Soft and hard nanomaterials for restoration and conservation of cultural heritage
Piero
Baglioni
* and
Rodorico
Giorgi
Department of Chemistry and CSGI, University of Florence, Via della Lastruccia 3, Sesto Fiorentino (Florence), Italy. E-mail: baglioni@csgi.unifi.it; Fax: +390554573032; Tel: +390554573033
First published on 10th February 2006
Abstract
In this review we report the most recent applications of nanotechnology to the conservation and restoration of the world’s cultural heritage. Nanoparticles of humble calcium and magnesium hydroxide and carbonate can be used to restore and protect wall paints and to de-acidify paper and wood. We highlight the synthetic pathways that can be used to produce nanoparticles, and some applications for the conservation of Maya wall paints in Mexico and to the de-acidification of paper documents and wood.
 Piero Baglioni | Piero Baglioni is a Full Professor of Physical Chemistry at the Department of Chemistry and CSGI of the University of Florence. He is the author of over 250 publications in the field of colloids and interfaces and a pioneer in the application of soft matter to the conservation of cultural heritage. He has produced several innovative methods which are applied worldwide. |
 Rodorico Giorgi | Rodorico Giorgi graduated in Chemistry in 1996, with a PhD in science for cultural heritage conservation in 2000 from the University of Florence. He is currently a Research Fellow at the CSGI Consortium, University of Florence. He is the author of 30 publications in the field of conservation of cultural heritage materials, his background is in the physical chemistry of colloid and interface science. He is currently extending his activity on the application of nanotechnology to the conservation of cultural heritage |
The study of soft and hard nanostructured materials is considered an emerging field for the next decades. Nanomaterials are characterized by scale lengths below 100 nm in one or more dimensions. The conventional granular materials are made up of grains whose dimensions range from microns to a few millimeters, each grain containing billions of atoms. Nanostructures represent a state of matter in between molecules and bulk structures, and are usually characterized by a large surface area that affects their physico-chemical properties. The innovative applications of nanostructures are based on at least two types of unique properties associated with nanostructures: 1) novel optical properties due to quantum confinement effects; 2) changes in reactivity and mechanical properties due to the small physical dimensions and large surface area. In addition to opto-electronic and surface properties, the small particle size results in improved mechanical properties, important for a variety of applications. The advantages of smaller particle grain size compared to bulk materials include low-temperature sintering, superplasticity, enhanced diffusivity, improved dielectric properties, and tribological properties. These attributes lead to better ceramics and may allow production of low-loss soft magnetic materials and improved ferro- and piezo-electrics properties. Nanostructures also confer high hardness, improved wear resistance, and higher materials density, all of which are desirable characteristics in ultrahigh-strength structural materials. The high surface area also imparts interesting properties as improved chemical reactivity, catalyst activity, and gas absorbency.
It is obvious how the research of new materials and devices based on nanometric structures represents a primary objective for the science of soft and hard materials. The creation of new materials starting from precursors at the nanometric scale offers opportunities for industrial, biomedical and environmental applications, which were inconceivable just a few years ago. The main targets include the synthesis of crystalline rather than amorphous products (or in some cases amorphous rather than crystalline), lower size heterogeneity, and improved purity and stability of the final product. A successful synthetic procedure should limit particle growth to the nanometer range while maintaining desirable traits such as low sample heterogeneity of size and shape. Although numerous techniques have been developed, the synthesis of nanoparticles is not a simple task due to their thermodynamic tendency to agglomerate into bulk structures, and specific pathways should be envisaged to produce nanostructures,1 as reported in the following chapter. Moreover, most practical applications require large quantities of suitable nanomaterial powders.
Nowadays, these limitations restrict the very large potential of nanotechnology to a few selected applications, one of these is the conservation of the cultural heritage.
Applications of nanotechnology to wall paintings consolidation and paper de-acidification have recently provided clear evidences of the huge potentiality of this emerging science for cultural heritage conservation.2,3 Nanodispersions of solids, micelle solutions, gels and microemulsions offer new reliable ways to restore and preserve works of art by merging together the main features and properties of soft-matter and hard-matter systems, allowing the synthesis of systems specifically tailored for the works of art to fight the deterioration processes which threaten the preservation of the world cultural heritage.
This review deals with the synthesis and application of nanostructures of calcium and magnesium hydroxide and carbonate, which have been recently applied to the conservation of wall paintings, paper and wood conservation. We will highlight in the following the most suitable methods for nanoparticle synthesis and some recent successful applications to the conservation of the world’s cultural heritage.4–9
Nanoparticle preparation
In order to produce nanomaterials in sufficient amounts for industrial needs, the development of innovative procedures to synthesize nanostructured systems is extremely important. Several excellent reviews are reported in the literature on the synthesis of nanoparticles, with special emphasis on the use of self-assembling systems (micelles and microemulsions).10–12 In this review we focus attention on contributions specifically addressing the synthesis of hydroxides and carbonates of magnesium and calcium, which were (until now) chemicals with important applications for the restoration and preservation of works of art. We also give a short account of the main limitations of the preparation of nanostructures and how soft matter can address these problems.Two fundamental processes are generally used to prepare inorganic particles. The first is a “break-down process” where bulk solids are broken down by grinding them into fine particles (i.e. mechanical synthesis) or to molecular or atomic size (i.e. by thermal decomposition of solids). The other process is a “bottom-up process” in which the particles are built up to a required size by the external collection of atoms or ions, by deposition and growth of crystals from liquid or vapour phase, or by solid-state sintering and reaction among nanoparticles.
The process of physically dividing solids into fine pieces by grinding in a mill is the best known and probably the most used method for the manufacture of powders.13 However, the grinding method is usually associated with contamination of the particle surface by atmospheric gases and by materials used for the mill. Moreover, this method suffers a limitation in the accessible particle size since the smallest size that can be produced by grinding is limited by the tendency to re-aggregate, and particles below a few microns cannot usually be produced.
The thermal decomposition of inorganic or organic metal salts is another well-known method for making fine powders, mainly used in ceramics manufacturing. For example, according to this method MgO is produced at temperatures around 500 °C from the decomposition of Mg(OH)2 (decomposition starts at 380 °C and is completed at 500 °C). The formation of nuclei of oxide in the skeleton lattice of the hydroxide can be controlled by the heating rate, since higher rates promote the formation of numerous nuclei characterized by a rapid growth. The thermal decomposition of Mg(OH)2 produces ultra-fine grade MgO particles, as shown by large changes in the specific surface area.14
The synthesis of nanosized particles of Mg(OH)2 is assuming great importance since, as reported above, it is used as a precursor for magnesium oxide synthesis, and its particle size, shape and aggregation level constitute key parameters in the sintering steps and the processing of the final products. Moreover, Mg(OH)2 is also largely used in flame-retardant composite formulations due to its ability to undergo endothermic dehydration.15 Mg(OH)2 particles synthesized through a heterogeneous phase reaction, starting from coarse MgO powders dispersed into water, might assume excellent properties and good features for different applications. Rod-, tube-, and lamella-like morphologies of Mg(OH)2 nanoparticles have been obtained through a hydrothermal reaction by using different Mg(OH)2 precursors as the reactants. Subsequent thermal decomposition of precursors produces nanosized MgO particles. The morphological features of the starting products are well retained during this thermal transformation process, particles are nanocrystallines and with a large surface area.16 Porous magnesium hydroxide nanoplates can be prepared by a simple hydrothermal treatment directly from commercial bulk magnesium oxide crystals. The plate-like morphology is retained after calcination with a wormhole-like porous structure with high surface area.17 However, the decomposition of Mg(OH)2, or different salts, usually leads to broad grain size distributions with sometimes inhomogeneous morphologies and small surface area.
The sol–gel technique is one possible method exploited to better control the size and shape of particles.18,19 This involves the phase transition of a system from a liquid “sol” (mostly colloidal) into a solid “gel” phase. The starting materials are usually inorganic metal salts or metal organic compounds such as metal alkoxides. In a typical sol–gel process, the precursor is subjected to a series of hydrolysis and polymerisation reactions to form a colloidal suspension, or a “sol”. When the “sol” is cast into a mold a wet “gel” is formed. With further drying and heat-treatment the “gel” is converted into an inorganic product like a glass, polycrystalline powder or a dry gel. Highly porous and extremely low density materials, called “aerogels”, can be obtained by drying a wet “gel” at supercritical conditions.
Nanosized MgO particles have been prepared via the sol–gel technique by different authors. Klabunde et al.20–22 prepared ultra-high surface area Mg(OH)2 and MgO powders with sizes of about 5 nm via a sol–gel technique followed by a hypercritical drying procedure. MgO nanoparticles were also prepared by ultrasound-enhanced hydrolysis of Mg-alkoxides to obtain large specific surface areas and small particle sizes.23
Aerogels of MgO and CaO nanoparticles have also been prepared through a sol–gel approach by conversion of methoxides to hydroxide gels followed by hypercritical drying and vacuum dehydration. These particulate materials exhibit unexpectedly high surface chemical reactivities, and are used as high-capacity destructive adsorbents for toxic chemicals such as chlorocarbons, organophosphorus compounds, and acid gases. The pore size distribution, unusual surface morphologies with a high ratio of edge ion/surface ions, and trace residual surface –OH and –OCH3 were hypothesized to be the main factors allowing these nanoparticulates to be isolable, stable but highly reactive.24
The deposition of solid particles from the liquid phase through a precipitation reaction is probably the simplest and reliable method for obtaining nanostructures with good control of the particles' characteristics and in a cheaper way.25 Several studies26–28 have been published on the synthesis and characterization of metal oxide, hydrous oxide, and hydroxide nanoparticles obtained by precipitation from salt solutions.
However, the literature on the preparation of moderately water-soluble inorganic nanomaterials (i.e. calcium hydroxide, calcium sulfate, magnesium hydroxide etc.) is relatively scarce5,6,8,29 as compared to the studies concerning nanoparticles of insoluble compounds (sulfides, oxides, metals, etc.).30–34 The synthesis of hydroxides particles can be complicated because it is usually difficult to achieve the high supersaturation degree necessary for nucleation and the effect of co-ions at these concentrations can be critical. By changing the conditions of reaction, size, shape, structure and composition of particles are adjustable with good yield. The effects of control parameters, such as reaction temperature, concentration of reacting species, and aging time of the nanostructure characteristics have been reported.35 It has been shown that the chemical nature of the base precipitant is of primary importance: the use of sodium hydroxide at 60 °C leads to the globular cauliflower-like agglomerates, while synthesis driven with aqueous ammonia promotes the formation of platelet-shaped particles.
The controlled double-jet precipitation (CDJP) method allows the production of colloidal particles with a good control of their monodispersity.36,37 In this method, reactant solutions, giving a precipitation reaction, are introduced into a reactor at the same time by a peristaltic pump, where temperature and reaction time can be easily adjustable. Oxides and other compounds have been synthesized according to this method.
Calcium hydroxide is one of mankind’s oldest and most important art and building materials. It is used for several applications (i.e. industrial, environmental, chemical) and is extensively studied by many scientists. Calcium hydroxide is synthesized via homogeneous and heterogeneous phase reactions that can take place in water and also in nonaqueous solvents.
In industrial manufacturing, calcium hydroxide (Ca(OH)2, slaked lime) is obtained by the hydration of quicklime, CaO. In this process, irregularly shaped crystals of about 1 µm are produced. The physical properties of hydrated lime after prolonged storage under water improve due to particle size reduction and morphology changes (from prism to plate-like crystals). Higher carbonation rates, in the case of aged lime, are also promoted by lime aging.38
Ca(OH)2 particles with uniform size and shape can be also obtained through homogeneous phase reactions in water (see for example Fig. 1). The relationship between the precipitation conditions (temperature and initial reactant concentrations) and the size and shape of the precipitated crystals, has been accurately described. Reactants concentrations of about 0.75–1 M at 80 °C reaction temperature give particles ten times smaller than those obtained at room temperature. The presence of a third component (for example methanol) in the reaction mixture was shown also to exert an influence on the crystal shape.39
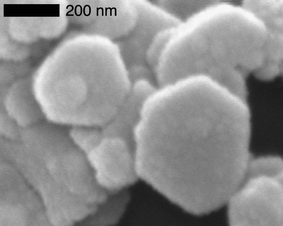 | ||
| Fig. 1 SEM picture of Ca(OH)2 nanoparticles synthesized through an homogeneous phase reaction at 90 °C. | ||
High temperatures have been shown to be necessary in order to obtain very fine particles (a few nanometers). The synthesis of spherical nanoparticles of In(OH)3 has been achieved at high temperatures by using 1,2-ethanediol (bp 195 °C at atmospheric pressure) as the reaction medium.40 This method involves several peptizations of the synthesized particles, because the diols tend to be adsorbed on the particle surface keeping them aggregated in micron-sized agglomerates.
Natural carbonates occur widely as the main mineral components in rocks and sediments and as inorganic components in exoskeletons and tissues of many mineralizing organisms. Most of the processes occurring in Nature are a never-ending source of suggestions for conceiving new ways to produce nanosystems with better physico-chemical and mechanical characteristics. For example, the hard tissues of organisms (e.g. bone, teeth, mollusk shells) are composed of minerals that are typically in close association with an organic polymeric phase, and thus are biocomposites. The mineral crystals that are formed by organisms (bio-minerals) have frequently physico-chemical and mechanical characteristics different from crystals produced inorganically. In the process of biomineralization, a living organism provides a chemical environment that controls the nucleation and growth of mineral phases. Often these materials exhibit an hierarchical structural order, leading to superior physical properties, not found either in their inorganic counterparts or in synthetic materials. The control of crystal shape is only one of the many puzzling features of biomineralization. Overall, it is seen that control over biomineral properties can be accomplished at a myriad of levels, including the regulation of particle size, shape, crystal orientation, polymorphic structure, defect texture, and particle assembly.41,42
A fundamental concept in the study of bio-mineralization concerns the molecular recognition of inorganic materials at organized organic macromolecular substrates. Mann (et al.)43 investigated this point through the use of stearic acid monolayers in the controlled crystallization of CaCO3 from supersaturated solutions. While crystallization in the absence of a monolayer results in rhombohedral calcite crystals, the presence of an organized monolayer gives rise to oriented vaterite formation. These results support current ideas on bio-mineralization, as well as being of potential significance in the crystal engineering of microscopic inorganic assemblies.
Chemical and biological precipitations of calcium carbonate polymorphs have been studied in some detail.44–47
Recently, it was described how different calcium carbonate precipitates could be obtained in solutions of calcium salts by catalytic decomposition of urea at 90 °C48 and by enzyme-catalyzed decomposition of urea by urease at lower temperatures.49,50 Enzymes can yield various calcium carbonate polymorphs and exert significant influence on the development of calcite in different habitats.51 Catalytic decomposition of urea by urease has also been used in the preparation of several inorganic products.52–57 Urease has an effect also on the precipitation of anhydrous carbonates from the aragonite group, that is, witherite (BaCO3) and strontianite (SrCO3), in solutions containing strontium and barium chloride and urea at room temperature. Urease plays a significant role in the formation of intracellular crystals, in the microbial precipitation of calcite, in the remediation of the surface and subsurface porous media,58 and in cracks of granite and concrete.59
More recently, a new method for the consolidation of limestone based on bacterially induced carbonate mineralization has been proposed. Tests showed that newly formed calcite grains grow epitaxially on the pre-existing substrate.60 Bacterial mediation in mineral carbonate formation, the so-called carbonatogenesis, is assuming an increasing importance in the field of cultural heritage conservation.
A common synthetic pathway for the synthesis of nanoparticles of inorganic materials as oxides, hydroxides, metals, and sulfides is from w/o microemulsions or reverse micelles.61–70
The use of microemulsions for the production of nanostructured materials consists of employing micelles as nanoreactors, so that it is possible to control the growth of the obtained particles. The most important feature of microemulsions lies in the capacity of exchanging the content of the droplets. In particular, the inverted micelles (water droplets in oil) are nanocompartments, which can be used for the reduction or the precipitation “in situ” of metallic salts or ceramic materials. It is sufficient to mix two microemulsions, one containing the metallic salt and the other the reductive or precipitating agent, to obtain particles on the nanodimension.10–12,71 There are several parameters which influence the final dimensions of clusters: the concentration of reagents, the nature of the reducing agent and, above all, the relationship between the content of water and surface-active agent of the microemulsion. Once the particles have reached the final dimensions, they are stabilised by the surface-active agent that adsorbs on the nanoparticles surface, prevents further growing and therefore allows the formation of a stable suspension. Such a suspension can be de-stabilised using a coalescing agent, in order to precipitate the solid content and separate it from the suspension. However, the separation of nanopowders from the surface-active agent, which constitutes the drops of the microemulsion, is an extremely complicated process and, even after a long and difficult procedure, the quantity of powders obtained is scarce.
An alternative to the separation of metallic or oxide nanoclusters (even though the method can be extended to other materials chemically resistant to high temperature) from the surface-active agent through washing is represented by the decomposition of the surfactant through a thermal process. A recently formulated72 new method, called “flame-spraying”, allows the synthesis of nanopowders and nanostructured coatings by using the suspension obtained through the synthesis of nanoparticles inside inverted micelles or microemulsions. In brief, the suspension is introduced as aerosol into the flame produced by the flame spraying system. In this phase, the whole organic content of the suspension is decomposed and the nanoparticles, at this point free from the surface-active agent which covered them, can be deposited on the support to be covered or directly collected in the form of powders.71 This method allows the control of the particle size distribution of the powders (or coatings) and, at the same time, allows a great flexibility in terms of the chemical composition of the deposited material.
Most of these compounds are insoluble in water and, therefore, the concentration of the reactant species in the microdroplets can be kept quite low. The analogous preparation of nanoparticles of substances with moderate solubility in water is rather problematic, due to the high ionic strength inside the droplets, which complicates the microemulsion/micellar system formulation. In the bulk aqueous phase, because of the difference in the solubilities of reactants and products, the mixture of some compounds induces flocculation of bulk materials.1 Recently, an attempt to synthesize nanoparticles of relatively high Kps materials (CaSO4) has been performed by using different types of w/o microemulsion.28 Calcium sulfate nanoparticles with different shapes have been reported working with ions concentrations inside the aqueous pool of the w/o microemulsion varying in the range 0.025–0.20 M. The synthesis is quite problematic due to the strong pH conditions inside the water pool where alkaline nanoparticles are synthesized. This can be a crucial factor for the surfactant stability: some surfactants can be hydrolyzed at high pH values, with possible subsequent phase separation of the microemulsion systems.
For this reason, as above reported, colloidal particles of hydroxides with moderate water solubility (i.e., Ca(OH)2) were usually obtained by means of other synthetic routes, as homogeneous hydrolytic reactions,5 or synthesis in diols at high temperature.6 Colloidal calcium hydroxide was also synthesized73 by hydrolysis of calcium hydride in a hydrocarbonated medium (a mixture of mineral oil, toluene, and methanol) in the presence of a surfactant. The authors claimed that the resulting product can be defined as a distribution of crystallized Ca(OH)2 surrounded by surfactants in a reverse type micelle association. These colloidal systems are generally achieved by synthesizing the inorganic product at the same time of the micellization step. In this way, calcium carbonate was prepared by the reaction of calcium hydroxide with CO2 in presence of a surfactant.74–77 Recently, calcium hydroxide nanoparticles have been synthesized in a w/o microemulsion, even though the yield is very poor.78
Most basic research in the field is focused on particle formation in microemulsions or related “templates”. The main drawback, even if the method is successful, is a poor yield. For most real applications one still relies on co-precipitation of salts, for which control of particle parameters is a problem.
The technique of microemulsions allows a more precise control of the dimensions of the nanoparticles obtained; on the other hand, the synthesis through over-saturation is at an industrial level and involves lower costs. At present, we can say that these two techniques are complementary: the synthesis in aqueous solution is ideal for most conventional industrial applications (inks, loudspeakers, contrasting agents, etc.), while the technique of microemulsion is more suitable for applications of high technological content, where it is necessary to have a precise control of the dimensions of the nanoparticles and where a high monodispersity of these nanoparticles is required.
Nanostructured hydroxides and carbonates: new tools for art conservation
Conservation science is probably one of the most complex topics in materials science since it requires different expertise that spans from archaeology and history of art to very sophisticated physico-chemical and analytical skill.The scientists' contribution to conservation of cultural heritage has grown to a great extent in the last decade. Chemists and physicists can greatly contribute to the “controlled death” of artefacts because they can provide useful and reliable predictions of the degradation of cultural heritage and delay, as far as possible, the complete degradation of the artefacts themselves.
Until a few years ago, most of the methods for the restoration or protection of artefacts used commercial products, mainly synthetic polymers such as Paraloid B72, Mowilith 30, and Primal AC 33, and were not tailored for specific applications to the artefacts. In controlled environments, the application of these polymers to fix powdered and flaked paints, or to re-adhere detached modelled polychrome stucco fragments, produced acceptable results. However, in most cases the use of synthetic polymers produced just after a few years dramatic effects on the artefacts as detachments, flaking of surfaces and a strong acceleration of the chemical reactions involved in the paintings degradation.79–84
Recently, important progress has been achieved in the application of nanomaterials to the field of cultural heritage preservation.
Chemical and physical degradation, promoted by rain, wind, dust, pollutants and other environmental causes, induces the weakening of the porous structure and of the surface layers of stones or wall paintings. Restoration should provide the reinforcement of the porous structure and the consolidation of the surface layer of artefacts. A few simple principles can be considered to define the most appropriate restoration method:85 1) the treatment should be reversible so that one can revert to the original status of the work of art at any desired time; 2) all the applied chemicals must ensure the maximum durability and the chemical inertness; 3) the applied chemicals must invert the degradation processes without altering the chemical composition of the artefacts and their physico-chemical and mechanical properties, i.e. the applied chemicals must be as compatible as possible with the artefacts' materials.
Nowadays, the concept of reversibility in a conservation/restoration treatment is still debated.86 For example, during the 1960s several chemicals such as polymeric resins were applied for consolidation or as protection agents. The accepted idea was that these substances could be removed at any time, leaving a completely unaltered substrate (i.e. the work of art). Numerous experiences accumulated during the years have shown that this assumption is wrong and the polymers lead to a consistent damage in almost all the restored artefacts.79–84 Unfortunately, the removal of polymers is not easy and in some cases is not possible. The prediction of the physico-chemical stability of most organic chemicals not compatible with the substrate is very difficult. Organic chemicals usually considered reliable are, in most cases, harmful in the long term. The rule of thumb is simple: only inorganic materials should be used for conservation treatments of inorganic artefacts as stones, wall paintings, and so on. In this “similia similibus curantur” approach the concept of reversibility and compatibility assumes similar meaning.
European wall paintings are usually made with slaked lime according to the fresco technique. Although very stable over a long period of time, wall paintings often suffer several decay processes due to environmental pollution. Natural ageing originates the flaking of the paint layer and the powdering of the painted surface. This is due to the “chemical corrosion” of the binder, usually calcium carbonate, with the loss of cohesion between pigments and substrate. Consolidation of mural painted surfaces (or stones) by inorganic treatments should provide the right content of carbonate binders to confer long-term preservation to the works of art. Historically, the Ferroni–Dini method, also called the “barium” method, is the first method that provided reliable results and its success is mainly related to the removal of salts that threaten the paintings, reinforcing at the same time the porous structure.87–91
The most common cause for wall painting degradation is due to the slow transformation of the binding CaCO3 into selenite (CaSO4·2H2O sulfatation process). Before the Ferroni–Dini method, restorers were forced to adopt the most drastic solution for the conservation of sulfated paints: the detachment. The detachment can be considered the “last chance” since in the detachment process most of the pigments is lost. The Ferroni–Dini method allows cleaning and consolidation of mural paintings affected by sulfates, avoiding the detachment of the fresco from the wall. The method consists of two steps: the first is the application to the painted layer of a saturated solution of ammonium carbonate, (NH4)2CO3; the second is the treatment with a barium hydroxide solution, Ba(OH)2. The selenite is converted into calcium carbonate with the formation of water soluble (NH4)2SO4 according to the following chemical scheme: (NH4)2CO3 + CaSO4·2H2O → (NH4)2SO4 + CaCO3 + 2H2O
When the reaction is completed, two different points need to be addressed: i) the elimination of the soluble ammonium sulfate, that otherwise would produce efflorescences onto the painted surface of the fresco; and ii) the reconsolidation of the wall painting because calcium carbonate formed in the first reaction is powdery and has a poor binding capacity.
In the second step of the Ferroni–Dini method, a solution of barium hydroxide, Ba(OH)2, is applied. Ba(OH)2 eliminates the ammonium sulfate and produces consolidation of the wall painting. The chemical reaction between ammonium sulfate and barium hydroxide is: (NH4)2SO4 + Ba(OH)2 → BaSO4 + 2NH3 + 2H2O
Ammonium sulfate is converted into insoluble BaSO4, while the volatile ammonia and water evaporate from the wall. BaSO4 crystals (if not removed) partly fill the empty spaces created by the re-conversion of selenite into calcite.
Consolidation of the mural painting is due to another series of chemical reactions promoted by the barium hydroxide. In fact, the excess of Ba(OH)2 is subjected to carbonation according to the reaction: CO2 + Ba(OH)2 → BaCO3 + H2O
The barium carbonate crystals act as ‘filler’ (if they are not removed by gentle washing) for the empty spots created by the reconversion of selenite into calcite and produce cohesion. The main consolidation effect is due to the reaction:89 Ba(OH)2 + CaCO3 → Ca(OH)2 + BaCO3 and: Ca(OH)2 + CO2 → CaCO3 + H2O
This is the same reaction, and with the same kinetics, of the “original” setting process. In fact, barium hydroxide converts calcium carbonate into a newly formed calcium hydroxide (slaked lime), which begins a slow setting process that produces the cohesion of the painting. Fig. 2 reports an illustrative example on the efficacy of this treatment. On the left are clearly visible the detachments due to calcium sulfate that is not correctly treated lead to the loss of the pigment. On the right side of the figure is reported the same area treated with Ferroni–Dini method, where the conversion of calcium sulfate to calcium carbonate “restore” the Beato Angelico masterpiece to its original aspect.
 | ||
| Fig. 2 Crucifixion by Beato Angelico (15th century, Florence). On the left, a pre-restoration image of the wall painting. On the right, a post-restoration image. Desulfatation and consolidation was performed with the Ferroni–Dini method. (Courtesy of Daniela Dini). | ||
Nanoparticles for conservation of lime based artefacts
The evolution of Ferroni–Dini is based on calcium hydroxide that is the best binder for limestone and wall paintings. However, the direct use of aqueous solutions of calcium hydroxide is limited by the low solubility of the salt (1.6 g L−1).92 A way to increase the lime concentration would be the use of lime dispersions in water.Commercially available earth alkaline hydroxide powders have a broad size distribution and the mean dimensions are larger than several micrometers. These particles are inappropriate for the application on the paint surface because the most of surface pores are smaller; therefore, there is a consistent risk of white glaze formation on it. These limitations favoured the inappropriate use of polymeric compounds that produced in most cases consistent damage.
Dispersions of kinetically stable Ca(OH)2 nanoparticles in non-aqueous solvents solved most of the drawbacks mentioned above. We were among the first able to synthesize nanoparticles in non aqueous solvents with the optimal properties for application to cultural heritage conservation.5,93 Kinetically stable dispersions can be obtained in short-chain aliphatic alcohols. Alcohols are environmentally friendly, volatile, and, compared to other solvents, have a low toxicity. Surface tension is small enough to ensure optimal wetting that is responsible for high penetration of the dispersions within the porous structure of the wall paint. According to the features of the porous materials, the dispersing solvent can be selected as pure or in a mixture to achieve the ideal penetration inside the artefact and the ideal rheological properties for the application purposes.
The features of the solvent make the methodology very simple and available to everybody. Nanodispersion of calcium hydroxide have been applied by using several simple techniques as brushing or spraying, and have been successfully tested over several porous materials. The dispersions of nanoparticles are similar to an extremely concentrated solution of lime water (up to 30% volume fraction), well above the physico-chemical limit imposed by the solubility of calcium hydroxide in water.
Stable dispersions of calcium hydroxide have been successfully applied (replacing polymers) as fixatives to re-adhere lifted paint layers during many restoration workshops in Italy and in Europe, and as a consolidant.93–97
Nanoparticles can replace the Ferroni–Dini method in most cases. However, when the degradation of the artefacts is very severe, or the paint is heavily contaminated with sulfates, a combination of nanoparticles and the Ferroni–Dini method is the most appropriate treatment. A complete conservative treatment of wall paintings is often performed in two steps: i) the classical Ferroni–Dini method to revert chemically the sulfate salts to carbonate, inhibiting their migration, and ii) the application of a nanoparticle dispersion, which provides the right content of calcium hydroxide binder. In this way, a whole reversible treatment can be achieved since the degraded material is reverted to its original un-degraded status.
“In situ” conservation is a challenge for archaeologists and restorers. Nanoparticle technology has been used with excellent results for the conservation of stucco and paints in the archaeological site of the “Antigua Ciudad Maya de Calakmul” in the Yucatan peninsula, a UNESCO World Heritage Site since year 2002 (Campeche, Mexico).98
The very unfavourable environmental conditions (very high relative humidity all over the year and short periods of rain typical of sub-tropical climatic regime) accelerate the degradation processes of the paintings, in particular of those treated with non-compatible materials, as polymers.
Several paintings in Calakmul showed a friable and powdered paint layer, which needed to be consolidated. This was done using nanoparticle dispersions, from ten to thirty times more concentrated than lime water, that were applied by gentle brushing. After a first application of Ca(OH)2 nanoparticles, a wood poultice soaked with water was applied for 8 h over the treated surface in order to maintain wet the first paint layers to favour a slow carbonation of hydroxide. A few days after, a second application of nanoparticles was performed. At the end of the treatments a simple testing (i.e. resistance to abrasion) showed a complete fixation of colour (see Fig. 3 and Fig. 4).
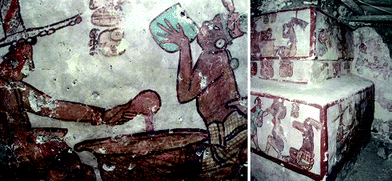 | ||
| Fig. 3 Recently discovered Maya paintings that decorate the inner parts of the pyramids in “La Antigua Ciudad Maya de Calakmul”, a UNESCO World heritage Site (Campeche, Mexico). Dispersions of Ca(OH)2 nanoparticles are used to consolidate the paint layer suffering for de-cohesion and powdering phenomena. (Courtesy of Proyecto Arqueologico Calakmul). | ||
 | ||
| Fig. 4 Recently discovered Maya paintings in “La Antigua Ciudad Maya de Calakmul”, a UNESCO World heritage Site (Campeche, Mexico). Dispersions of Ca(OH)2 nanoparticles are used to consolidate the paint layer suffering for de-cohesion and powdering phenomena. After restoration the paint recovered its original colour tonality because the re-cohesion of pigments in the surface layer minimized the diffuse light scattering that conferred opacity to the wall paintings. | ||
Stucco is another handmade material, based on the setting process of lime that has been successfully treated with nanoparticle dispersions. This material has a low porosity and the application of nanosized particles allows the reinforcement of the surface layer, providing protection from wind, rain, and dust from atmosphere.96,97 Similar effects are also conferred to some limestones that have been previously treated with polymers as protective agents.93–95
Paper and wood deacidification
Hydroxides or carbonates, can be also used for conservation of paper and wood. Alkaline nanosized particles, applied from nonaqueous dispersions have found to be particularly efficient for the preservation of cellulose-based materials, which degradation is catalyzed by acidification processes developing in the paper and leading to chemical disruption of the cellulose polymer. Fig. 5 reports a typical example of a sheet of paper from the beginning of 20th century where acid inks promoted the degradation of cellulose. In fact, it has been shown that acid-catalyzed hydrolysis is the main chemical route for cellulose de-polymerization.99 The overall effect of several reactions taking place in the cellulose fibres, and promoted by the presence of acids, is the shortening of the average chain length of cellulose that leads to a catastrophic loss of paper strength. | ||
| Fig. 5 Yellowed paper, from the beginning of 20th century, with oxidation effects on cellulose due to ink corrosion promoted by both paper and ink acidity. | ||
These processes can be stopped or consistently slowed down by a de-acidification treatment. Many different techniques and products have been studied or developed in order to eliminate acidity from paper.100 Aqueous solutions of calcium and barium hydroxide have been widely used for many decades, but, unfortunately, they induced undesirable side-effects, mainly related to paper (which is hydrophilic) exposure to strongly alkaline conditions with subsequent alkaline cellulose depolymerization. To overcome cellulose hydrolysis in aqueous alkaline solutions, a gas-phase method was developed and used at the Library of Congress in Washington.101 This method used diethylzinc that, after paper impregnation, neutralized acids to give ethane. The excess slowly reacted with water to produce hydroxide, which changed to carbonate after exposure in the air. Unfortunately, the control of the reaction rate into the reactor was difficult and the contact with oxygen in the air produced a series of accidents that this technology being banned.
Most of the limitations described above can be overcome by using de-acidification processes based on non-aqueous solvents that, nowadays, are used worldwide.
Wei T'o102 is a deacidification process that uses methoxymagnesium methylcarbonate (MMC) solutions in nonaqueous solvents. MMC forms in situ magnesium hydroxide after hydrolysis promoted by moisture. In a comparative study, the Wei T'o process was tested by the Canadian Conservation Institute; investigations showed a complete neutralization of acid, good penetration of the de-acidification agent, improved chemical and, to a lesser extent, mechanical stability during accelerated ageing. On the other hand, the Wei T'o treatment led to inhomogeneous distribution of the alkaline reserve, and appeared to lead some damage to books and to increase absorbency of the paper, possibly caused by the removal of the initial paper sizing, which might stimulate the reactivity of the paper to moisture.103,104
In the Battelle method,105 magnesium and titanium ethoxide in hexamethyldisyloxane solutions are used. Magnesium ethoxide and titanium ethoxide hydrolyze easily in the presence of the water soaking the paper. The overall chemical reaction is a two-step process: in the first step a metal hydroxide is formed under the influence of the environmental air; the subsequent reaction forms magnesium carbonate (MgCO3) and titanium dioxide (TiO2). The magnesium carbonate and the residual metal hydroxides neutralize the acids present in the paper. The excess of magnesium carbonate in the paper forms the alkaline reserve, protecting the paper against future acidification.
The Battelle de-acidification is a positive contribution to the permanence of the paper both for books and for archival materials.105 However, despite these positive results it was found out that the Battelle process suffers from a number of side effects that hamper a large-scale application. For example, a large part of the tested material (40% of the books and 2 out of 3 archival materials) showed a significant immediate decrease in paper strength as result of the de-acidification treatment.106 There were also undesired side effects in the de-acidified materials, such as discolorations, white deposits, Newton rings, bleeding of inks and dyes, and a different “touch” of the paper. Finally, there was no homogeneous distribution of the deacidification agent in the books and compact stacks of paper.
The Bookkeeper process, based on a liquid-phase process using magnesium oxide (MgO) particles suspended in an organic solvent (perfluoro heptane), is probably the most used method for de-acidification.107 In this method, the magnesium oxide is converted to magnesium hydroxide, by reaction with moisture. Accelerated ageing tests and folding endurance measurements showed a significant decrease in the rate of paper degradation as a result of the treatment. On the other hand, white deposits on coated paper were sometimes found and thick books, treated as a single item, showed often insufficient alkaline reserve in the inner (gutter) margins.108
All the methods described above are based on the usage of magnesium compounds, which after hydrolysis form magnesium hydroxide in situ. A real challenge was the introduction of magnesium or calcium hydroxide directly into the cellulose fibres network. This has been possible, and with great benefits for some old acid paper samples7,109 by using nanosized particles that present high surface reactivity and a narrow grain size distribution (Fig. 6).
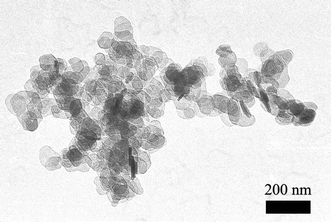 | ||
| Fig. 6 TEM picture of Mg(OH)2 nanoparticles synthesized through a homogeneous phase reaction at 90 °C. | ||
Mg(OH)2 and Ca(OH)2 are excellent de-acidifying agents, ensure a good physico-chemical compatibility with the support, and after their transformation into the corresponding carbonate work very well as alkaline reservoir7,109 without originating any undesirable side-products.
Mg(OH)2 and Ca(OH)2, which form nanoparticle dispersions in alcohols (although the method is not restricted to these solvents and other less polar solvents can be employed as well) may be applied on paper by spraying or by impregnation in a chamber similarly to the Bookkeeper, Battelle and Wei t'o methods. Solvents are volatile, environmentally friendly and have low surface tension so that they properly work as carrier for solid particles, ensuring a homogeneous and penetration depth within the paper fibres (Fig. 7).
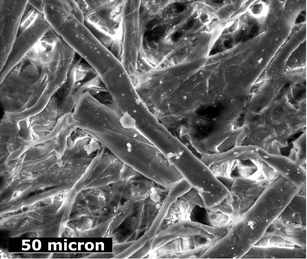 | ||
| Fig. 7 SEM picture of an acid paper sheet (19th century) treated with a Mg(OH)2 nanoparticles dispersion. Deacidification provided 3 pH unit variation. | ||
Acidity affects other materials used in the field of cultural heritage such as canvas and wood, both cellulose-based materials. Flax fabric degradation can be monitored by the fall in pH and may reach approximately 3.5–4 pH units.110 The acidity neutralization can be inhibited by the application of alkaline material. Deacidification treatment of the canvas support is a preventive treatment. Unfortunately, the “already lost” mechanical properties of the canvas cannot be restored.
The application on the back of painted canvas of a buffer is not straightforward. The simplest method is to use dry powdered chalk of magnesium carbonate. The disadvantage is that the powder does not penetrate deeply inside the canvas and therefore it is not very effective against the internal acidity of the canvas.
Aqueous treatments of canvas have been reported although the addition of moisture has obvious drawbacks. It causes, even if sprayed, swelling of the canvas, glue, and paint. Vapour phase methods devised for large-scale treatment of books are not appropriate for paintings. In spite of several drawbacks, the methoxy magnesium methyl carbonate (Wei t'o), has been the only chemical used so far to fight acidity of canvases.111 Nanoparticles dispersions in nonaqueous solvent offer some advantages and can be considered an efficient alternative.
Another interesting application of hydroxide nanoparticles to the conservation of cultural heritage was the deacidification treatment of acidic wood from the famous shipwreck Vasa, recovered 44 years ago after 333 years spent in the seabed of Stockholm harbour. During the last decade Vasa wood developed a large amount of sulfuric acid that consistently decreased wood pH.112 The application of nanoparticles of calcium hydroxide and magnesium hydroxide113,114 conferred a neutralization effect and provided an alkaline reserve that protected wood from aging.
Conclusions
Nanotechnology is based on the recognition that particles of size below 100 nm impart to nanostructures formed from them new behaviour and properties. Although nanotechnology is considered the most important theoretical and applicative framework of human knowledge for the near future, breakthroughs are restricted to few applications, one being the conservation and restoration of cultural heritage. The most recent applications of nanotechnology to the conservation and restoration of the world cultural heritage are discussed in this review and some specific applications of humble calcium and magnesium hydroxide and carbonate nanoparticles, showing their efficacy for the restoration and protection of wall paints and for the de-acidification of paper and wood, are highlighted.References
- Y. Wang and N. Herron, J. Phys. Chem., 1991, 95, 525–532 CrossRef CAS.
- News@Nature, Nanotechnology restores flaking frescos – An off-the-wall application of tiny particles re-unites paint and plaster, Philip Ball, 11 July 2001.
- News@Nature, Nanoparticles save paper – A sprinkling of slaked lime conserves old documents, Philip Ball, 21 October 2002.
- R. Giorgi, L. Dei and P. Baglioni, Stud. Conserv., 2000, 45, 154 Search PubMed.
- M. Ambrosi, L. Dei, R. Giorgi, C. Neto and P. Baglioni, Langmuir, 2001, 17, 4251–4255 CrossRef CAS.
- B. Salvadori and L. Dei, Langmuir, 2001, 17, 2371–2374 CrossRef CAS.
- R. Giorgi, L. Dei, M. Ceccato, C. V. Schettino and P. Baglioni, Langmuir, 2002, 18, 8198–8203 CrossRef CAS.
- R. Giorgi, C. Bozzi, L. Dei, C. Gabbiani, B. W. Ninham and P. Baglioni, Langmuir, 2005, 21, 8495–8501 CrossRef CAS.
- R. Giorgi, D. Chelazzi and P. Baglioni, Langmuir, 2005, 21, 10743–10748 CrossRef CAS.
- M. P. Pileni, J. Phys. Chem. B, 2001, 105, 3358–3371 CrossRef CAS.
- M. P. Pileni, Langmuir, 1997, 13, 3266–3276 CrossRef CAS.
- M. P. Pileni, in Handbook of Surface and Colloid Chemistry, ed. K. S. Birdi, CRC press, 1997, pp. 495–532 Search PubMed.
- M. Sternitzke, J. Eur. Ceram. Soc., 1997, 17, 1061–1082 CrossRef CAS.
- Y. Arai, Gypsum Lime, 1977, 146, 55 Search PubMed.
- R. N. Rothon, in Particulate-Filled Polymer Composites, ed. R. H. Rothon, Longmans, Harlow, UK, 1995; ch. 6 Search PubMed.
- Y. Ding, G. Zhang, H. Wu, B. Hai, L. Wang and Y. Qian, Chem. Mater., 2001, 13, 435–440 CrossRef CAS.
- J. C. Yu, A. Xu, L. Zhang, R. Song and L. Wu, J. Phys. Chem. B, 2004, 108, 64–70 CrossRef CAS.
- S. Mann, S. L. Burkett, S. A. Davis, C. E. Fowler, N. H. Mendelson, S. D. Sims, D. Walsh and N. T. Whilton, Chem. Mater., 1997, 9, 2300–2310 CrossRef CAS.
- H. Schmidt, M. Menning and R. Naß, in Sol–Gel Processing and Application, ed. Y. A. Attia, Plenum Press, New York, 1994, pp.185–186 Search PubMed.
- S. Utamapanya, K. J. Klabunde and J. R. Schlup, Chem. Mater., 1991, 3, 175 CrossRef CAS.
- J. A. Wang, O. Novaro, X. Bokhimi, T. Lopez, R. Gomez, J. Navarrete, M. E. Llanos and E. Lopez-Salinas, J. Phys. Chem. B, 1997, 101, 7448 CrossRef CAS.
- J. A. Wang, O. Novaro, X. Bokhimi, T. Lopez, R. Gomez, J. Navarrete, M. E. Llanos and E. Lopez-Salinas, Mater. Lett., 1998, 35, 317 CrossRef CAS.
- V. Stengl, S. Bakardjieva, M. Marìkova, P. Bezdicka and J. Subrt, Mater. Lett., 2003, 57, 3998–4003 CrossRef CAS.
- O. B. Koper, I. Lagadic, A. Volodin and K. J. Klabunde, Chem. Mater., 1997, 9, 2468–2480 CrossRef CAS.
- Y. Arai, in Chemistry of Powder Production, ed. B. Scarlett, Powder Technology Series, Chapman & Hall, London, 1996, ch. 4 Search PubMed.
- V. Chhabra, V. Pillai, B. K. Mishra, A. Morrone and D. A. Shah, Langmuir, 1995, 11, 3307 CrossRef CAS.
- J. E. Rodriguez-Paez, A. C Caballero, M. Villegas, C. Moure, P. Duran and J. F Fernandez, J. Eur. Ceram. Soc., 2001, 21, 925–930 CrossRef CAS.
- P. D. Southon, J. R. Bartlett, J. L. Woolfrey and B. Ben-Nissan, Chem. Mater., 2002, 14, 4313–4319 CrossRef CAS.
- G. D. Rees, R. Evans-Gowing, S. J. Hammond and B. H. Robinson, Langmuir, 1999, 15, 1993 CrossRef CAS.
- S. Hamada and E. Matijevic, J. Chem. Soc., Faraday Trans. 1, 1982, 78, 2147 RSC.
- K. Kurihara, J. Kizling, P. Stenius and J. H. Fendler, J. Am. Chem. Soc., 1983, 105, 2574 CrossRef CAS.
- R. P. Bagwe and K. C. Khilar, Langmuir, 1997, 13, 6432 CrossRef CAS.
- R. Kumar, K. H. Prakash, P. Cheang and K. A. Khor, Langmuir, 2004, 20, 5196–5200 CrossRef CAS.
- L. Suber, I. Sondi, E. Matijeviç and D. V. Goia, J. Colloid Interface Sci., 2005, 288, 489–495 CrossRef CAS.
- C. Henrist, J.-P. Mathieu, C. Vogels, A. Rulmont and R. Cloots, J. Cryst. Growth, 2003, 249, 321–330 CrossRef CAS.
- S.-H. Lee, Y.-S. Her and E. Matijevic, J. Colloid Interface Sci., 1997, 186, 193–202 CrossRef CAS.
- Q. Zhong and E. Matijevic, J. Mater. Chem., 1996, 6, 443–447 RSC.
- K. Elert, C. Rodriguez-Navarro, E. S. Pardo, E. Hansen and O. Cazalla, Stud. Conserv., 2002, 47, 62–75 Search PubMed.
- T. Yasue, Y. Tsuchida and Y. Arai, Gypsum Lime, 1984, 189, 17 Search PubMed.
- L. A. Perez-Maqueda, L. Wang and E. Matijevic, Langmuir, 1998, 14, 4397 CrossRef CAS.
- P. Calvert and P. Rieke, Chem. Mater., 1996, 8, 1715–1727 CrossRef CAS.
- S. Mann, in Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford, 2002 Search PubMed.
- S. Mann, B. R. Heywood, S. Rajam and J. D. Birchall, Nature, 1988, 334, 692 CrossRef CAS.
- S. Weiner and L. Addadi, J. Mater. Chem., 1977, 7, 689 Search PubMed.
- G. Xu, N. Yao, I. A. Aksay and J. T. Groves, J. Am. Chem. Soc., 1998, 120, 11977 CrossRef CAS.
- S. M. De Souza, C. Alexander, S. W. Carr, A. M. Waller, M. J. Whitcombe and E. N. Vulfson, Nature, 1999, 398, 312 CrossRef CAS.
- L. Brecevic, J. Chem. Soc., Faraday Trans., 1996, 92, 1017 RSC.
- L. Wang, I. Sondi and E. Matijevic, J. Colloid Interface Sci., 1999, 218, 545 CrossRef CAS.
- I. Sondi and E. Matijevic, J. Colloid Interface Sci., 2001, 238, 208 CrossRef CAS.
- K. L. Bachmeier, A. M. Williams, J. R. Warmington and S. S. Bang, J. Biotechnol., 2002, 93, 171 CrossRef CAS.
- I Sondi and E. Matijevic, Chem. Mater., 2003, 15, 1322–1326 CrossRef CAS.
- H. Unuma, S. Kato, T. Ota and M. Takahashi, Adv. Powder Technol., 1988, 9, 181 Search PubMed.
- F. Kara and G. Sahin, J. Eur. Ceram. Soc., 2000, 20, 689 CrossRef CAS.
- R. E. Simpson, C. Habeger, A. Rabinovich and J. H. Adair, J. Am. Ceram. Soc., 1998, 81, 1377.
- M. Ogawa and H. Kaiho, Langmuir, 2002, 18, 4240 CrossRef CAS.
- D. Bayraktar and A. C. Tas, J. Mater. Sci. Lett., 2001, 20, 401 CrossRef CAS.
- A. C. Tas, P. J. Majewski and F. Aldinger, J. Am. Ceram. Soc., 2002, 85, 1414 CrossRef CAS.
- S. Stocks-Fischer, J. K. Galinat and S. S. Bang, Soil Biol. Biochem., 1999, 31, 1563 CrossRef CAS.
- U. K. Gollapudi, C. L. Knutson, S. S. Bang and M. R. Islam, Chemosphere, 1995, 30, 695 CrossRef CAS.
- C. Rodriguez-Navarro, M. Rodriguez-Gallego, K. B. Chekroun and M. T. Gonzalez-Munoz, Applied and Environmental Microbiology, 2003, 69, 2182–2193 Search PubMed.
- C. Sangregorio, M. Galeotti, U. Bardi and P. Baglioni, Langmuir, 1996, 12, 5800–5802 CrossRef CAS.
- I. Lisiecki and M. P. Pileni, J. Phys. Chem., 1995, 99, 5077 CrossRef CAS.
- Y. Tina, C. Wu and J. H. Fendler, J. Phys. Chem., 1994, 98, 4913 CrossRef CAS.
- C. R. Bowers, T. Pietrass, E. Barash, A. Pines, R. K. Grubbs and A. P. Alivisators, J. Phys. Chem., 1994, 98, 9400 CrossRef CAS.
- S. K. Haram, A. R. Mahadeshwar and S. G. Dixit, J. Phys. Chem., 1996, 100, 5868 CrossRef CAS.
- P. K. Nair, V. M. Garcia and A. M. Fernandez, J. Phys. D: Appl. Phys., 1991, 24, 441 CrossRef CAS.
- M. Boutonnet, J. Kizling and P. Stenius, Colloids Surf., 1982, 5, 209 CrossRef CAS.
- H. Yamauchi, T. Ishikawa and S. Kondo, Colloids Surf., 1989, 37, 71 CrossRef.
- A. R. Kortan, R. Hull, R. L. Opila, M. G. Bawendi, M. L. Steingerwald, P. J. Carroll and L. E. Brus, J. Am. Chem. Soc., 1990, 112, 1327 CrossRef CAS.
- C. Tojo, M. C. Blanco, F. Rivadulla and M. A. Lopez-Quintela, Langmuir, 1997, 13, 1970 CrossRef.
- M. Bonini, U. Bardi, D. Berti, C. Neto and P. Baglioni, J. Phys. Chem. B, 2002, 106, 6178–6183 CrossRef CAS.
- Eur. Pat., 1134302, 2001 Search PubMed.
- B. Delfort, M. Born, A. Chivé and L. Barre, J. Colloid Interface Sci., 1997, 189, 151–157 CrossRef CAS.
- U.S. Pat., 4 427 559, 1984 Search PubMed.
- C. Belle, C. Beraud, D. Faure, R. Gallo, P. Hornaert, J. M. Martin and C. Rey, J. Chim. Phys. Phys.-Chim. Biol., 1990, 87, 93 CAS.
- J. P. Roman, P. Hoornaert, D. Faure, C. Bivier, F. Jaquet and J. M. Martin, J. Colloid Interface Sci., 1991, 144, 324 CrossRef CAS.
- J. L. Mansot, M. Hallouis and J. M. Martin, Colloids Surf., A, 1993, 71, 123 CrossRef CAS.
- A. Nanni and L. Dei, Langmuir, 2003, 19, 933–938 CrossRef CAS.
- R. L. Feller, Accelerated Ageing: Photochemical and Thermal Aspects, The Getty Conservation Institute, Los Angeles, CA, 1994 Search PubMed.
- E. Carretti, L. Dei and P. Baglioni, Langmuir, 2003, 19, 7867–7872 CrossRef CAS.
- E. Carretti, L. Dei, C. Miliani and P. Baglioni, Prog. Colloid Polym. Sci., 2001, 118, 63 CAS.
- E. Carretti, L. Dei and P. Baglioni, Prog. Colloid Polym. Sci., 2004, 123, 280–283 CAS.
- E. Carretti and L. Dei, Prog. Org. Coat., 2004, 49, 282–289 CrossRef CAS.
- S. Bracci and J. M. Melo, Polym. Degrad. Stab., 2003, 80, 533–541 CrossRef CAS.
- P. Mora, L. Mora and P. Philipot, in Conservation of Wall Painting, Butterworths, 1984 Search PubMed.
- L. Dei, P. Baglioni and M. Mauro, Preprints of the Conference, Reversibility: Does it Exist?, London 1999 Search PubMed.
- E. Ferroni, Atti del Convegno Internazionale di Studi ‘Piero della Francesca ad Arezzo’, Arezzo, 1990 Search PubMed.
- E. Ferroni, V. Malaguzzi-Valerj and G. Rovida, Proceedings of the ICOM Conference, Amsterdam, 1969 Search PubMed.
- E. Ferroni, OPD Restauro, 1999, 11, 97 Search PubMed.
- P. Baglioni, E. Carretti, L. Dei and R. Giorgi, in Self-Assembly, ed. B. H. Robinson, IOS press, 2003, pp. 32–41 Search PubMed.
- E. Ferroni and P. Baglioni, Proceedings of the symposium “Scientific Methodologies Applied to Works of Art”, Florence, 1984 Search PubMed.
- I. Brajer and N. Kalsbeek, Stud. Conserv., 1999, 44, 145–156 Search PubMed.
- M. Ambrosi, P. Baglioni, L. Dei, R. Giorgi and C. Neto, Prog. Colloid Polym. Sci., 2001, 118, 68–72 CAS.
- P. Croveri, L. Dei, R. Giorgi and B. Salvadori, Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, 2004 Search PubMed.
- M. Ambrosi, P. Baglioni, P. R. David, L. Dei, R. Giorgi, C. Lalli, G. Lanterna, A. Mairani, M. Matteini, M. Rizzi and G. Schonhaut, Proceedings of the International Symposium Science and Technology for the Safeguard of Cultural Heritage in the Mediterranean Basin, Paris, 1999 Search PubMed.
- P. Baglioni, C. Cesari, L. Dei, R. Giorgi, R. Grassi, M. Lorenzetti, M. Mauro, D. Pinzani, P. Ruffo and G. Schonhaut, Atti del XVII Convegno Internazionale Scienza e Beni Culturali -Lo Stucco- Cultura, Tecnologia, Conoscenza, Bressanone, 2001 Search PubMed.
- L. Dei, M. Favaro, R. Giorgi and R. Portieri, Atti del XVII Convegno Internazionale Scienza e Beni Culturali -Lo Stucco- Cultura, Tecnologia, Conoscenza, Bressanone, 2001 Search PubMed.
- R. Carrasco, in Maya, Rizzoli, New York, 1998, pp. 372–385 Search PubMed.
- W. J. Barrow, in Permanence/Durability of the Book – VII. Physical and Chemical Properties of the Book Papers, pp. 1507–1949, W. J. Barrow Research Laboratory, Inc., Richmond (Virginia), 1974 Search PubMed.
- H. J. Porck, Mass Deacidification, An Update of Possibilities and Limitations, ECPA reports, ECPA – European Commission on Preservation and Access, Amsterdam, 1996 Search PubMed.
- K. E. Harris and C. J. Shahani, Mass Deacidification: An Initiative to Refine the Diethylzinc Process, Preservation Directorate – Library of Congress Washington, D. C., October 1994, http://www.loc.gov/preserv/deacid/dezeval.html Search PubMed.
- R. D. Smith, in Conservation of Library and Archive Materials and the Graphic Arts, ed. G. Petherbridge, Butterworth, London, 1987 Search PubMed.
- H. D. Burgess, E. M. Kaminska and A. Boronyak-Szaplonczay, Evaluation of Commercial Mass Deacidification Processes: Akzo-DEZ, Wei T'o and fmc-mg3. Phase I: Naturally Aged Papers, Canadian Conservation Institute, Ottawa, 1992 Search PubMed.
- E. M. Kaminska and H. D. Burgess, Evaluation of Commercial Mass Deacidification Processes: Akzo-DEZ, Wei T'o and fmc-mg3. Phase II: New and Artificially Aged Modern Papers, Canadian Conservation Institute, Ottawa, 1994 Search PubMed.
- J. Havermans, R. Van Deventer, S. Pauk and H. Porck, Deacidification of Books and Archival Materials with the Battelle Process, CNC-Publikaties, 9, Den Haag, 1996 Search PubMed.
- S. Pauk and H. Porck, Oriënterend onderzoek naar de effecten van de Battelle en Diethylzink massaontzuringsmethoden/Pilot research into the effects of the Battelle and diethylzinc massdeacidification methods. CNC-Publikaties, 8, Den Haag, 1994 Search PubMed.
- Preservation Technologies, L. P., Bookkeper Process, U.S., http:// www.ptlp.com.
- S. Buchanan, An Evaluation of the Bookkeeper Mass Deacidification Process. Technical Evaluation Team Report for the Preservation Directorate, Library of Congress. Preservation Directorate, Library of Congress, Washington-DC, 1994 Search PubMed.
- R. Giorgi, L. Dei, C. V. Schettino and P. Baglioni, Preprint of the IIC Congress 2002 – Works of Art on Paper, Books, Documents and Photographs: Techniques and Conservation, Baltimore, 2002.
- G. F. Davidson and T. P. Nevell, J. Text. Inst., 1965, 47, 439–444 Search PubMed.
- S. J. Hackney and G. A. Hedley, Proceedings of ICOM Committee for Conservation 7th Triennal Meeting, Copenhagen, 1984.
- M. Sandström, F. Jalilehvand, I. Persson, U. Gelius, P. Frank and I. Hall-Roth, Nature, 2002, 415, 893–897 CrossRef CAS.
- R. Giorgi, D. Chelazzi and P. Baglioni, Appl. Phys. A Search PubMed in press.
- R. Giorgi, D. Chelazzi and P. Baglioni, Langmuir, 2005, 21, 10743–10748 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
