From crystal engineering to cluster engineering: How to transform cadmium chloride from 2-D to 0-D†
Chun-Long
Chen
and
Alicia M.
Beatty
*
Department of Chemistry, Mississippi State University, Mississippi State, MS 39762, USA. E-mail: abeatty@ra.msstate.edu; Fax: +1 662 3251618; Tel: +1 662 325 3588
First published on 15th November 2006
Abstract
The transformation of a 2-D perovskite structure to “expanded” 2-D and finally to a 0-D hexanuclear cadmium chloride cluster by varying the size of substituents on the associated counterions (H vs. methyl vs. ethyl) is described.
A tenet of crystal engineering has been that the rational design of molecular building blocks is necessary for the control of network topology (1D, 2D, 3D), in order to create materials useful for applications in various areas such as gas storage, gas separation, catalysis, electronics, host–guest chemistry.1 Therefore, the design of inorganic networks (both coordination polymer2 and hydrogen-bonded3) usually depends on well-known coordination chemistry, for example building 1-D networks from e.g. linear Ag(I) ions, or 2-D networks from e.g. square planar Pt(II). Similarly, organic networks of desired dimensionality are created by using molecules or ions of correct size and shape (tetrahedral for diamondoid networks,4 flat molecules for 2-D5) with e.g. hydrogen bonding moieties placed at the appropriate positions for network propagation.
On the other hand, the Lee group6 has made the intriguing observation that the dimensionality of organic hydrogen-bonded networks can be controlled by changing the “hydrophobic to total-volume ratio” in amphiphiles. For example, a layered (2-D) organic arrangement can be transformed to a cylindrical (1-D) or a spherical (0-D) arrangement by increasing the hydrophobic content. It has been reported previously that the dimensionality (0-D, 1-D, 2-D, 3-D) of e.g. lead iodide networks can be affected by changing the counterions used in synthesis,7 but so far there has not been a report of a rational method of effecting 2-D vs. 1-D vs. 0-D metal halide architectures.
An interesting question is whether or not the metrics and dimensionality of inorganic assemblies can be controlled by systematically tuning the steric demands of the counterion. We began to address this question by using a recurring inorganic pattern (anionic metal halide layers) and adding organic substituents on associated cations that might interrupt the inorganic assembly.
Metal halides having the perovskite sheet structure in which the structures comprise anionic metal–halide sheets with ammonium counterions (H3NRNH3)2+MX42− (M = divalent metal; X = halogen atoms; R = organic moiety) have been the focus of a number of investigations in recent years.1d,8 The cations span the distance between perovskite layers, so that the interlayer metrics increase with the length of the cation.1d The variable bridging units allow investigations of structure–function relationships.
Here we report a systematic transformation in anionic cadmium chloride assemblies, where the layers (2D) are expanded, then condensed into a hexanuclear (0-D) cluster by increasing the size of the hydrophobic substituents on the counterions. The synthesis and structural characterization of four dianilinium cadmium chloride complexes: [CdCl4(H2A)]n (1), [CdCl4(H2B)]n (2), {[CdCl4(H2C)2]Cl2}n (3) and [Cd6Cl19(H2D)4](Cl)(H2O)12 (4) (A = benzidine, B = 4,4′-diaminostilbene, C = 3,3′-dimethylbenzidine, D = 3,3′-diethylbenzidine) are given, demonstrating how the cadmium chloride layer can be transformed into a very unusual discrete [Cd6Cl19]7− cage compound.
Complexes 1–4 are prepared by reaction of excess of CdCl2 with HCl and the corresponding diammonium chloride in methanol solution.‡ X-Ray quality single crystals are obtained by evaporating clear solutions slowly at room temperature.§
Both 1 and 2 crystallize in the monoclinic P21/c space group, and consist of [CdCl4]n2n− perovskite layers pillared by [H2L]2+ dianilinium cations as shown in Fig. 1, with one CdCl42− unit for each dication. The cadmium ion is located on an inversion center and adopts an octahedral coordination environment (dCd–Cl = 2.5–2.7Å), and each ammonium is connected via hydrogen bonds to three chloride ions from the same layer, similar to other (H3NRNH3)MX4 complexes reported previously.1d,8b It is worthwhile to mention that the extension of benzidine to 4,4′-diaminostilbene did not change the overall structure; this can be attributed to the similar steric requirements (substituent ortho to ammonium = H) for these two dianilinium cations.
 | ||
| Fig. 1 Crystal structure of 1, with anionic cadmium chloride layers separated by dianilinium cations. Represented by CdCl6 octahedra and dashed lines to indicate NH⋯Cl hydrogen bonds. | ||
When 3,3′-dimethylbenzidine (substituent ortho to ammonium = CH3) was used under the same reaction conditions, 3 was obtained. Single crystal X-ray diffraction results show that the asymmetric unit in 3 has one unique half of the CdCl4 dianion and one 3,3′-dimethylbenzidine diammonium cation, giving one CdCl42− for two dications, with two uncoordinated chloride ions (one Cl− per asymmetric unit) also present. Unlike the uniform distribution of Cd–Cl bond lengths in the layers of 1 and 2, the perovskite layer in 3 is “expanded,” containing discrete distorted tetrahedral CdCl42− units (dCd–Cl = 2.5–2.6Å) that are relatively weakly inter-connected (dCd⋯Cl = 3.1–3.9 Å), Fig. 2. In complex 3, each [CdCl4]n2n− layer is separated by two equivalents of dianilinium ions that are further separated by the free chloride anions. All cations and anions are interconnected via ammonium–chloride hydrogen bonds.
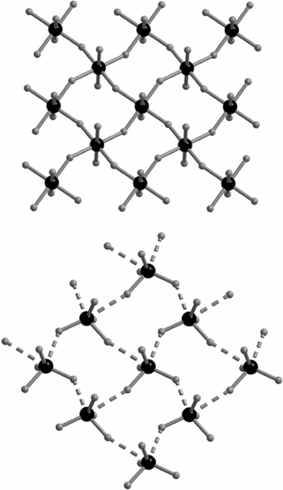 | ||
| Fig. 2 Cadmium chloride layers in 1 and 2 (top) vs. the “expanded” layers in 3. | ||
This “expansion” of layered MX42− compounds (M = Pb and Sn) has previously been attributed to stereochemically active lone pairs,9 which seems unlikely in the present case involving d10 Cd(II). In fact, similarly substituted methyl-anilinium (mono-ammonium rather than bridging di-ammonium) counterions yield a nearly identical expanded layer in the structure of “bis(2-methyl-4-nitroanilinium) tetrachloro-cadmate,”10 even though it has no additional chloride ions (as in 3). Therefore, we attribute the layer expansion specifically to the methyl-substituent on the anilinium counterion.
Complex 4 is synthesized under identical conditions with the 3,3′-diethylbenzidine (substituent ortho to ammonium = CH2CH3). In 4, the infinite layer has been forgone altogether in favor of a cadmium chloride cluster having the formula [Cd6Cl19]7−. These clusters are bridged in the solid assembly by cations, water, and chloride ions. The asymmetric unit of 4, which crystallizes in the tetragonal P4/mnc space group, contains one eighth of the hexameric cluster, one half of the dianilinium counterion, one and a half uncoordinated water molecules and an eighth of a free chloride ion that is disordered over two positions. As shown in Fig. 3, the structure of [Cd6Cl19]7−, which contains a central μ6-Cl, is reminiscent of the well-known hexamolybdate ion,11 [Mo6O19]2−, consisting of a μ6 bridging chloride surrounded by six cadmium ions, with an additional twelve bridging and six terminal chlorides. The Cd–Cl bond lengths of 2.862(1) and 3.043(1) Å in the cluster are significantly longer than normal Cd–Cl bond lengths. As far as we can determine, [Cd6Cl19]7− is the first example of a hexameric cadmium halide cluster, and is the first metal halide having a framework structure akin to that of hexamolybdate.
![Top: Thermal ellipsoid plot of [Cd6Cl19]7− from crystal structure data of 4. Bottom: Hydrogen-bonded layered network of hexamers and water molecules in 4, OH⋯Cl hydrogen bonds shown as dotted lines.](/image/article/2007/CC/b613761j/b613761j-f3.gif) | ||
| Fig. 3 Top: Thermal ellipsoid plot of [Cd6Cl19]7− from crystal structure data of 4. Bottom: Hydrogen-bonded layered network of hexamers and water molecules in 4, OH⋯Cl hydrogen bonds shown as dotted lines. | ||
Comparing the structures of 1–4, we can conclude that the length of the diammonium cation did not affect the overall architecture, but the substituent ortho to the ammonium moiety (H vs. Me vs. Et) did play an important role in the inorganic assembly. That is, increasing the size of ortho substituents led to expansion of the layer and finally to the formation of a large 0-D chloride cage anion. It is possible that, by changing the nature of the anilinium cation (i.e. adding substituents of different sizes ortho and meta to the ammonium substituent), we will be able to further change the size and shape of the inorganic assembly from 2-D to 1-D to 0-D. This may also open up access to other polymeric anions (dimers, trimers, tetramers, etc.) through use of ortho-substituted anilinium and dianilinium cations as templates.
We gratefully acknowledge the financial support of this work by Mississippi State University, NSF-EPSCoR and the ACS-Petroleum Research Fund (Grant #43466-AC10).
Notes and references
- (a) J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670 CrossRef CAS; (b) D. Bradshaw, T. J. Prior, E. J. Cussen, J. B. Claridge and M. J. Rosseinsky, J. Am. Chem. Soc., 2004, 126, 6106 CrossRef CAS; (c) H. L. Ngo and W. Lin, Top. Catal., 2005, 34, 85 CrossRef CAS; (d) D. B. Mitzi, in Functional Hybrid Materials, ed. P. Gomez-Romero and C. Sanchez, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2004, pp. 347–386 Search PubMed.
- Representative recent coordination polymer reviews: (a) S. L. James, Chem. Soc. Rev., 2003, 32, 276 RSC; (b) C. Janiak, Dalton Trans., 2003, 2781 RSC; (c) C. N. R. Rao, S. Natarajan and R. Vaidhyanathan, Angew. Chem., Int. Ed., 2004, 43, 1466 CrossRef CAS; (d) C.-L. Chen, B.-S. Kang and C.-Y. Su, Aust. J. Chem., 2006, 59, 3 CrossRef CAS; (e) S. J. Loeb, Chem. Commun., 2005, 1511 RSC; (f) S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS.
- (a) A. M. Beatty, Coord. Chem. Rev., 2003, 246, 131 CrossRef CAS; (b) A. M. Beatty, B. A. Helfrich, G. A. Hogan and B. A. Reed, Cryst. Growth Des., 2006, 6, 122 CrossRef CAS.
- J. D. Wuest, Chem. Commun., 2005, 5830 RSC.
- (a) M. D. Ward, Chem. Commun., 2005, 5838 RSC; (b) S. Oshita and A. Matsumoto, Chem.–Eur. J., 2006, 12, 2139 CrossRef CAS; (c) K. Biradha, D. Dennis, V. A. MacKinnon, C. V. K. Sharma and M. J. Zaworotko, J. Am. Chem. Soc., 1998, 120, 11894 CrossRef CAS; (d) A. M. Beatty, K. E. Granger and A. E. Simpson, Chem.–Eur. J., 2002, 8, 3254 CrossRef CAS; (e) A. M. Beatty, C. M. Schneider, A. E. Simpson and J. L. Zaher, CrystEngComm, 2002, 4, 282 RSC.
- S. Lee, A. B. Mallik, Z. T. Xu, E. B. Lobkovsky and L. Tran, Acc. Chem. Res., 2005, 38, 251 CrossRef CAS.
- (a) H. Krautscheid and F. Vielsack, Angew. Chem., Int. Ed. Engl., 1995, 34, 2035 CrossRef CAS; (b) Z. Tang and A. M. Guloy, J. Am. Chem. Soc., 1999, 121, 452 CrossRef CAS; (c) X.-H. Zhu, N. Mercier, A. Riou, P. Blanchard and P. Frère, Chem. Commun., 2002, 2160 RSC; (d) J. Guan, Z. Tang and A. M. Guloy, Chem. Commun., 2005, 48 RSC.
- (a) X.-H. Zhu, N. Mercier, P. Frère, P. Blanchard, J. Roncali, M. Allain, C. Pasquier and A. Riou, Inorg. Chem., 2003, 42, 5330 CrossRef CAS; (b) S. A. Bourne and Z. Mangombo, CrystEngComm, 2004, 6, 437 Search PubMed.
- Z. Tang, J. Guan and A. M. Guloy, J. Mater. Chem., 2001, 11, 479 RSC.
- R. Azumi, K. Honda, M. Goto, J. Akimoto, Y. Oosawa, H. Tachibana, M. Tanaka and M. Matsumoto, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1996, 52, 588 CrossRef.
- M. T. Pope, Polyoxo Anions: Synthesis and Structure, in Comprehensive Coordination Chemistry II,, ed. A. G. Wedd, Elsevier, Oxford, 2004, vol. 4, p. 635 Search PubMed.
- (a) SMART Version 5.625, SAINT + Version 6.22, Bruker Analytical X-ray Systems, Inc., Madison, Wisconsin, USA, 2001 Search PubMed; (b) G. M. Sheldrick, SHELXTL Version 6.1, Bruker Analytical X-ray Systems, Inc., Madison, Wisconsin, USA, 2000 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization details for 1–4. See DOI: 10.1039/b613761j |
| ‡ General procedure for preparation of 1–4: A solution of CdCl2 (0.2 mmol) with two drops of concentrated HCl (37%) in methanol (2 mL) was added to a solution of the diammonium chloride (0.1 mmol) in methanol (2 mL). The resulting solution was stirred for 0.5 h and filtered. Colorless single crystals 1–4 suitable for X-ray diffraction analyses were obtained by slow evaporation of the clear reaction filtrate. Yield: 52% (1), 56% (2), 40% (3), 38% (4). Bulk phase purity was established by powder X-ray diffraction for complexes 1, 2 and 4. Detailed synthetic procedures, additional figures, hydrogen bond tables and powder XRD information can be found in the ESI.† The agreement between the calculated and measured diffraction patterns, shown in Fig. S5, supports phase purity of the complexes and rules out the possibility of a second phase. |
| § Single-crystal X-ray diffraction: Bruker SMART 1000 CCD diffractometer.121 C24H28Cd2N4Cl8M = 880.90, monoclinic, space group P2(1)/c, a = 14.765(5), b = 7.271(3), c = 7.363(3) Å, β = 97.447(6)°, V = 783.8(5) Å3, Z = 1, ρcalc = 1.866 g cm−3, T = 299 (2) K, F(000) = 432, μ(Mo-Kα) = 2.061 mm−1, 5557 reflections measured, 1393 unique (Rint = 0.0282), 88 parameters, R1 = 0.0187 (I > 2σ(I)), wR2 = 0.0505 (all data), GOF = 1.045. 2 C14H16CdN2Cl4M = 466.49, monoclinic, space group P2(1)/c, a = 17.079(3), b = 7.359(2), c = 7.274 (2) Å, β = 97.423(3)°, V = 906.5(3) Å3, Z = 2, ρcalc = 1.709 g cm−3, T = 300 (2) K, F(000) = 460, μ(Mo-Kα) = 1.788 mm−1, 6414 reflections measured, 1609 unique (Rint = 0.0342), 97 parameters, R1 = 0.0296 (I > 2σ(I)), wR2 = 0.1047 (all data), GOF = 1.195. 3 C112H144Cd4N16Cl24M = 3014.83, orthorhombic, space group Pnma, a = 8.094(2), b = 48.185(11), c = 8.239(2) Å, V = 3213.1(13) Å3, Z = 1, ρcalc = 1.558 g cm−3, T = 299 (2) K, F(000) = 1528, μ(Mo-Kα) = 1.208 mm−1, 22004 reflections measured, 2862 unique (Rint = 0.0775), 181 parameters, R1 = 0.0430 (I > 2σ(I)), wR2 = 0.1312 (all data), GOF = 1.167. 4 C128H224Cd12N16O24Cl40M = 5138.03, tetragonal, space group P4/mnc, a = 12.546(3), b = 12.546(3), c = 32.740(9) Å, V = 5154(2) Å3, Z = 1, ρcalc = 1.656 g cm−3, T = 301 (2) K, F(000) = 2552, μ(Mo-Kα) = 1.656 mm−1, 23117 reflections measured, 2328 unique (Rint = 0.0602), 153 parameters, R1 = 0.0333 (I > 2σ(I)), wR2 = 0.0769 (all data), GOF = 1.064. CCDC 612085–612089. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b613761j |
| This journal is © The Royal Society of Chemistry 2007 |
