrossRef CAS; (j) C. Belton, D. F. O'Brien, W. J. Blau, A. J. Cadby, P. A. Lane, D. D. C. Bradley, H. J. Bryne, R. Stockmann and H. H. Horhold, Appl. Phys. Lett., 2001, 78, 1059 CrossRef CAS.
- (a) H. Tong, Y. Dong, M. Häußler, Z. Li, B. Mi, H. S. Kwok and B. Z. Tang, Mol. Cryst. Liq. Cryst., 2006, 446, 183 CrossRef CAS; (b) H. Tong, Y. Q. Dong, M. Haussler, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, J. Sun and B. Z. Tang, Chem. Commun., 2006, 1133 RSC; (c) J. Chen, B. Xu, X. Ouyang, B. Z. Tang and Y. Cao, J. Phys. Chem. A, 2004, 108, 7522 CrossRef CAS; (d) H. Tong, Y. Dong, M. Häußler, Y. Hong, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, H. S. Kwok and B. Z. Tang, Chem. Phys. Lett., 2006, 428, 326 CrossRef CAS; (e) H. Tong, Y. Hong, Y. Dong, M. Haussler, J. W. Y. Lam, Z. Li, Z. F. Guo, Z. H. Guo and B. Z. Tang, Chem. Commun., 2006, 3705 RSC.
- (a) Z. Li, Y. Q. Dong, B. Mi, Y. Tang, M. Haussler, H. Tong, Y. P. Dong, J. W. Y. Lam, Y. Ren, H. H. Y. Sung, K. S. Wong, P. Gao, I. D. Williams, H. S. Kwok and B. Z. Tang, J. Phys. Chem. B, 2005, 109, 10061 CrossRef CAS; (b) Y. Ren, J. W. Y. Lam, Y. Dong, B. Z. Tang and K. S. Wong, J. Phys. Chem. B, 2005, 109, 1135 CrossRef CAS; (c) Y. Ren, Y. Dong, J. W. Y. Lam, B. Z. Tang and K. S. Wong, Chem. Phys. Lett., 2005, 402, 468 CrossRef CAS; (d) S. Yin, Q. Peng, Z. Shuai, W. Fang, Y. Wang and Y. Luo, Phys. Rev. B, 2006, 73, 205409 CrossRef.
- In the silole system, the absolute quantum yields (ΦF,a) of the AIE-active siloles are higher than their relative ones (ΦF,r): for example, ΦF,a of 1-methylpentaphenylsilole (85%)6a is four-fold higher than its ΦF,r (21%)6c.
- Y. Q. Dong, J. W. Y. Lam, A. Qin, Z. Li, J. Sun, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Chem. Commun., 2006 10.1039/b613157c.
- N. J. Turro, Modern Molecular Photochemistry, University Science Books, Mill Valley, CA, 1991 Search PubMed.
- (a) W. Zhu, M. Hu, R. Yao and H. Tian, J. Photochem. Photobiol. A: Chem., 2003, 154, 169 CrossRef CAS; (b) Y. Liu, G. Yu, H. Li, H. Tian and D. Zhu, Thin Solid Films, 2002, 417, 107 CrossRef CAS.
- P. P. S. Lee, Y. Geng, H. S. Kwok and B. Z. Tang, Thin Solid Films, 2000, 363, 149 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation and characterization details for 1–6; change of PL intensity of biphenyl with water content in water–DMF mixtures; PL spectra of solutions, aggregates and films of 1–6; temperature effect on PL intensity in THF solutions of 1–6; dependence of fluorescence quantum yield of 4–6 on water content in water–acetone mixtures. See DOI: 10.1039/b613522f |
| This journal is © The Royal Society of Chemistry 2007 |
