Flexible chocolate
Yu Wen
Chen
and
Malcolm Robert
Mackley
*
Department of Chemical Engineering, University of Cambridge, New Museums Site, Pembroke Street, Cambridge, CB2 3RA, UK. E-mail: mrm5@cam.ac.uk
First published on 10th February 2006
Abstract
Chocolate is a complex soft solid that undergoes a series of physical transformations in the mouth during consumption. Normally chocolate products are manufactured by liquid processing techniques that include ‘melting and casting’ liquid chocolate into moulds as well as enrobing sweet centres with a chocolate outer shell. There is however another method of shape forming chocolate and this review discusses the key elements of a so called ‘cold extrusion’ process. This process involves a combination of pressure and flow, which results in a product with a temporary flexibility. The review covers the basic aspects of the composition and microstructure of chocolate together with experimental observations on the cold extrusion process and the subsequent post extrusion flexibility of the extrudate. Finally some physical insight into the unusual behaviour of chocolate during and after cold extrusion is presented.
 Yu Wen Chen | Yu Wen Chen is currently completing a PhD within the Department of Chemical Engineering at Cambridge University on aspects relating to the shape forming of cold extruded chocolate. She obtained her MEng degree in Chemical Engineering at Imperial College London. |
 Malcolm Mackley | Malcolm Mackley is a Professor of Process Innovation and heads a Polymer Fluids Group within the Dept. of Chemical Engineering at Cambridge University. His research interests include the rheological aspects of polymer melt extrusion processing, the development of plastic MicroCapillary Film (MCF) and continuous OFM (Oscillatory Flow Mixing) biodiesel and mesoreactors. Prof. Mackley is also currently the President of the British Society of Rheology. |
Introduction
An essential component of chocolate comes from the cocoa beans of the Theobroma cacao tree. Cocoa butter, the fat extract obtained by pressing ground cocoa beans, is a primary ingredient that allows chocolate to melt and flow easily in the mouth and this greatly influences the texture of the chocolate. One key element of the texture is that chocolate remains essentially a semi-solid at room temperature, but melts readily in the mouth at the normal body temperature of 37 °C. Due to its distinct and pleasant flavour and texture, chocolate is universally popular and a steady increase in demand has raised the sophistication of chocolate processing. Liquid forming techniques such as ‘melt and cast’ have been commonly used to produce chocolate shapes and this usually involves pouring a tempered chocolate mass into moulds or over sweet centres to form a chocolate shell, a process known as enrobing. While most of the chocolate products are still manufactured by these conventional methods, other ways of producing chocolate shapes have emerged. It was discovered that chocolate could be shape formed by extrusion processing with methods now widely associated with polymer,1,2 metal3 or ceramic paste processing.4 Normally polymers are extruded in the melt but extrusion below the melting point of polymers has been reported before5,6 and this forms a starting point for chocolate cold extrusion.The idea behind the extrusion of chocolate dates back to the early 1920's when the semi-solid processing of chocolate was first carried out by Laskey, who showed that chocolate could be extruded at high pressures through a die.7 No further work on the semi-solid extrusion of chocolate had been reported until 1994, when Mackley et al.8 described the isothermal extrusion of chocolate below its peak melting temperature and the resulting product possessed a certain short-term flexibility. This temporary flexibility and shape retainment ability of the extrudate immediately after extrusion offer interesting opportunities to form shapes that cannot be manufactured by traditional chocolate production methods. It also raised scientific questions as to why the material behaved in this unexpected and unusual way.
This review briefly describes the basic microstructure of chocolate and the behaviour of chocolate during and after cold extrusion. In addition, the possible link between the mechanical properties of the material and its microstructure is given.
1. Chocolate composition and microstructure
Chocolate is a complex material with physical characteristics that can vary significantly within a relatively small temperature range. At room temperature, chocolate is mainly in the semi solid state. However by the time it reaches the body temperature of 37 °C, the material is predominantly viscous and can possess a low yield stress of approximately 10–20 Pa. Chocolate can be variously described as a soft solid, a concentrated suspension or a paste. Chocolate consists of a high concentration (50–60% by vol.) of suspended solid particles, which comprise mostly of sugar crystals, cocoa and milk solids and these are dispersed in a continuous fat matrix made of cocoa butter and milk fats (Fig. 1). Another ingredient commonly found in commercial chocolate is an emulsifier, usually soya lecithin, which is added to promote the coating of the hydrophilic sugar particles with the hydrophobic fat molecules. Cocoa butter is an essential ingredient in chocolate and this component is mainly responsible for the rheological properties of the material, in both the molten and the solid form. One reason for the complexity of chocolate is due to the polymorphic nature of the primary fat constituent. Cocoa butter is known to be capable of existing in various crystalline forms and the standard ‘Wille and Lutton’ polymorph numbering system (I–VI) describes the existence of cocoa butter in six different crystalline configurations, with an increase in thermal stability from forms I to VI.9 Alternatively the convention developed by Larsson10 can be used to describe the different polymorphs and crystal packings in cocoa butter. In practice, Form V crystals are the most desirable crystal forms of cocoa butter as they provide good demoulding properties and give the final chocolate products a stable glossy finish as well as a pleasant texture. In order to ensure the formation of crystals of the right polymorph V, good tempering,11 a procedure involving a series of careful temperature controlled steps, is therefore needed. Failure to do so can result in a product that is more susceptible to fat bloom, a physical imperfection that often manifests itself as a white or greyish white layer on the surface of the chocolate product during storage. Fat bloom occurs when a lower and unstable crystal form of cocoa butter changes into a higher and more stable form such as Form VI.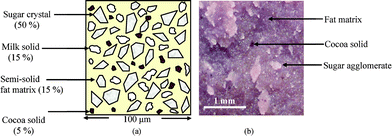 | ||
| Fig. 1 (a). Schematic representation of the microstructure of chocolate. (b) An optical microscope image of a typical milk chocolate recipe. | ||
Cocoa butter is composed mainly of triacylglycerols (TAGs) or triglycerides, which are found in most natural oils and fats. TAGs are esters of three fatty acid molecules joined to a glycerol molecule backbone (Fig. 2). The physical and chemical properties of oils and fats are determined by the composition and crystal packing arrangements of the triglycerides. Palmitic (P), stearic (S) and oleic (O) fatty acids, which make up more than 95% (Table 1) of the fatty acids found in cocoa butter,11 combine to form the three main triglycerides POP, SOS and POS (Fig. 2b).
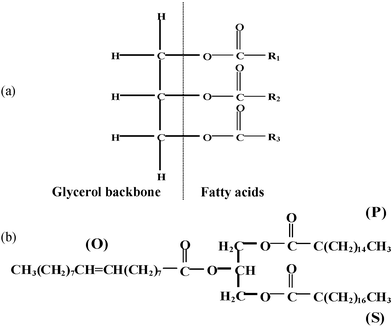 | ||
| Fig. 2 (a) Basic structure of a triglyceride molecule with a glycerol backbone connected to three fatty acids. (b) Structure of triglyceride POS with the three main fatty acids. | ||
| Fatty acid | Chemical formula | Cocoa butter (%) by wt |
|---|---|---|
| Palmitic acid (P) | CH3(CH2)14COOH | 26.0 |
| Oleic acid (O) | CH3(CH2)7CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH2)7COOH CH(CH2)7COOH |
34.8 |
| Stearic acid (S) | CH3(CH2)16COOH | 34.4 |
It has been proposed that a certain degree of molecular ordering still exists among the triglygeride molecules of the most stable form just above the melting point of the cocoa butter, usually above 40 °C.12 The chains adopt a chair-like structure which is distorted.13 However the actual structural arrangement of the triglycerides is not yet fully understood and is still a subject of much debate. Figs. 3a and b show, highly schematically, the arrangement of triglyceride molecules in both the solid and the liquid states.
 | ||
| Fig. 3 (a). Schematic structure of cocoa butter in the solid crystalline state (b) A more ‘disorganised’ molecular structure of liquid cocoa butter. | ||
Most of the melting of cocoa butter occurs over a relatively wide temperature range of between 15 °C and 40 °C (Fig. 4). At 20 °C, about 16% of the triglyceride fats are in the liquid form, hence the semi-solid state of cocoa butter and chocolate at room temperature. Temperatures above 40 °C will result in cocoa butter being predominantly in the molten state. Previous work has proposed that fat crystals and aggregates form a three dimensional network made of interlinked chains and between these solid crystal regions, pools of liquid regions are entrapped.14
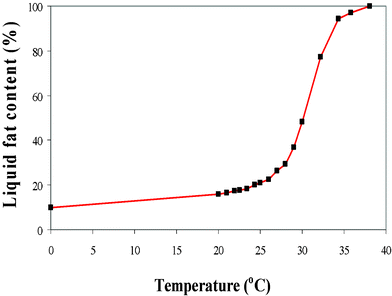 | ||
| Fig. 4 Liquid fat composition in cocoa butter as a function of temperature (ref. 29). | ||
2. The cold extrusion process
Ram or screw extrusion is commonly applied to materials such as polymer melts,1,2 ceramic pastes,4,15,16 sweets or sugary products,17 pasta dough18 and ice cream19 among others. The cold extrusion process of chocolate is carried out in a processing window between the temperatures of 18 °C and 28 °C in order for shape retaining extrusion to be achieved. For temperatures below about 18 °C, the chocolate will extrude as a crumbly mass and the pressures involved will be very high, whereas extrusion temperatures above approximately 28 °C will yield a molten extrusion with little or no shape retention.Fig. 5a shows the schematic diagram of the cold extrusion process using a ram extruder. Filaments of chocolate are extruded with the use of an orifice die (Fig. 5b). Immediately after extrusion, the fresh filaments possess a temporary flexibility that is sufficiently high for the chocolate filament to be tied into knots or coiled into a spring frame (Fig. 6).
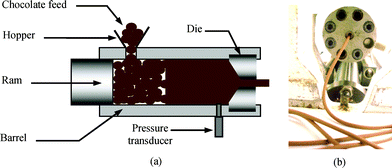 | ||
| Fig. 5 (a) Schematic representation of a ram extruder used in the cold extrusion process. (b) Freshly extruded flexible chocolate filaments at the die exit. | ||
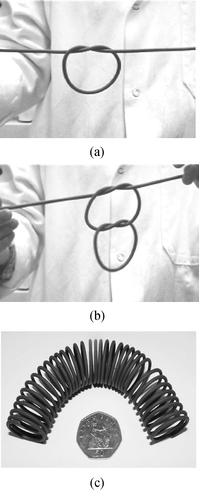 | ||
| Fig. 6 (a–b) Freshly extruded chocolate filaments flexible enough to be tied into knots. (c) A spring coil made from extruded chocolate. | ||
Fig. 7 shows a pressure against time profile obtained during extrusion. The chocolate first goes through a compaction phase where little or no flow occurs. As the piston moves forward, a sharp increase in pressure is observed until the pressure reaches a maximum yield value where the material starts to extrude. At this point, there is a decrease in pressure to a constant value known as the flow pressure. When the piston is stopped, there is an instantaneous decrease in pressure with a further, slow time dependent, relaxation towards a residual pressure. The existence of a residual pressure shows that part of the flow deformation has a characteristic of plastic flow. It has been found that the extrusion pressure is usually independent of the extrusion flow rate20,21 although at temperatures above 26 °C a dependence of the flow pressure with the flow rate develops.8 The flow behaviour of chocolate can therefore be described by a perfect plastic constitutive model20,23,24 where the shear stress of the material is independent of its shear rate, but dependent on the specific composition of the chocolate and temperature.
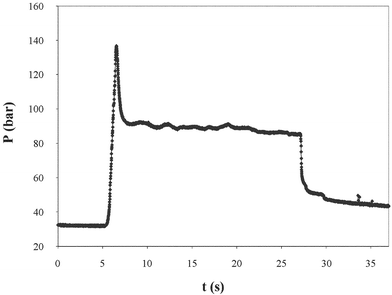 | ||
| Fig. 7 Typical variation of pressure with time during a ram extrusion. Piston movement starts at t = 5 s and stops at t = 27 s. (Extrusion T = 20 °C). | ||
Fig. 8 shows the effect of the piston speed on the flow pressure. The flow pressures for both milk and dark chocolates are essentially independent of the piston speed and the milk chocolate requires lower extrusion pressures than dark chocolate. Studies have shown that the amount of fat phase has a significant effect on the extrusion pressure,20,25 with lower extrusion pressures being associated with high fat phase. The effect of composition on the extrusion pressure has been linked to the amount of liquid or ‘free’ mobile fat present in the chocolate recipe.20 Under similar temperature conditions, milk chocolate has an overall higher liquid fat content than dark chocolate due to the high amount of milk fat present, therefore making it easier to extrude than dark chocolate.
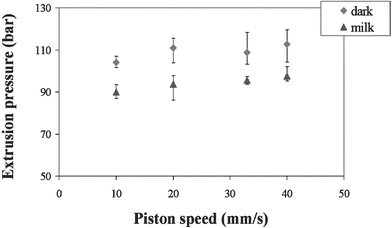 | ||
| Fig. 8 Effect of piston speed on extrusion pressure. | ||
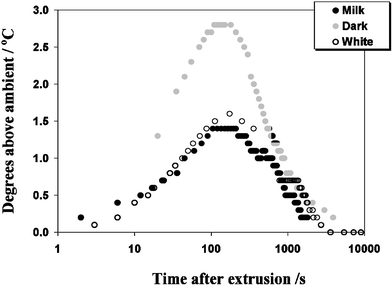
Surprisingly, the cold extrusion process itself was found to be essentially isothermal.8,20,23 This resulted in the hypothesis that the considerable work done during extrusion leads to a partial isothermal melting of some crystalline fat and not an increase in the temperature of the extrudate, as might be originally expected. It was however discovered that a transient rise of up to 3 °C in the temperature of the extrudate could occur 2 to 3 min after extrusion as shown in Fig. 9. The latter effect was related to the recrystallisation of the additional liquid fat that had been formed during extrusion.23
Ram extrusion has been used for most of the experimental studies of the cold extrusion process. However screw extrusion is also possible.20,22 The main advantage of cold extrusion is that it offers the possibility of a continuous process for complex shape formation, which was previously difficult to attain with the conventional liquid processing techniques.
3. Post extrusion mechanical characterisation
Studies of the mechanical characterisation of post extruded chocolate have been reported by a number of authors.21,23,26,27 Beam bending, penetrometry and cube compression were among the tests used for determining the degree of flexibility or hardness of the post extruded samples. In general, the tests showed that with increased post extrusion time, the flexibility decreased and the hardness of the material increased (Figs. 10a,b, 11). The ‘hardening’ of the post extruded product has been associated with the re-crystallisation of the liquid fat produced during extrusion. The mechanical tests showed that both flexibility and hardness followed an initial rapid transition and then a slower transition to its preprocessed state suggesting that the re-crystallisation process occurred in two stages: one with a rapid short term hardening kinetics and the other a slower long term kinetics. Processing conditions such as the extrusion speed and temperature were also found to affect the post extrusion properties. The flexibility of the material was found to increase and be prolonged with both the extrusion speed and the increase of temperature within the cold extrusion processing temperature window.21 Both factors have been shown to raise the amount of liquid fat content within the extruded sample. This provides evidence for a strong link between the liquid fat content present and the mechanical properties of the post extruded chocolate. Cube compression tests also indicated that the post extruded chocolate had a small level of mechanical anisotropy and was found to be structurally weaker along the direction of extrusion.21,23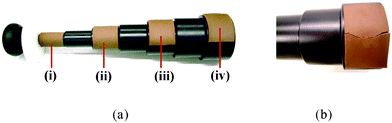 | ||
| Fig. 10 Beam bending tests carried out with a series of solid discs of increasing diameters from left to right. Variation in the flexibility of extruded chocolate shown by the minimum disc curvature the chocolate can sustain without cracking at different post extrusion times. (a) (i)–(iv) tpost = 12 s, 2 min, 5 min, 10 min. (b) Severe cracking and brittle fracture observed in the chocolate at longer post extrusion time tpost = 3 h. | ||
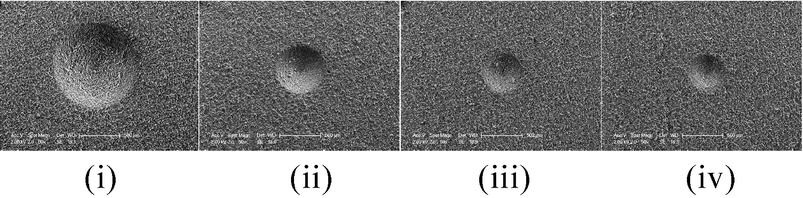 | ||
| Fig. 11 Change in the hardness of extruded chocolate with post extrusion time during 45° cone penetrometry tests. (i)–(iv) tpost = 20 s, 5 min, 1 h, 1 day. | ||
4. Microstructure and physical insight
X-Ray diffraction techniques have shown that cold extrusion does not change the crystallographic unit cell structure of cocoa butter within the chocolate during or after extrusion and the crystal phase remains in the original form V state.28 Recent in situ X-ray measurements have however shown that the fraction of crystalline material decreases during cold extrusion and that after extrusion the crystal fraction recovers to its original value with kinetics similar to the mechanical hardening recovery.24, 29 p-NMR studies have also indicated that the liquid fat fraction within a cold extruded sample decreases with time after extrusion.24 All the physical and mechanical measurements are consistent with a mechanism that cold extrusion occurs with a decrease in the fraction of crystalline content in the chocolate, thus producing a higher than normal liquid phase. As a consequence of this enhanced liquid fat content, the chocolate bears a temporary flexibilty. This short term flexibility recovers to the original hardness of the chocolate with kinetics that follow the recrystallisation of the cocoa butter. The exact location of the liquid phase in the cocoa butter is not known and optical microscopy provides little insight into the distribution of the liquid and crystalline phase. Higher resolution using AFM imaging has also been attempted, but again little information in relation to the microstructure has been obtained.A postulated mechanism for the mechanical and physical processes underlying the cold extrusion is shown schematically in Fig. 12. Before extrusion, the liquid fat is believed to exist in isolated sub-micron domains surrounded by the non-fat solid particles. Once the material reaches yield point under applied pressure, those previously isolated liquid fat domains deform and bond to form ‘internal slip planes’ to allow plastic flow of the material. Indirect experimental evidence20 has shown that some liquid fat migrates to the wall of the barrel and acts as a ‘lubricating layer’ at the wall, hence reducing the frictional resistance at the wall boundary and easing the flow through the die. The creation and the presence of the liquid fat slip planes are thought to make the material temporarily flexible. The chocolate extrudate soon regains its usual hardness and brittleness as the liquid fat recrystallises and reverts back to essentially its original sub-micron domains or microstructure.
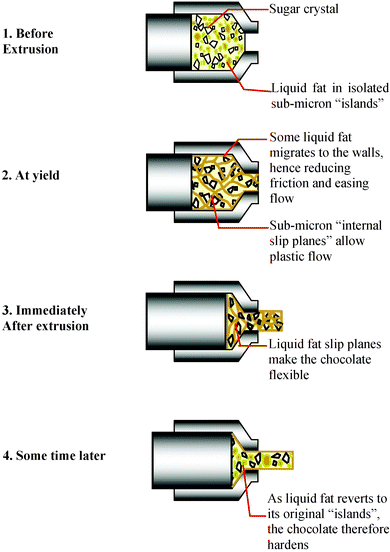 | ||
| Fig. 12 Proposed mechanism governing the cold extrusion process. | ||
A simple calculation equating the work done during extrusion with the amount of work required to melt a certain crystalline fraction of cocoa butter fat gives a consistent result with the measured changes in the liquid phase during extrusion.8,30 It is therefore clear that cold extrusion involves a mechanically enhanced transition of the crystalline cocoa butter fat to liquid fat phase and hence an associated change in the mechanical properties of the material. Experimental observations8,20,23 have shown that the mechanical work done does not go into heating and increasing the temperature of the chocolate material as might first be expected. At this stage a quantitative model to couple the mechanical characteristics with the phase structure has not been possible and will probably require the future development of experimental techniques that can identify the changes in nano length scale structure within the cocoa butter.
Strategically the cold extrusion processing of chocolate has a number of benefits in that the process is predominantly isothermal and obeys a near perfect plastic extrusion response. This indicates that the extrusion pressure is independent of the extrusion speed and as a result, there is no energy or pressure penalty in extruding at high speed. In addition the extrudate is shape retaining, which means that high precision profiles can be produced from intricate dies. It is plausible to think of cold extruding other materials with a matrix that is the same or comparable to that of cocoa butter, thereby developing a cost effective and continuous shape forming process for different materials with similar interesting soft solid behaviour as described in this review.
Summary
This review has summarised work carried out on a cold extrusion process for chocolate. It is clear that chocolate is a complex soft solid where its room temperature mechanical properties are intimately related to the physical state of the cocoa butter fat within the chocolate composite. Isothermal cold extrusion processing below the material's main melting peak results in the chocolate having an increased liquid cocoa butter fat content, which in turn gives rise to a temporary flexibility of the material. The work done during extrusion goes mainly into producing a phase change in the cocoa butter, rather than simply heating the material. As with many soft solids, the exact link between this microstructural change and the resulting physical properties is still not fully understood and awaits further experimental investigation and modelling.Acknowledgements
We would like to thank Dr. Steve Beckett (Nestlé, PTC, York UK) for his support, guidance and interest in this work. We also thank Mr Robert Marshall for his assistance with the graphics of Figs. 3 and 12.References
- J. M. Dealy and K. F. Wissbrun, in Melt Rheology and Its Role in Plastic Processing, Van Nostrand Reinhold, New York, 1990 Search PubMed.
- C. Rauwendaal, in Polymer Extrusion, Hanser Publishers, NewYork, 3rd edn., 1994 Search PubMed.
- N. Inoue and M. Nishihara, in Hydrostatic Extrusion: Theory and Applications, Elsevier Applied Science Publishers, London, 1985 Search PubMed.
- J. Benbow and J. Bridgwater, in Paste Flow and Extrusion, Clarendon Press, Oxford, 1993 Search PubMed.
- C. Anton, M. R. Mackley and S. B. Solbai, Polym. Bull. (Berlin), 1987, 17, 175–177 Search PubMed.
- J. H. Southern and R. S. Porter, J. Appl. Polym. Sci., 1970, 14(9), 2305–2317 CrossRef CAS.
- W. Laskey, US Patent, 1 628, 251, 1927 Search PubMed.
- S. T. Beckett, M. A. Craig, R. J. Gurney, B. S. Ingleby, M. R. Mackley and T. C. L. Parsons, Food Bioprod. Process, 1994, 72(Part C), 47–54 Search PubMed.
- R. L. Wille and E. S. Lutton, J. Am. Oil Chem. Soc., 1966, 43, 491–496 CrossRef CAS.
- K. Larsson, Acta Chem. Scand., 1966, 20, 2255–2260 CrossRef CAS.
- G. Talbot, in Industrial Chocolate Manufacture and Use, ed. S. T. Beckett, Blackwell Science Ltd., Oxford, 2nd edn., 1994, ch. 11, pp. 156–166 Search PubMed.
- A. E. Bailey, in Melting and Solidification of Fats, Interscience Publishers, New York, 1950 Search PubMed.
- K. Larsson, Fette Seifen Anstrichm., 74(3), 136–142 Search PubMed.
- S. S. Narine and A. G. Marangoni, Food Res. Int., 1999, 32, 227–248 CrossRef CAS.
- A. S. Burbidge and J. Bridgwater, Chem. Eng. Sci., 1995, 50(16), 2531–2543 CrossRef CAS.
- M. J. Ribeiro, J. M. Ferreira and J. A. Labrincha, Ceram. Int., 2005, 31(4), 515–519 CrossRef CAS.
- D. J. van Zuilichem, W. J. Tempel, W. Stolp and K. van't Tiet, J. Food Engineering, 1985, 4(1), 37–51 Search PubMed.
- F. Sarghini, S. Cavella, E. Torrieri and P. Masi, J. Food Eng., 2005, 68(4), 497–503 CrossRef.
- S. Bolliger, B. Kornbrust, H. D. Goff, B. W. Tharp and E. J. Windhab, Int. Dairy J., 2000, 10(7), 497–504 CrossRef.
- S. J. Crook and Ph. D. Thesis, University of Cambridge, 1997.
- H. Ovaici and Ph. D. Thesis, University of Cambridge, 1999.
- M. Jury and S. T. Beckett, EU Patent, 0 781, 510 1997 Search PubMed.
- N. Mulji, M. E. Miquel, L. D. Hall and M. R. Mackley, Food Bioprod. Process, 2003, 81(Part C), 97–105 Search PubMed.
- J. Engmann and Ph. D. Thesis, University of Cambridge, 2002.
- A. L. Papadopoulos and A. P. Harvey, Part II Research Project, Department of Chemical Engineering, University of Cambridge, 1994.
- J. L. Campbell and L. M. Dee, Part II Research Project, Department of Chemical Engineering, University of Cambridge, 1994.
- N. Campbell and P. Mann, Part II Research Project, Department of Chemical Engineering, University of Cambridge, 1998.
- L. Stoimenof and Ph. D. Thesis, University of Cambridge, 1996.
- J. Engmann and M. R. Mackley, Semi-solid processing of chocolate and cocoa butter. 1. The experimental correlation of process rheology with microstructure, Food Bioprod. Process, 2005 Search PubMed , in press.
- J. Engmann and M. R. Mackley, Semi-solid processing of chocolate and cocoa butter. 2. Modelling rheology and microstructure changes during extrusion, Food Bioprod. Process., 2005 Search PubMed in press.
| This journal is © The Royal Society of Chemistry 2006 |