Mycosporines from freshwater yeasts: a trophic cul-de-sac?
Patricia
Pérez
*a,
Diego
Libkind
b,
María del Carmen
Diéguez
a,
Monika
Summerer
c,
Bettina
Sonntag
c,
Ruben
Sommaruga
c,
María
van Broock
b and
Horacio E.
Zagarese
d
aLaboratorio de Fotobiología, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, U. P. Universidad, 8400 Bariloche, Argentina. E-mail: perezp@arnet.com.ar; Fax: 54-2944-461021; Tel: 54-2944-461601
bLaboratorio de Microbiologia aplicada y Biotecnología, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, U. P. Universidad, 8400 Bariloche, Argentina
cLaboratory of Aquatic Photobiology and Plankton Ecology, Institute of Zoology and Limnology, University of Innsbruck, 6020 Innsbruck, Austria
dInstituto de Investigaciones Biotecnológicas-Instituto Tecnológico de Chascomús, CONICET, Chascomús, Argentina
First published on 11th November 2005
Abstract
Mycosporine-like amino-acids (MAAs) are found in aquatic bacteria, algae, and animals. A related compound, the mycosporine-glutaminol-glucoside (myc-glu-glu), has recently been reported in freshwater yeasts. Although animals depend on other organisms as their source of MAAs, they can efficiently accumulate them in their tissues. In this work we assessed the potential transfer of the yeast mycosporine myc-glu-glu from the diet into the copepod Boeckella antiqua and the ciliate Paramecium bursaria. For this purpose, we performed experiments to study the feeding of B. antiqua and P. bursaria on the yeast Rhodotorula minuta and their ability to bioaccumulate myc-glu-glu. Bioaccumulation of myc-glu-glu in B. antiqua was assessed through long-term factorial experiments manipulating the diet (Chlamydomonas reinhardii and C. reinhardii + yeasts) and radiation exposure (PAR and PAR + UVR). Shorter term experiments were designed in the case of P. bursaria. The composition and concentration of MAAs in the diet and in the consumers were determined by HPLC analyses. Our results showed that even though both consumers ingested yeast cells, they were unable to accumulate myc-glu-glu. Moreover, when exposed to conditions that stimulated the accumulation of photoprotective compounds (i.e. UVR exposure), an increase in MAAs concentration occurred in copepods fed C. reinhardii plus yeasts as well as in those fed only C. reinhardii. This suggests that the copepods were able to modify their tissue concentrations of MAAs in response to environmental clues but also that the contribution of yeast mycosporines to total MAAs concentration was negligible.
Introduction
Many aquatic organisms that are regularly exposed to potentially damaging levels of solar ultraviolet radiation (UVR) accumulate photoprotective compounds (PPC) that serve as sunscreens and/or antioxidants. A particularly diverse family of such substances, collectively referred to as mycosporine-like amino-acids (MAAs), includes at least 19 different chemical species.1–4 MAAs are believed to act as sunscreens filtering out the most damaging UV wavelengths of solar radiation and releasing the excess energy as harmless heat.5 MAAs owe their name from a related group of compounds called mycosporines. The latter compounds were first discovered in fungal sporulating mycelia.6,7 Mycosporines are water-soluble compounds showing absorption maxima between 310 and 320 nm. Their chemical structure consists of an aminocyclohexenone unit bound to an amino-acid or amino-alcohol group.1,8 In contrast, in MAAs molecules the aminocyclohexenone is typically replaced by an aminocyclohexenimine unit, except for mycosporine-glycine and mycosporine-taurine where an aminocyclohexenone ring binds glycine and taurine, respectively2 Although MAAs are known to be present in a great variety of aquatic organisms (bacteria, algae, and animals),1,2 the occurrence of mycosporines has only recently been reported to occur in freshwater yeasts.3Fungal mycosporines are putatively synthesized through a diversion of the shikimic acid metabolic pathway,1 which is the main route of synthesis of aromatic amino-acids in most microorganisms and plants. Animals purportedly lack this pathway, therefore they are thought to depend on other species (either symbiotic or food organisms) as their sources of MAAs.4 Despite the inability of animals to synthesize MAAs, the concentration of these compounds in animal tissues might be much higher than that present in their food, suggesting that certain consumers are highly efficient at sequestering and accumulating MAAs. However, not all aquatic animals are able to accumulate MAAs. For example, the presence of MAAs has never been detected in cladocerans.9,10 Interestingly, in animals that do accumulate these compounds, such as most copepods tested so far, the total MAAs concentration might be many times higher than in the corresponding phytoplankton samples.11
The mycosporine found in yeasts has been identified as mycosporine-glutaminol-glucoside (myc-glu-glu), a compound originally found in terrestrial fungi.12 Following the first report from a limited set of aquatic yeasts, Libkind et al.13 reported the presence of myc-glu-glu in a wide variety of yeast species from a large range of freshwater habitats. Despite the seemingly common occurrence of mycosporine-producing yeasts in freshwater habitats, myc-glu-glu has never been identified from any other group of organisms. For example, Tartarotti et al.11 surveyed the presence of UV-absorbing compounds (hereinafter, this term is used to refer indistinctly to MAAs or myc-glu-glu) in zooplankton samples from a set of lakes that overlapped those studied by Libkind et al.13,14 but they did not detect the presence of myc-glu-glu, and neither did other researchers9 that performed similar surveys in other regions of the world.
Yeasts are a rather minor component of pelagic communities, their abundance typically range from 0 to 500 cells L−1 (Libkind et al.14). Thus, it is not surprising that yeast metabolites are not detected on water samples when low volumes of lake water are filtered (i.e., as customarily done for the assessment of MAAs in seston). However, given the ability of many consumers to accumulate photoprotective compounds, we wondered why not even trace amounts of myc-glu-glu had ever been shown in chromatographs performed on zooplankton extracts. Here, we experimentally assessed two of four potential alternatives: (i) are mycosporine-rich yeasts ingested by consumers, i.e. copepods and ciliates? and (ii) are consumers able to incorporate myc-glu-glu when they are offered high density cultures of mycosporine-rich yeasts as food? Answering affirmatively to both previous questions would imply that either (iii) myc-glu-glu function as a precursor of other mycosporines or MAAs or that (iv) yeasts are too scarce in nature for consumers to be able to bioaccumulate myc-glu-glu above the analytical detection limit.
Materials and methods
Source of organisms
Rhodotorula minuta (CRUB-76) was selected from a large collection of wild yeast strains (CRUB, Microbiology Laboratory) based on their high ability to synthesize myc-glu-glu under experimental induction with UVR and PAR.3 This strain was originally isolated from lakes located in the Nahuel Huapi National Park (North Patagonia, Argentina) as described by Brizzio and van Broock.15 The strain was cultured in potato dextrose liquid medium (PD: yeast extract 3 g L−1; malt extract 3 g L−1; peptone 5 g L−1; dextrose 10 g L−1 supplemented with 1 g L−1 (NH4)2SO4).The copepod Boeckella antiqua was collected from Laguna Los Juncos (41°03′38″S 71°00′38″W, 907 m a.s.l.), a shallow (maximum depth ∼ 1.5 m) freshwater lake located in the Northwest of the Patagonian steppe. B. antiqua was selected as the study organism because it has a remarkable high tolerance to UV exposure. Such resistance is likely to result from its dark-brown pigmentation as well as from high potential for photoreactivation.16 Laboratory cultures of B. antiqua were initiated using individuals collected in June 2002 with a plankton net of 55 µm mesh size. The copepods were placed into 2 L flasks filled with filtered (20 µm) freshwater water from River Gutierrez and fed Chlamydomonas reinhardii (wild type, Carolina Biological Supplies) grown in modified (i.e. without NaCl) Marine Biological Laboratory (MBL) medium. Every two days, the content of the flasks was poured through a 220 µm mesh to separate the nauplii and set up new cultures. The cultures were maintained in an environmental chamber at 18 ± 1 °C (Sanyo, model MLR-350), under a 14 : 10 h light : dark cycle (two fluorescent lamps, PAR: 0.04 mE m−2 s−1; UVA: 0.07 W m−2. The copepods were raised for several generations in the absence of ultraviolet radiation before they were used in experiments.
Paramecium bursaria (Ehrenberg, 1831) Focke, 1836 is a relatively large (ca. 150 µm) hymenostome ciliate which usually bears symbiotic algae of the genus Chlorella sp. For our experiments, we used the strain KM2 (kindly provided by Dr I. Miwa, Ibaraki University, Japan to R.S.), from which we obtained Chlorella-free clones (KM2w) by growing the ciliate in the dark for several weeks at 20 °C on a bacterial diet supported by a lettuce culture medium enriched with 1.5% Chalkley's Medium. Afterwards, the aposymbiotic strain was grown in an environmental chamber at 17 °C with a 14 : 10 h light : dark cycle, including 1 h exposure to UV radiation (UVR) per day provided by one Q-Panel lamp (PAR: 0.1 mE m−2 s−1; UVA: 1.1 W m−2; UVB: 0.88 W m−2).
Feeding experiments
The feeding of the copepod B. antiqua and the ciliate P. bursaria on R. minuta was analyzed in the laboratory by means of two different experiments.Experiment 1 was designed to assess the feeding of B. antiqua on yeasts grown under two different radiation regimes which promoted markedly different concentrations of myc-glu-glu in yeast cells. Prior to the experiment, R. minuta cells were incubated for 72 h in 38 ml quartz test tubes with PD liquid culture media at 18 °C, and a photoperiod of 12 : 12 h light : dark. Yeasts grew under two different radiation regimes: (i) UVR plus PAR, provided by five Q-Panel 340 lamps and ten fluorescents lamps, respectively (PAR: 0.11 mE m−2 s−1;UVA: 1.75 W m−2; UVB: 0.39 W m−2, 66 W m−2, 15 W m−2 and 0.7 W m−2), and (ii) dark, wrapped in aluminium foil (for details see Libkind et al.3). The feeding rate of Boeckella antiqua was estimated for three different initial yeast concentrations. Initial cell concentrations of the irradiated yeasts were 1.4 × 105, 4.6 × 104, and 1.4 × 104 cells ml−1, whereas those of dark-grown yeasts were slightly lower 1.2 × 105, 2.9 × 104 and 1.2 × 104 cells ml−1. A total of 18 test tubes were set up in a plankton wheel (2 rpm). For each radiation treatment and for each yeast concentration, two replicates, with ten females of B. antiqua and one control without copepods were set up. The experiment was run for 2 h in an environmental chamber at 18 °C and PAR provided by two lamps (PAR: 0.04 mE m−2 s−1). Several test trials had been run before in order to establish the optimum experiment duration. After the exposure, the replicates were preserved in 4% formalin and counted according to Utermöhl.17
Experiment 2 tested the ingestion and digestion of R. minuta by P. bursaria. Before starting the experiments, several tests were run to ensure that the yeasts were ingested and digested by the ciliates. In addition, these previous trials served to select the appropriate duration of the experiment. Prior to the experiment, R. minuta and the ciliates were grown for several months under the same conditions. The uptake of R. minuta by P. bursaria was assessed by collecting sub samples of the ciliate culture at regular intervals during 2 h and inspecting their vacuoles under a microscope. To assess the digestion of the yeasts, the cells were previously stained with Congo Red and then offered to the ciliate. The characteristic decrease of pH occurring inside the vacuoles during digestion was observed in the microscope as a change from red to blue.
Bioaccumulation of myc-glu-glu
The bioaccumulation of myc-glu-glu was assessed in experiments 3 and 4 that tested the ability of the copepod and the ciliate to incorporate the compound produced by the yeasts.Experiment 3 tested the accumulation of myc-glu-glu by B. antiqua during 20 days. The experiment consisted of incubations of copepods (150 adults per flask) in a 2 × 2 factorial design. The treatments were two different radiation conditions: (i) PAR and (ii) PAR + UVR crossed with two diets: (i) Chlamydomonas reinhardii (1 × 104 cells ml−1) and (ii) C. reinhardii (5 × 103 cells ml−1) plus R. minuta (5 × 104 cells ml−1). For each treatment three replicates were used. C. reinhardii grew under PAR (0.04 mE m−2 s−1) only and R. minuta under PAR plus UVR following the same conditions as in experiment 1. C. reinhardii was used as a control diet because it does not produce MAAs (even when grown under UVR) and sustains high copepod growth rates.
For the PAR + UVR treatment the copepods were raised in 2 L UV transparent cylinders (Plexiglas UVT, GS 2458, 74 Röhm and Haas, Darmstadt, Germany). For the PAR-only treatment, the copepods were maintained in 2 L glass flasks covered with Ultraphan film (Digefra, UV Opak, 50% transmission at 395 nm). The experiments were run in an environmental chamber at 18 °C and a 12 :12 light : dark photoperiod (UVR plus PAR provided by five Q-Panel 340 lamps and ten fluorescents lamps, respectively, PAR: 0.11 mE m−2 s−1;UVA: 1.75 W m−2; UVB: 0.39 W m−2). Cultures were continuously aerated and cleaned every three days. At the end of the experiments, the copepods were concentrated using a 47 µm mesh size net and placed in Eppendorf vials, kept at −20 °C for a few days and lyophilized for shipping to the laboratory at Innsbruck. Cultures of C. reinhardii were set up in parallel under the same experimental conditions to confirm the absence of MAAs production. Every day during one week, a volume of ca. 500 ml was sampled with two replicates from each culture, filtered (Whatman GF/F) and prepared for MAAs extraction. Similarly myc-glu-glu production was checked in concentrated and lyophilized samples of R. minuta exposed to PAR + UVR. Concentration of myc-glu-glu and MAAs in the samples of R. minuta and C. reinhardii was assessed by means of both UV-visible spectrophotometer scans and HPLC analyses.
Experiment 4 was performed to assess the ability of P. bursaria KM2w to accumulate myc-glu-glu via the ingestion of R. minuta. Feeding experiments were conducted over 48 h in an environmental chamber at 17 °C under PAR + UVR as described above. This period was selected to minimise the dilution of myc-glu-glu in the ciliates due to cell division in the case where the compound was assimilated. Ciliate cultures in the stationary growth phase were fed with the yeast culture for 0.5 h. After feeding, ciliates were thoroughly washed with sterile tap water to remove non-ingested yeast cells. This washing step was repeated several times to remove yeast cells possibly egested by the ciliates. After 48 h, 70 ciliates were withdrawn from the medium with a micropipette. To eliminate yeast cells attached to the ciliate, each individual was cleaned five times on a microscopic slide by transferring each single cell from one drop of sterile-filtered tap water to the next. Finally, the ciliates were placed into a 2 ml Eppendorf vial. Throughout the experiment, we followed the feeding and digestion processes under the microscope and controlled the absence of yeast cells in the medium. Samples of P. bursaria KM2w and R. minuta cultures were taken prior to be used in the feeding experiment as controls for the absence and presence of myc-glu-glu, respectively. All samples were stored at −80 °C for further HPLC analysis.
Extraction and analysis of myc-glu-glu and MAAs
Spectrophotometric determinations were performed on 20% aqueous methanol extracts (24 h at 4 °C, followed by 2 h at 45 °C) following Sommaruga and Garcia-Pichel9 and Laurion et al.18 Qualitative and quantitative analysis of UV-absorbing compounds in yeasts, copepods, microalgae, and ciliates were made by HPLC in lyophilized samples. In all cases, the freeze-dried samples were extracted three times consecutively in 25% aqueous methanol (v : v; MeOH) for 2 h in a water bath at 45 °C. At the beginning of the first extraction, samples were placed on ice and treated with a tip sonicator (diameter: 2 mm) for 1 min at 0.5 cycles and 20% amplitude (UP 200 S, Dr Hielscher GmbH, Germany). The extracts were then cleared by centrifugation at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g and stored at −80 °C. For separation and quantification of the myc-glu-glu and MAAs, 20–60 µl aliquots were injected in a Phenosphere 5 µm pore size C8 column (250 × 4.6 mm, Phenomenex) protected with a RP-8 (Brownlee) guard column, for isocratic reverse-phase HPLC analysis. During the analysis, samples in the autosampler were kept at 15 °C, while the column was maintained at 20 °C. The mobile phase consisted of 0.1% acetic acid in 25% aqueous MeOH (v : v) running at a flow rate of 0.70 ml min−1. The UV-absorbing compounds in the eluate were detected with a diode array detector (Dionex UVD340 S) using four pre-selected channels (310, 320, 334, and 360 nm). Peak purity was checked by analysis of the spectrum over the entire wavelength range. Quantification of myc-glu-glu through spectrophotometric and HPLC analyses was based on the 310 nm absorbance values and the extinction coefficient of the UV-absorbing compound (25
000 g and stored at −80 °C. For separation and quantification of the myc-glu-glu and MAAs, 20–60 µl aliquots were injected in a Phenosphere 5 µm pore size C8 column (250 × 4.6 mm, Phenomenex) protected with a RP-8 (Brownlee) guard column, for isocratic reverse-phase HPLC analysis. During the analysis, samples in the autosampler were kept at 15 °C, while the column was maintained at 20 °C. The mobile phase consisted of 0.1% acetic acid in 25% aqueous MeOH (v : v) running at a flow rate of 0.70 ml min−1. The UV-absorbing compounds in the eluate were detected with a diode array detector (Dionex UVD340 S) using four pre-selected channels (310, 320, 334, and 360 nm). Peak purity was checked by analysis of the spectrum over the entire wavelength range. Quantification of myc-glu-glu through spectrophotometric and HPLC analyses was based on the 310 nm absorbance values and the extinction coefficient of the UV-absorbing compound (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, taken from Bouillant et al.19). The concentration of shinorine, porphyra-334, mycosporine-glycine and an unidentified compound with an absorbance maximum at 332 nm (hereinafter referred to MAA-332) was calculated from HPLC peak areas, using published molar extinction coefficients2 and an average molar extinction coefficient of 40
000 M−1 cm−1, taken from Bouillant et al.19). The concentration of shinorine, porphyra-334, mycosporine-glycine and an unidentified compound with an absorbance maximum at 332 nm (hereinafter referred to MAA-332) was calculated from HPLC peak areas, using published molar extinction coefficients2 and an average molar extinction coefficient of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the latter.20 All values were expressed as µg mg−1 of dry weight.
000 for the latter.20 All values were expressed as µg mg−1 of dry weight.
Data analysis
The consumption of yeast cells (F) by B. antiqua was calculated from results of experiment 1 as:| F = (Ci − Cf)/(n × t) |
Where F is the feeding rate, Ci is the initial yeast concentration, Cf is the final yeast concentration, n is the number of copepods and t is the duration of the experiment.
The consumption rate of B. antiqua on exposed and unexposed yeasts was compared by testing differences between treatments regression coefficients using Student's t (α = 0.05). In experiment 3, the effect of diet and radiation treatment on total concentration of MAAs as well as the relative concentration of myc-glu-glu, shinorine, porphyra-334 and MAA-332 in Boeckella were compared using Two-Way ANOVA. The significance level (α = 0.05) was adjusted to P = 0.015 with the Dunn-Sidák formulae to account for the non-independence of the separate ANOVAs.
Results
The results of the feeding experiments showed that both consumers readily ingested Rhodotorula minuta. The mean yeast feeding rate of B. antiqua changed between 187![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 877 ± 14
877 ± 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 and 4869 ± 2848 cells ind−1 h−1 depending on initial yeast concentration (experiment 1). Regardless of the initial yeast concentration, B. antiqua fed on exposed and unexposed yeasts cells at similar rates (t = 1.028, df = 8, P = 0.05, Fig. 1). In addition, microscopic observations (experiment 2) showed that P. bursaria started to ingest R. minuta within 5 min.
493 and 4869 ± 2848 cells ind−1 h−1 depending on initial yeast concentration (experiment 1). Regardless of the initial yeast concentration, B. antiqua fed on exposed and unexposed yeasts cells at similar rates (t = 1.028, df = 8, P = 0.05, Fig. 1). In addition, microscopic observations (experiment 2) showed that P. bursaria started to ingest R. minuta within 5 min.
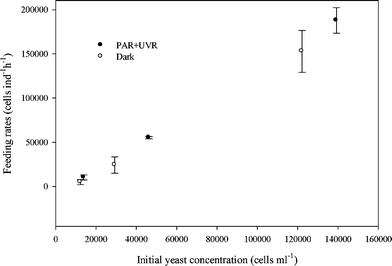 | ||
| Fig. 1 Feeding rates (F) of Boeckella antiqua on Rhodotorula minuta grown in PAR + UVR and dark as a function of initial yeast concentration (Experiment 1). Values are means (±SD) of two replicates. Differences in the feeding rates of B. antiqua between exposed and unexposed yeasts were not significant (t = 1.028, df = 8, P = 0.05, n = 12). | ||
B. antiqua presented a basal level of MAAs of about 1.33 ± 0.01 µg mg−1 dry weight which was maintained even when the copepods were cultured on a C. reinhardii diet and unexposed to PAR plus UVR (Fig. 2). The bioaccumulation experiment showed that the amount of MAA increased significantly when the copepods were exposed to PAR plus UVR (F = 42.95, df = 1, P = 0.0001, Fig. 3). The addition of R. minuta to the diet did not result in higher levels of MAAs (F = 0.34; df = 1, P = 0.57; Fig. 2), nor in changes in the relative concentration of each compound (P > 0.015; Fig. 3). Individuals from the different treatments contained mycosporine-glycine, porphyra-334, shinorine, and MAA-332. Mycosporine-glutaminol-glucoside was not detected in B. antiqua.
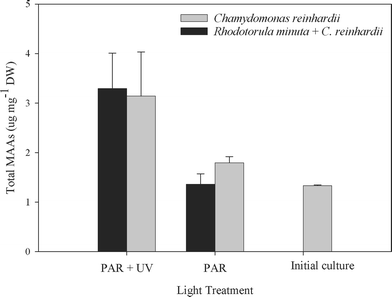 | ||
| Fig. 2 Total MAA concentration in Boeckella antiqua after 20 days of feeding on either Chlamydomonas reinhardii alone or C. reinhardii plus the yeast Rhodotorula minuta. Initial culture bar shows the basal level of MAAs present in B. antiqua at the beginning of the experiment. The copepods were exposed to PAR only or PAR plus UVR. The total MAA concentration (means ± SD) was significantly higher in copepods exposed to PAR + UVR as compared to those exposed to PAR alone (F = 42.95, df = 1, P = 0.0001, n = 12), but there were no significant differences between diets (F = 0.34, df = 1, P = 0.57, n = 12). | ||
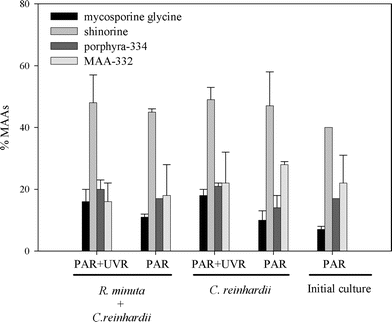 | ||
| Fig. 3 Relative MAA concentration in Boeckella antiqua at the end of the bioaccumulation experiment. Initial culture shows the relative MAA concentration present in B. antiqua at the beginning of the experiment. No significant differences in relative MAAs concentration (means ± SD of three replicates) were observed across diets and treatments (P > 0.015). | ||
The yeast cells were ingested by P. bursaria (Fig. 4a) and digested as indicated by the colour change of Congo Red within the digestive vacuoles (Fig. 4b). However, even after 24 h it was still possible to observe some ciliates with 1 to 2 yeast cells left in food vacuoles. After 48 h, yeasts were undetectable inside the ciliates as confirmed by mechanical disruption and microscopical observations. Despite the high concentration of myc-glu-glu found in R. minuta, the compound was undetectable in P. bursaria.
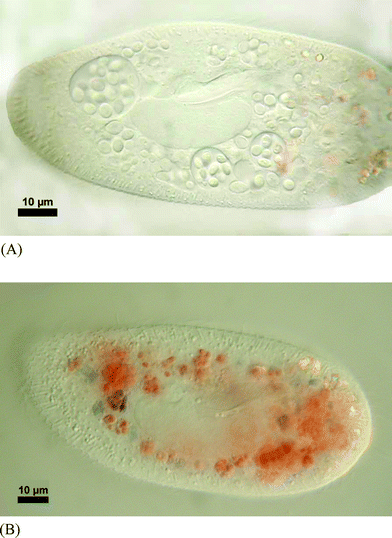 | ||
| Fig. 4 (A). Micrograph showing the ingestion of Rhodotorula minuta inside the food vacuoles of Paramecium bursaria. (B). Paramecium bursaria with ingested Rhodotorula minuta showing the start of digestion. The blue colour indicates a pH < 3. | ||
Discussion
The results of our experiments demonstrated that two different aquatic consumers, B. antiqua and P. bursaria, are able to ingest aquatic yeasts. In addition, B. antiqua was found to feed at similar rates on yeasts grown under PAR or PAR plus UVR. On the other hand, the results obtained from the bioaccumulation experiment provide strong evidence that none of the consumers accumulate myc-glu-glu. Under exposure to PAR, the total MAAs concentration was actually lower in the copepods fed Chlamydomonas + yeast as compared to those fed only Chlamydomonas, strongly suggesting that the yeast are not sources of MAAs or MAA precursors.Moeller et al.21 suggested that UVR exposure enhances the uptake of MAAs in Leptodiaptomus minutus feeding the same diet. But, even though the copepods in our experiments were exposed to conditions stimulating the accumulation of photoprotective compounds (i.e. UVR exposure), they were unable to accumulate myc-glu-glu. Certain consumers may be highly efficient in sequestering and accumulating MAAs. In fact, in those animals that accumulate these compounds, such as most copepods tested so far, the total MAAs concentration might be many times higher than in the corresponding phytoplankton samples.11 While HPLC analysis performed to B. antiqua identified the presence of shinorine, mycosporine-glycine, porphyra-334 plus the unknown compound MAA 332, myc-glu-glu was never detectable in the chromatographs. The experiment with ciliates provided further direct evidence since it was observed they digested the yeast cells, however, myc-glu-glu was absent from ciliates extracts. Although the ciliates may have preferred the bacterial diet, our observations clearly indicated that R. minuta was rapidly ingested and digested. Usually, filter feeding ciliates of the genus Paramecium digest their food (e.g., bacteria) in food vacuoles within 20 min.22 Under our experimental conditions digestion of R. minuta took longer in the order of hours and after complete digestion there was no sign of accumulation of myc-glu-glu.
One explanation for the absence of myc-glu-glu in our HPLC analysis of copepods and ciliates may be a potential transformation into a different chemical species. For example, Shick and Dunlap1 have suggested the possibility of transformation of ingested MAAs into different ones by bacteria. However, the fact that an increase in concentration of the same MAAs occurred both in copepods fed C. reinhardii + yeasts, as well as in those fed only C. reinhardii suggests that myc-glu-glu is unlikely to be transformed into a different MAA. Thus, the most probable fate of myc-glu-glu in B. antiqua and P. bursaria is its complete degradation or excretion.
Collectively, the evidence gathered in this study points to an impossibility of the consumers to accumulate the yeast metabolite myc-glu-glu or to use it as a precursor for other MAAs. However, at the same time, it raises several questions that warrant further investigation. First, the fact that copepods increased their levels of MAAs when exposed to UVR in the absence of a dietary source was completely unexpected (it must be recalled that the HPLC analyses failed to detect even trace amounts of MAAs in C. reinhardii even when it was grown under UVR exposure). Actually no reports of MAAs in Chlamydomonas have been performed at present. Although, the copepods cultures were not axenic (i.e., they might contain bacteria and a few ciliates), the HPLC analysis was performed on the whole culture, thus the evidence suggests that not only the algae, but also the minor contaminant of the culture lack the ability to synthesize MAAs. Therefore, the available evidence suggests that MAAs present in the copepods are not directly derived from their diet. Several groups of animals including ascidians and corals23–26 are thought to obtain their MAAs from symbiotic microorganisms. However to the best of our knowledge, there is not reported evidence of MAA-producing symbionts in copepods, although Chang and Jenkins27 found plastid endosymbionts in Daphnia obtusa and suggested that plastids uptake is facultative and released photosynthetic and organic products to the host. Secondly, the results from this study combined with previous data raise a number of biochemical questions. Previous work9,11 suggested that cladocerans are unable to bioaccumulate MAAs. Here, we present evidence suggesting that both copepods and ciliates are unable to utilize a mycosporine directly or indirectly as precursors of other MAAs. The characteristics that make certain PPC assimilable by certain animals but not by others remains an open question.
Acknowledgements
This work was supported by Universidad Nacional del Comahue (Grant B940 and B091), Fundación Antorchas (Grant 14156-82), Interamerican Institute for Global Change (IAI-CNR 026), PIP-CONICET 02135, and FONCyT PICT 01-13550 to H. Z. and by the Austrian Science Foundation (Project FWF 14153-BIO) to R.S.References
- J. M. Shick and W. C. Dunlap, Mycosporine-like amino acids and related gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms, Annu. Rev. Physiol., 2002, 64, 223–262 CrossRef CAS.
- D. Karentz, Chemical defenses of marine organisms against solar radiation exposure: UV absorbing mycosporine-like amino acids and scytonemin, in Marine Chemical Ecology, ed. J. B. McClintock and B. J. Baker, CRC Press Inc, 2001 Corporate Blvd NW/Boca Raton/FL 33431/USA, 2001, 481-520 Search PubMed.
- D. Libkind, P. A. Perez, R. Sommaruga, M. C. Diéguez, M. Ferraro, S. Brizzio, H. Zagarese and M. R. Rosa Giraudo, Constitutive and UV-inducible synthesis of photoprotective compounds (carotenoids and mycosporines) by freshwater yeasts., Photochem. Photobiol. Sci., 2004, 3, 281–286 RSC.
- A. T. Banaszak, Photoprotective physiological and biochemical responses of aquatic organisms, in UV effects in aquatic organisms and ecosystems, ed. W. E. Helbling and H. E. Zagarese, The Royal Society of Chemistry, Cambridge, UK, 2003, 329-356 Search PubMed.
- F. R. Conde, M. S. Churio and C. M. Previtali, The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution, J. Photochem. Photobiol., 2000, 56, 139–144 Search PubMed.
- C. M. Leach, Ultraviolet absorbing substances associated with light-induced sporulation in fungi, Can. J. Bot., 1965, 43, 185–200 CrossRef CAS.
- E. J. Trione, C. M. Leach and J. M. Mutch, Sporogenic substances isolated from fungi, Nature, 1966, 212, 163–164 CAS.
- W. M. Bandaranayake, Mycosporines: are they nature's sunscreens?, Nat. Prod. Rep., 1998, 15, 159–172 RSC.
- R. Sommaruga and F. Garcia Pichel, UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake, Arch. Hydrobiol., 1999, 144, 255–269 Search PubMed.
- B. Tartarotti, I. Laurion and R. Sommaruga, Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient, Limnol. Oceanogr., 2001, 46, 1546–1552 CAS.
- B. Tartarotti, G. Baffico, P. Temporetti and H. E. Zagarese, Mycosporine-like amino acids in planktonic organisms living under different UV exposure conditions in Patagonian lakes, J. Plankton Res., 2004, 26, 753–762 Search PubMed.
- R. Sommaruga, D. Libkind, M. van Broock and K. Whitehead, Mycosporine-glutaminol-glucoside, a UV-absorbing compound of two Rhodotorula yeast species, Yeast, 2004, 12, 1077–1081 CrossRef.
- D. Libkind, R. Sommaruga, H. Zagarese and M. R. van Broock, Mycosporines in carotenogenic yeasts, Syst. Appl. Microbiol., 2005, 28, 749–754 Search PubMed.
- D. Libkind, S. Brizzio, A. Ruffini, M. Gadanho, M. R. van Broock and J. P. Sampaio, Molecular characterization of carotenogenic yeasts from aquatic environments in Patagonia, Argentina, Antonie Leeuwenhoek, 2003, 84, 313–322 CrossRef CAS.
- S. Brizzio and M. van Broock, Characterization of wild yeast killer from Nahuel Huapi National Park (Patagonia, Argentina), J. Food Technol. Biotechnol., 1998, 4, 273–278 Search PubMed.
- H. E. Zagarese, M. Feldman and C. E. Williamson, UV-B induced damage and photoreactivation in three species of Boeckella (Copepoda, Calanoida), J. Plankton Res., 1997, 19, 357–367 Search PubMed.
- H. Utermöhl, Zur Vervollkommung der quantitativen phytoplankton-methodik, Mitt Int. Ver. Limnol., 1958, 9, 1–38 Search PubMed.
- I. Laurion, M. Ventura, J. Catalan, R. Psenner and R. Sommaruga, Attenuation of ultraviolet radiation in mountain lakes: Factors controlling the among- and within-lake variability, Limnol. Oceanogr., 2000, 45, 1274–1288.
- M. L. Bouillant, J. L. Pittet, J. Bernillon, J. Favre-Bonvin and N. Arpin, Mycosporines from Ascochyta pisi, Cladosporium herbarum and Septoria nodorum, , Phytochemistry, 1981, 20, 2705–2707 CrossRef CAS.
- B. Tartarotti and R. Sommaruga, The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton, Arch. Hydrobiol., 2000, 154, 4, 691–703 Search PubMed.
- R. E. Moeller, S. Gilroy, C. E. Williamson, G. Grad and R. Sommaruga, Dietary acquisition of photoprotective compounds (mycosporine-like amino acids, carotenoids) and acclimation to ultraviolet radiation in a freshwater copepod, Limnol. Oceanogr, 2005, 50(2), 427-439.
- R. D. Allen and L. A. Staehelin, Digestive systems membranes: freeze-fracture evidence for differentiation and flow in Paramecium, J. Cell Biol., 1981, 89, 9–20 CrossRef CAS.
- M. L. Dionisio-Sese, M. Ishikura, T. Maruyama and S. Miyachi, UV-absorbing substances in the tunic of a colonial ascidian protect its symbiont, Prochloron sp., from damage by UV-B radiation, Mar. Biol., 1997, 128, 455–461 CrossRef CAS.
- J. M. Shick, S. RomaineLioud, C. FerrierPages and J. P. Gattuso, Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates, Limnol. Oceanogr., 1999, 44, 1667–1682 CAS.
- A. T. Banaszak and R. K. Trench, Effects of ultraviolet (UV) radiation on marine microalgal-invertebrate symbioses. 2. The synthesis of mycosporine-like amino acids in response to exposure to UV in Anthopleura elegantissima and Cassiopeia xamachana, J. Exp. Mar. Biol. Ecol., 1995, 194, 233–250 CrossRef CAS.
- A. T. Banaszak, T. C. LaJeunesse and R. K. Trench, The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates, J. Exp. Mar. Biol. Ecol., 2000, 249, 219–233 CrossRef CAS.
- N. Chang and D. G. Jenkins, Plastid endosymbionts in the freshwater crustacean Daphnia obtuse, J. Crustacean Biol.,, 2000, 20, 231–238 Search PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |
