Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2005
United Nations Environment Programme, Environmental Effects Assessment Panel†
First published on 29th November 2005
Abstract
Since the first assessments in 1989, the complexity of the linkages between ozone depletion, UV-B radiation and climate change has become more apparent. This makes it even clearer than before that we are dealing with long-term developments, which can be complicated by large year-to-year variability.
Introduction
The Vienna Convention for the Protection of the Ozone Layer (1985) exerts its specific actions via the Montreal Protocol on Substances that Deplete the Ozone Layer (1987). Almost all countries are involved; only six, with limited release of ozone depleting substances, have not yet ratified the Protocol.The Parties to the Montreal Protocol are informed by three panels of experts. One of these is the Environmental Effects Assessment Panel (EEAP), which deals with effects of ozone depletion and its interactions with climate change. The EEAP produces an extensive assessment report for the Parties to the Montreal Protocol every four years, and in the intermediate years a brief Progress Report is prepared. These assessments aim at readability for non-specialists, but are based on scientific information published in the scientific literature. The latest full report was published in Photochem. Photobiol. Sci., 2003, 2, 1–72 and the previous progress report in Photochem. Photobiol. Sci., 2005, 4, 177–184.
Ozone and UV changes
International measures to control ozone depleting substances are working and the beginning of ozone recovery is no longer controversial
Statistical trend analyses have demonstrated that, globally, the average column of ozone reached a minimum in the late 1990s, and since then has started to recover at mid-latitudes in both hemispheres.1 The analysis included the effects of variations in atmospheric circulation patterns. The observed changes in ozone are consistent with those expected from changes in atmospheric chemistry that would result from the actions mandated by the Montreal Protocol. However, the correlations with changes in atmospheric circulation1–3 may indicate an interaction between ozone depletion and climate.Low concentrations of springtime ozone are predicted to persist in polar regions
In Antarctica, the springtime stratospheric ozone “hole” will continue to recur for the next decades. There will continue to be a large year-to-year variability. For example, the ozone hole in 2005 was large (Fig. 1) and developed early, like the record sized hole in 2003, but was rather small in 2004. Very large year-to-year variability is expected to continue in the Arctic, depending on the minimum temperatures reached. With global climate change, temperatures in the Arctic stratosphere are expected to continue to decrease, increasing the likelihood of severe ozone depletion on the surfaces of polar stratospheric clouds. For every degree of stratospheric cooling, a reduction in ozone of 15 Dobson Units can be expected.4 This sensitivity is three times larger than had been estimated previously from model calculations, making future polar ozone depletion more susceptible to climate change.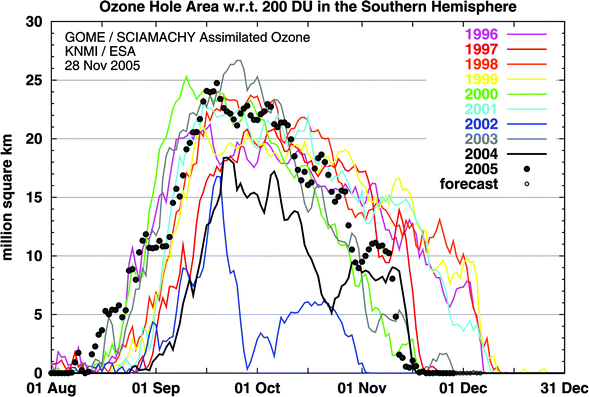 | ||
| Fig. 1 Area (millions of km2) where the total ozone column in the Southern Hemisphere is less than 220 DU (Dobson Unit, where 1 DU = 0.01 mm thickness at standard temperature and pressure). All the years from 1996 to 2005 (black dots) are shown. Graphic prepared by the KNMI, Het Koninklijk Nederlands Meteorologisch Instituut and ESA (European Space Agency) using data from GOME (Global Ozone Monitoring Experiment) a satellite based instrument and the scanning imaging absorption spectrometer for atmospheric chartography (SCIAMACHY) on board the ESA Envisat satellite. | ||
Our understanding of interactions between ozone depletion and climate change has improved significantly
The effects of climate change on ozone depletion may be most pronounced—yet least understood—at high latitudes,5 where springtime ozone losses are expected to continue up to ∼2020.6 The role of changes in atmospheric circulation seems increasingly important in modulating ozone variability.7–10 Ozone heats the stratosphere by absorbing incoming solar energy and outgoing infrared from the surface of the earth. A significant component of the observed stratospheric cooling (−0.17 °C decade−1) can be attributed to ozone depletion, rather than being solely a radiative effect of climate change.11 Consequently, the future impact of climate change on the ozone layer at mid-latitudes may be diminished. Interactions with solar activity may also be more important for ozone depletion and UV increases than previously thought.12–19Further contributions towards understanding the effects of aerosols on UV radiation reaching the Earth's surface are now available
The UV absorbing properties of aerosols are now quantified20–24 leading to improvements of satellite algorithms for more effective treatment of the interference of tropospheric aerosols in the derivation of surface UV irradiances.25,26 This will enable more accurate satellite estimates of UV in the future.Reconstruction of historical UV records has improved, and the observed changes of UV radiation in recent decades are consistent with those expected from changes in ozone and atmospheric transmission
Time series of UV irradiances, extended several decades back from the start of the ozone depletion, are now available. The longest of these goes back to the 1920s when ozone measurements first became available,27 depicting fluctuations of similar magnitude to observations in the last two decades.Measurements of UV irradiance at various locations over northern mid-latitudes show that surface UV has increased in the 1980s and 1990s as a consequence of ozone depletion and increasing atmospheric transmission.27–33 The period since the “turnaround” in ozone is too short to reveal unequivocal changes in UV radiation. However, data since the late 1990s from some Southern Hemisphere sites indicate that surface UV radiation has levelled off34 or may even be decreasing.35
Attempts have been made to reconstruct historical ozone or UV-B amounts for periods prior to the modern instrumented records and prior to human impacts
These include: (1) measurements from historical records of star spectra, back to about 1900;36–38 (2) analyses of pigments in herbarium specimens,39,40 over periods of up to ∼100 years, and (3) analyses of pigments in lake sediments41,42 and analyses of tree rings,43,44 over thousands of years. Most of these attempts are still in a method development stage, but some show promise for the future.Methyl bromide constitutes the largest source of bromine atoms to the stratosphere and therefore plays an important role in the depletion of stratospheric ozone45
There are still large uncertainties in the identification and quantification of significant sources and sinks of methyl bromide (and other simple organic halides).46 New sources47–50 for these compounds are still being discovered but uncertainties prevail. The phase-out of methyl bromide usage is important for the short-term recovery of stratospheric ozone concentrations. Our knowledge of its effects over longer periods is limited by uncertainties in the magnitudes of sources and sinks.51 New information that becomes available about these will improve existing models and our understanding of its involvement in atmospheric processes.52,53Health
The incidence rates of sunlight-induced skin cancer continue to rise rapidly in light-skinned populations
Results from the Netherlands, the UK, Australia and the USA document these trends in basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and cutaneous malignant melanoma (CMM).54–57 Forecast modelling of these tumours in the Netherlands predicts that their rates will double by 2015.58 In Minnesota, USA, the increases in BCC and SCC were most apparent in men and women under the age of 40 years and there was a disproportionate increase in BCC in young women.55 In the UK between 1975 and 2000, CMM has shown the largest increase in incidence rates compared with other major tumours, and modelling results suggest that, even with sun avoidance and use of sunscreens, the melanoma incidence rate will not decrease for about another 30 years, by which time the rate may be twice that found currently.56 Similar projections for Australia indicate that, by 2011, melanomas will represent 11% of all new cancer cases in men and will have overtaken lung cancer (10%) as the third most common cancer for men of all ages.57 These findings emphasize the need for skin cancer prevention programs, particularly those aimed at young adults, in order to lessen the adverse impacts of increasing UV-B exposure.Recent animal experiments confirm that it is UV-B and not UV-A radiation that initiates melanomas
In a study using transgenic mice that develop melanomas which closely resemble those of humans, UV-B radiation induced CMM while UV-A did not, even at doses considered biologically relevant.59 These experiments suggest that reducing exposure to UV-B is important in reducing CMM incidence in human populations.Adverse solar UV effects on the eye can be enhanced by the presence of clouds
The eye is protected from most direct solar UV radiation by its anatomical location and physiological defence mechanisms (e.g., squinting, pupil constriction) to bright light.60 Indirect, i.e., reflected and/or diffuse UV, however, plays a major role in acute solar photokeratitis and is also important in cataract formation. Under cloud cover (when light levels are lower and greater scatter occurs), the natural defence mechanisms of the eye relax, and the surface and interior of the eye receive more of the incident UV. At the same time, the effective UV-B incident on the eye can be increased under cloud cover.61,62 These results confirm the need for eye protection from solar UV radiation even when it is not sunny.Recent reports confirm that sunlight-associated pterygium occurs in people of all skin colours
On the basis of in vitro studies, pterygium (a common inflammatory, proliferative and invasive lesion of the human cornea that can severely impair vision; Fig. 2) appears to result from UV-B-induced intracellular damage to epithelial cells.63 The accumulated exposure to sunlight64,65 and a variety of genes, such as those involved in the repair of DNA damage,66–68 influence the development of pterygia in human populations. All outdoor workers, regardless of skin colour, would thus benefit from eye protection.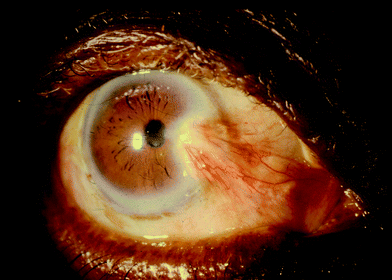 | ||
| Fig. 2 Photograph of a pterygium. | ||
Evidence suggesting a role for sunlight-induced vitamin D production in the prevention of several cancers is accumulating
A number of recent epidemiologic studies have concluded that self-estimated sun exposure was associated with reduced risk of some internal tumours, e.g., non-Hodgkin lymphoma and prostate cancer.69–71 In addition, one report found an increased 5-year survival following the diagnosis of early stage CMM with increasing solar exposure assessed not only by self-estimated solar exposure but also through the measurement of solar elastosis (one measure of skin aging).72 A common suggestion from the above studies was that the observed protective effects might be due to an increase in vitamin D which has been shown to have a role in controlling the proliferation of tumour cells. However, it should be noted that at least one report, of a prospective epidemiologic study, found no association between plasma concentrations of active vitamin D or its precursor and the incidence of prostate cancer.73Many studies indicate that there is widespread vitamin D insufficiency and deficiency, especially in populations living at mid to high latitudes. The exposure to UV-B required to provide sufficient vitamin D to maintain optimal immune and other functions is not currently known but is likely to vary from person to person depending on skin characteristics (colour, thickness), health and nutritional status and vitamin D receptor genotype.74 It has been estimated that, for healthy light-skinned individuals, the exposure required would be about 5 minutes per day 3 times a week while darker skinned individuals the exposure required would be 10 minutes per day 3 times a week.75,76 Others believe that a greater solar exposure is required.77 Indeed it is thought by some that where there is insufficient solar UV-B radiation due to season, latitude or lifestyle, various foods may require fortification with vitamin D.78,79
For several internal cancers and particular autoimmune diseases, the benefits from increases in ambient UV-B are hard to predict
There are inadequacies of personal UV dosimetry in human population studies and a lack of suitable animal experiments. However, although the incidence rates of skin cancers have risen in association with increased levels of sun exposure, there has not been a trend towards a decrease in internal cancers such as prostate and breast or in autoimmune diseases such as multiple sclerosis (for example the incidence of prostate cancer is increasing steadily in almost all countries80). Thus caution is required before encouraging people to expose themselves to full sunlight in order to reduce their risk of developing these types of diseases. More research is needed in this challenging and complex area.Additional diseases and conditions have been identified that may be adversely affected by exposure to UV-B
There is evidence that UV-B exposure may induce the skin lesions characteristic of the autoimmune disease, systemic lupus erythematosus, in patients with gastrointestinal cancer (adenocarcinoma) who have been treated with the anti-cancer drug, fluorouracil.81 In addition, based on the results of animal experiments, individuals exposed to arsenic may experience an increase in skin cancer incidence,82 while those undergoing chronic stress may experience an accelerated development of skin cancer83 when exposed to UV-B.Concerns have arisen about unintended environmental consequences from the increased use of sunscreens to protect against UV-B
A number of the UV-B absorbing components in sunscreens have been shown to have weak estrogenic activity (potencies in one report of 1/600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 or less compared to the reference estradiol84) and thus may have adverse consequences for reproductive function when released into the environment, concomitant with other endocrine disrupting chemicals.85 These observations lend strength to the recommendation that protection from UV-B may need to rely on strategies other than sunscreen use.84,86–92
000 or less compared to the reference estradiol84) and thus may have adverse consequences for reproductive function when released into the environment, concomitant with other endocrine disrupting chemicals.85 These observations lend strength to the recommendation that protection from UV-B may need to rely on strategies other than sunscreen use.84,86–92Terrestrial ecosystems
Biodiversity may be altered by UV-B radiation even though total community production and biomass are not necessarily affected
A further recent report of this nature shows changes in the species composition and biodiversity of micro-fungi growing in peat bogs of Tierra del Fuego.93New research in Antarctica shows that some native species are adversely affected by current levels of UV radiation
An endemic species of moss was found to have reduced levels of photoprotective pigments (compared to other widely distributed moss species), and current levels of UV radiation were reported to cause increased frequency of abnormalities in leaf morphology.94 These results are consistent with previous findings showing reduced growth as a result of exposure to ambient UV-B in native species of Antarctica and Tierra del Fuego.The interplay between UV radiation and periodic drought conditions may result in altered species composition in shortgrass-steppe ecosystems
In a field study in which solar UV was manipulated by filters,95 productivity was decreased and forage quality increased by UV radiation for the dominant grass species in a dry, but not in a wet year. In another grass species, productivity decreased due to UV, but only in wet years. Forage quality of this species did not change. Since forage quality increases decomposition, UV radiation and drought would reduce biomass and soil organic matter. The variable response observed among species grown under changing conditions of soil moisture availability and UV suggests that as drought becomes more prevalent with global change in the shortgrass-steppe ecosystem, the structure and function of this ecosystem may change substantially.Increasing nitrogen supply to plants may result in additional sensitivity to UV-B
In field experiments, supplemental UV-B simulating ozone depletion caused reductions in total biomass, nitrogen content and specific pigment concentrations for a South African legume species (Cyclopia maculata), but the UV-B effect was much more pronounced if the plants were supplied with supplemental nitrate.96 In another field study with simulated ozone reduction over Portugal, net photosynthesis of maize was less affected by UV-B at low nitrogen supply than at higher nitrogen levels.97 Interactions of nitrogen and UV-B may be important in regions experiencing nitrogen deposition or in agricultural systems with nitrogen applications.In a study with soybean, increased UV-B radiation, elevated CO2 and high temperature resulted in smaller flowers with less pollen and lower pollen germination rates98
Many earlier studies have shown that elevated CO2 ameliorates many injurious effects of UV-B. In another study with cotton, no interactive effect was found for elevated UV-B and CO2.99 These experiments were performed in sunlit, controlled-environment chambers that may have compromised the realism in relation to field conditions.UV-B radiation can alter plant responses to extreme temperatures
UV-B radiation increased heat tolerance considerably in cucumber plants grown in growth chambers.100 Freezing tolerance of jack pine was similarly increased by UV-B and this was linked to induction of secondary compounds (phenols) in plant tissues.101 These studies were conducted in growth chambers and may not necessarily apply to field conditions. However, in a field study, low-temperature tolerance was increased.102New studies on the genetic basis for plant responses to UV-B radiation contribute more detailed information on the effects of UV-B radiation, from the gene to the whole plant level
Recent studies confirm earlier reports that a sizeable portion of the plant genome can exhibit changes in gene expression due to UV-B and that many of the UV-B-regulated genes are plant organ-specific.103,104Aquatic ecosystems
Climate change may increase the sensitivity of aquatic organisms to UV-B radiation
In the oceans, future warming is likely to alter the spatial distribution of productivity, affecting ecosystems and placing additional stress on already depleted fish stocks and other marine resources.105 Field observations suggest that climate forcing plays a strong role in determining UV exposures to communities in snow and ice-covered environments.106 Climate change and ozone layer thinning simultaneously expose polar organisms to increasingly stressful conditions, including changes in temperature, salinity and UV radiation.107 Further, polar ecosystems may be more vulnerable to UV radiation because of the inhibiting effects of low temperatures on cellular repair of UV-B damage,108,109 decreases in protective sea ice coverage in areas of recent rapid warming110,111 and possible changing cloud cover.112Changes in underwater UV-B and temperature can significantly influence the composition of the zooplankton community and ultimately food web dynamics113
Laboratory studies on molluscan larvae have demonstrated that the effects of ultraviolet radiation were particularly marked, with mortality increasing up to 12-fold under temperature and salinity stress associated with climate change as compared with UV-B or temperature and salinity stress alone. It is likely that we are consistently underestimating the ecological impacts of climate change and enhanced UV-B radiation by failing to consider the complex interplay of environmental variables and their impact on organisms.114There remains a lack of consensus regarding the role of UV-B and other environmental stresses with respect to the causes of the worldwide decline in amphibian populations
Various experiments have led researchers to propose several potential causes for these declines, including disease, habitat destruction, environmental contaminants, introduced exotic species, global climate change and increased levels of UV-B radiation. Accumulating evidence suggests that high levels of UV-B radiation are harmful to many amphibian species. However, often in the natural environment where exposure is limited, UV-B radiation is less of a factor, although UV-B is believed to exacerbate a variety of these other listed stresses.115–117Long-term studies conducted in the South Atlantic Ocean have shown seasonal sensitivities of phytoplankton to solar UV radiation
Pre- and post-bloom phytoplankton assemblages are mainly composed of small-sized cells that are sensitive to and strongly inhibited by solar UV-B.118 During the bloom in winter and early spring, microplankton diatoms, which are likewise sensitive to solar UV-B, dominate, but due to low solar irradiances, inhibition of photosynthesis and DNA damage are low. In these Patagonian waters, the exposure to detrimental radiation is mainly governed by the nutrient status and high wind speeds that affect the depth of the upper mixed layer and, by this, the mean irradiance affecting phytoplankton. Vertical circulation of the phytoplankton within the mixed layer reduces the exposure of solar UV radiation to individual organisms.Recent investigations have revealed mechanisms for UV-B stress induction and avoidance by aquatic producers and consumers
In addition to direct DNA damage by solar UV-B,119–121 generation of reactive oxygen species (ROS) is a major factor in induced damage and mutation by UV-B.122 Significant advances in understanding the molecular mechanisms have been achieved by identifying genes responsible for UV induction of damage and repair mechanisms,123 as well as enzymes involved in ROS detoxification and defense mechanism induction.124Results of modeling, evaluation of paleorecords, and studies using microcosms and field research all suggest a complex influence of UV-B radiation at the ecosystem level
Paleorecords in the circumpolar Arctic reveal widespread changes in algal and invertebrate communities that are driven by climate warming.125 Predicted increases in warming and precipitation for polar regions will influence freshwater and oceanic systems. Shifts in temperature and likely increased input of dissolved organic carbon, and consequent changes in the penetration of UV radiation, have an impact on Arctic lakes and ponds.126 Modelling results are consistent with paleorecords. This suggests that global ocean productivity is sensitive to changes in whole ocean circulation127 and to large-scale iceberg discharges from Northern Hemisphere ice sheets as during the last ice age.128 The underlying processes linking these events with subantarctic productivity remain unclear but underpin the complexity of these extreme climate perturbations and their consequent influence of enhanced solar UV-B on aquatic systems.Biogeochemical cycles
UV-B accelerates the production of CO2 from organic matter that runs off from the land into freshwaters and the ocean
Global warming and precipitation changes can alter the transport of dissolved organic matter (DOM) from terrestrial to freshwater and marine systems (Fig. 3). Increased UV-B radiation can enhance CO2 production from DOM via direct and iron-catalyzed DOM photo-transformations.129–131 The net result could be a UV-mediated positive feedback to CO2 accumulation in the atmosphere.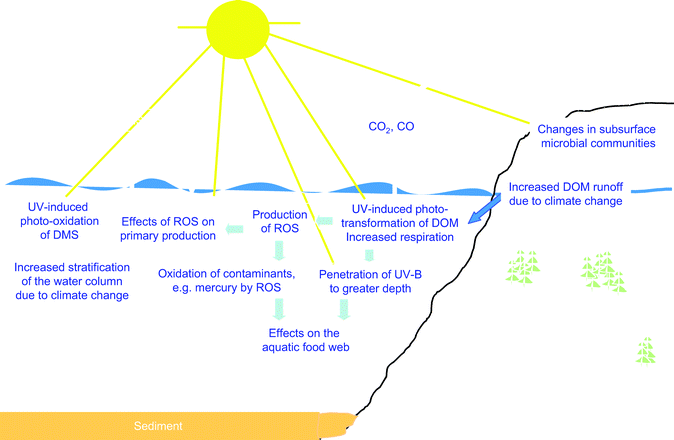 | ||
| Fig. 3 Diagrammatic representation of the effects of increased UV-radiation and climate change on biogeochemical cycles. | ||
Climate change causes increased stratification of lakes and the ocean, which intensifies UV effects on biogeochemistry in the surface layer
This important effect is illustrated by the extensive increase in transparency of the water to UV-B in the upper layer of stratified aquatic environments.132 Warming affects the quality of the DOM produced by microbial decomposition of seagrass, rendering DOM more susceptible to UV-induced degradation. These effects of climate change will amplify UV impacts on biogeochemical cycles in the upper part of aquatic systems, thus partially offsetting the beneficial effects of an ozone recovery.The toxicity of metals depends on their biological availability, which can be enhanced by reactions involving UV-B
For example, UV-induced oxidation of mercury has been demonstrated.133,134 This process increases the concentration of methylmercury,135 which bioaccumulates in the aquatic and terrestrial food chain.136UV radiation drives photoreactions that play an important role in marine sulfur cycling and thus in the production of atmospheric aerosols
Aerosols that influence atmospheric radiation and climate are affected by the UV radiation that penetrates into the ocean. Oceanic emissions of dimethyl sulfide (DMS) produce aerosols. Photolysis is an important transformation process for DMS in the upper ocean and the UV component of solar radiation induces this process.137 Marine photoproduction of carbonyl sulfide also is induced by solar UV-B that interacts with chromophoric DOM and dissolved organic sulfur in the upper ocean.138Climate change and UV-B influence the budget of halogen-containing compounds that alter ozone chemistry in the atmosphere
Generation of halogen atoms in atmospheric aerosols can be driven by processes involving UV radiation in the marine boundary layer.139,140 Emissions of halomethanes related to climate change may be interacting with the flux of UV-B to the Earth's surface to modulate trends in atmospheric ozone concentrations. For example, increased temperatures strongly influence emissions of methyl bromide and methyl iodide from rice; an increase of ∼10% was observed with warming of 1 °C.141 In contrast, photoreactions involving UV-B can degrade brominated and iodinated methanes in water.142,143UV-B increases the oxidative activity of aquatic environments, thereby affecting biogeochemical cycles and the fate of contaminants
Reactive oxygen species (ROS), important in the processing of natural and anthropogenic compounds, are produced by UV-induced photoreactions of organic matter,130,131,144 inorganic nitrogen and/or iron. Reactions involving ROS oxidize aquatic contaminants such as pharmaceuticals,145–147 certain herbicides148 and commercial dyes149 as well as naturally-occurring organic substances such as lignin150 and DOM.131,151 ROS can affect biogeochemical cycles by damaging aquatic microbiota and also by producing toxic species such as methylmercury.Carbon cycling in terrestrial systems can be affected by vegetation exposure to UV-B leading to alterations in subsurface microbial communities in the rhizosphere (soil around root systems)
Microbial communities in the rhizosphere play a key role in controlling plant nutrient supply. UV-B effects on plant root exudation and/or changes in litter quality were likely responsible for the alterations.94,152,153 These changes may modify utilization of different carbon substrates by the microbial communities.Air quality
Tropospheric ozone is influenced by UV-B radiation as well as by local stagnant high pressure systems and pollutant concentrations
A global chemical transport model study and analyses of measured total ozone and lower tropospheric ozone at three selected stations in Samoa, Hawaii, and Germany between the 1970s and 1990s agreed in terms of the dependency of lower tropospheric ozone on stratospheric ozone change.154 These studies showed that, in unpolluted air (marine locations—Samoa and Mauna Loa, Hawaii), a decrease in stratospheric ozone led to a decrease in ozone at ground level. However, in a more polluted continental site (Hohenpeissenberg, Germany), a decrease in stratospheric ozone led to an increase in ground level ozone. It was concluded that the sensitivity of lower tropospheric ozone to reduced total ozone column amounts was strongly linked to the large-scale weather situation (stagnant high pressure systems) as well as local emissions of pollutants.154 Thus, these factors must be considered in predicting effects of ozone depletion on lower tropospheric ozone concentrations.The changes in tropospheric hydroxyl radical (OH) caused by UV-B are now much better quantified
OH is one of the major oxidizing agents in the atmosphere, destroying about 3.7 petagrams (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 million tonnes) of trace gases each year, including many gases involved in ozone depletion, the greenhouse effect, and urban air pollution. The anomalously low global concentrations of OH from 1997–1999 may be explained by massive forest fires in Indonesia, Russia, and North America at these times.155 The sources and sinks of methyl chloroform, the primary molecule used to detect OH changes, are now better characterized,156,157 giving greater certainty to the analysis. Further, a second estimation method using carbon monoxide containing radiocarbon (14CO) agrees with the analysis based on methyl chloroform. The global averaged OH is observed to change on short time scales (months–years) but not in the longer term.158 Thus, the estimate for the long term trend in the change in OH (1979/1980–2003/2004) is small, and could be explained by changes in tropospheric composition rather than by UV-B radiation.
700 million tonnes) of trace gases each year, including many gases involved in ozone depletion, the greenhouse effect, and urban air pollution. The anomalously low global concentrations of OH from 1997–1999 may be explained by massive forest fires in Indonesia, Russia, and North America at these times.155 The sources and sinks of methyl chloroform, the primary molecule used to detect OH changes, are now better characterized,156,157 giving greater certainty to the analysis. Further, a second estimation method using carbon monoxide containing radiocarbon (14CO) agrees with the analysis based on methyl chloroform. The global averaged OH is observed to change on short time scales (months–years) but not in the longer term.158 Thus, the estimate for the long term trend in the change in OH (1979/1980–2003/2004) is small, and could be explained by changes in tropospheric composition rather than by UV-B radiation.Perfluoropolyethers, substances proposed as chloro-hydrofluorocarbon (CHFC) substitutes that have very large global warming potential show great stability to chemical degradation in the atmosphere
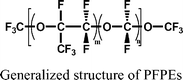
Perfluoropolyethers (PFPEs) are commonly used industrial heat transfer fluids that may be released to the atmosphere. In smog chamber studies on a PFPE, found in the commercial mixture of the heat-transfer fluid, Galden HT70®, reactivity with OH radical was very small (<2.28 × 10−16cm3 molecule−1 s−1) and that with Cl radical 10 times less.159 The minimum atmospheric lifetime was estimated to be about 1000 years. Over a 100 year time-frame, the global warming potential (GWP) of the PFPE was calculated to be 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (compared to the GWP of carbon dioxide of one), a value exceeded by only a few hydrofluoro ethers. These substances have been suggested as substitutes for CHFCs160 but they are very persistent and may be important contributors to global warming. They should be considered for further evaluation.
400 (compared to the GWP of carbon dioxide of one), a value exceeded by only a few hydrofluoro ethers. These substances have been suggested as substitutes for CHFCs160 but they are very persistent and may be important contributors to global warming. They should be considered for further evaluation.Although iodine-containing compounds have not been considered to be significant ozone-depleting substances (ODS), a recent assessment suggests that they may be important in ozone depletion in the polar boundary layer in synergy with other ODSs
It has been suggested that iodine-containing compounds are minor contributors to ozone depletion (<1% reduction of stratospheric ozone).161 However, recent modeling studies have suggested that iodine and iodine-containing substances from natural sources, such as the ocean, may increase ozone depletion in polar regions during spring.162 Measurements of IO- and I-containing precursor molecules as well as those of the important trace gasses, O3, CH2O, BrO, and Br-containing precursors may allow a realistic accounting to be made of the actual pathways that lead to the observed ozone depletion. Given the uncertainty of the fate of iodine in the stratosphere,161 these results may also be relevant for stratospheric ozone depletion and measurements of these substances should be considered in the future.Materials
Photodegradation in plastic-wood composite materials by solar UV radiation increases with their lignin content
Composites of plastics and wood, especially those with polyethylene and polypropylene as the plastic component, are being increasingly used outdoors.163 In these materials, the lignin chromophores in the wood powder were found to accelerate the photodamage to the plastics component.163 The acceleration increases with the fraction of wood in the composite. Conventional UV stabilizers can control the photodegradation of wood to a limited extent. For instance, UV absorbers used in mechanical pulps (newsprint paper) were found to be effective in significantly reducing the UV-induced loss in brightness of newsprint paper.164The role of additives in modifying the rates of photodegradation in common polymers is now better understood
Additives such as flame retardants,165 recycled plastics,166 and certain copolymers,167 are routinely used in plastics formulations. The mechanism by which each of these enhances the photodegradation of plastics has been better elucidated in recent findings. The use of certain UV stabilizers (sterically hindered-amine) along with bromine-containing flame retardants in the same formulation was shown to reduce stabilizer effectiveness and also promote photodegradation of the polypropylene plastics matrix.168 This new information can help in selecting additives for improved UV stability in common plastics formulations.Fillers generally retard the photodegradation of plastics. The newer nano-scale titanium dioxide filler, for instance, was shown to be particularly effective in stabilizing model epoxy polymers exposed to UV radiation.169
Recent studies confirm the synergistic interaction of solar UV and higher ambient temperatures acting together to cause increased photodamage to plastics
Polycarbonates undergo discoloration, weakening and changes in materials chemistry on exposure to solar UV radiation. This reduces both the transparency and strength of the glazing material. At higher temperatures (45 °C and 60 °C) an increase in such damage, by as much as 2–3 times compared to that at 25 °C, was obtained. The finding confirms synergistic effects of UV and temperature acting together causing increased photodamage to plastics.170New techniques allow more accurate modelling of photodamage at different depths in plastics exposed to UV radiation
Recent improvements in depth profiling techniques,171 especially those based on confocal microscopy, allow better quantification of the photodamage at different depths below the irradiated surface. The improved models allow for better design of plastic products as well as new stabilizer systems intended for materials destined for routine outdoor use.References
- G. C. Reinsel, A. J. Miller, E. C. Weatherhead, L. E. Flynn, R. M. Nagatani, G. C. Tiao and D. J. Wuebbles, Trend analysis of total ozone data for turnaround and dynamical contributions, J. Geophys. Res., 2005, 110, D16306 CrossRef.
- P. Hadjinicolaou and J. Pyle, The impact of Arctic ozone depletion on northern middle latitudes: Interannual variability and dynamical control, J. Atmos. Chem., 2004, 47, 25–43 CrossRef CAS.
- P. Hadjinicolaou, J. A. Pyle and N. R. P. Harris, The recent turnaround in stratospheric ozone over northern middle latitudes: A dynamical modeling perspective, Geophys. Res. Lett., 2005, 32, L12821 CrossRef.
- M. Rex, R. J. Salawitch, P. von der Gathen, N. R. P. Harris, M. P. Chipperfield and B. Naujokat, Arctic ozone loss and climate change, Geophys. Res. Lett., 2004, 31, L04116 CrossRef.
- B. Weatherhead, A. Tanskanen and A. Stevermer, Atmospheric Ozone and UV radiation, in Arctic Climate Impact Assessment, ed. C. Symon and B. Heal, Cambridge University Press, New York, USA, 2005, pp. 151–185 Search PubMed.
- B. M. Knudsen, N. R. P. Harris, S. B. Andersen, B. Christiansen, N. Larsen, M. Rex and B. Naujokat, Extrapolating future Arctic ozone losses, Atmos. Chem. Phys., 2004, 4, 1849–1856 CAS.
- G. Zeng and J. A. Pyle, Influence of El Nino Southern Oscillation on stratosphere/troposphere exchange and the global tropospheric ozone budget, Geophys. Res. Lett., 2005, 32, L01814 CrossRef.
- P. E. Huck, A. J. McDonald, G. E. Bodeker and H. Struthers, Inter-annual variability in Antarctic ozone depletion controlled by planetary waves and polar temperature, Geophys. Res. Lett., 2005, 32, L13819 CrossRef.
- D. Rind, J. Perlwitz and P. Lonergan, AO/NAO response to climate change: 1. Respective influences of stratospheric and tropospheric climate changes, J. Geophys. Res., 2005, 110, D12107 CrossRef.
- D. Rind, J. Perlwitz, P. Lonergan and J. Lerner, AO/NAO response to climate change: 2. Relative importance of low- and high-latitude temperature changes, J. Geophys. Res., 2005, 110, D12108 CrossRef.
- S. H. E. Hare, L. J. Gray, W. A. Lahoz, A. O'Neill and L. Steenman-Clark, Can stratospheric temperature trends be attributed to ozone depletion?, J. Geophys. Res., 2004, 109, D05111 CrossRef.
- U. Langematz, J. L. Grenfell, K. Matthes, P. Mieth, M. Kunze, B. Steil and C. Brühl, Chemical effects in 11-year solar cycle simulations with the Freie Universität Berlin Climate Middle Atmosphere Model with online chemistry (FUB-CMAM-CHEM), Geophys. Res. Lett., 2005, 32, L13803 CrossRef.
- Y. Kuroda and K. Kodera, Solar cycle modulation of the Southern Annular Mode, Geophys. Res. Lett., 2005, 32, L13802 CrossRef.
- C. E. Randall, V. L. Harvey, G. L. Manney, Y. Orsolini, M. Codrescu, C. Sioris, S. Brohede, C. S. Haley, L. L. Gordley, J. M. Zawodny and J. M. Russell, III, Stratospheric effects of energetic particle precipitation in 2003–2004, Geophys. Res. Lett., 2005, 32, L05802 CrossRef.
- K. Tourpali, C. J. E. Schuurmans, R. van Dorland, B. Steil, C. Bruhl and E. Manzini, Solar cycle modulation of the Arctic Oscillation in a chemistry-climate model, Geophys. Res. Lett., 2005, 32, L17803 CrossRef.
- G. Rohen, C. von Savigny, M. Sinnhuber, E. J. Llewellyn, J. W. Kaiser, C. H. Jackman, M.-B. Kallenrode, J. Schroter, K.-U. Eichmann, H. Bovensmann and J. P. Burrows, Ozone depletion during the solar proton events of October/November 2003 as seen by SCIAMACHY, J. Geophys. Res., 2005, 110, A09S39 CrossRef.
- S. K. Solanki, I. G. Usoskin, B. Kromer, M. Schüssler and J. Beer, Solanki et al. reply to: R. Muscheler et al. doi:10.1038/nature04045 (2005), Nature, 2005, 436, E4–E5 CrossRef CAS.
- S. K. Solanki, I. G. Usoskin, B. Kromer, M. Schüssler and J. Beer, Unusual activity of the Sun during recent decades compared to the previous 11,000 years, Nature, 2004, 431, 1084–1087 CrossRef CAS.
- R. Muscheler, F. Joos, S. A. Müller and I. Snowball, How unusual is today's solar activity? Arising from: S. K. Solanki, I. G. Usoskin, B. Kromer, M. Schüssler and J. Beer, Nature, 2004, 431, 1084–1087, Nature, 2005, 436, E3–E4 CrossRef CAS.
- A. F. Bais, A. Kazantzidis, S. Kazadzis, D. S. Balis, C. S. Zerefos and C. Meleti, Deriving an effective aerosol single scattering albedo from spectral surface UV irradiance measurements, Atmos. Environ., 2005, 39, 1093–1102 CrossRef CAS.
- R. W. Bergstrom, P. Pilewskie, J. Pommier, M. Rabbette, P. B. Russell, B. Schmid, J. Redemann, A. Higurashi, T. Nakajima and P. K. Quinn, Spectral absorption of solar radiation by aerosols during ACE-Asia, J. Geophys. Res., 2004, 109, D19S15 CrossRef.
- C. D. Goering, T. S. L'Ecuyer, G. L. Stephens, J. R. Slusser, G. Scott, J. Davis, J. C. Barnard and S. Madronich, Simultaneous retrievals of column ozone and aerosol optical properties from direct and diffuse solar irradiance measurements, J. Geophys. Res. Atmos., 2005, 110, D05204 CrossRef.
- N. A. Krotkov, P. K. Bhartia, J. R. Herman, J. R. Slusser, G. R. Scott, G. J. Labow, A. P. Vasilkov, T. Eck, O. Doubovik and B. N. Holben, Aerosol ultraviolet absorption experiment (2002 to 2004), part 2: Absorption optical thickness, refractive index, and single scattering albedo, Optic. Eng., 2005, 44, 18–34 Search PubMed.
- N. A. Krotkov, P. K. Bhartia, J. R. Herman, J. R. Slusser, G. J. Labow, G. R. Scott, G. T. Janson, T. Eck and B. N. Holben, Aerosol ultraviolet absorption experiment (2002 to 2004), part 1: Ultraviolet multifilter rotating shadowband radiometer calibration and intercomparison with CIMEL sunphotometers, Optic. Eng., 2005, 44, 1–17 Search PubMed.
- D. Meloni, A. di Sarra, J. R. Herman, F. Monteleone and S. Piacentino, Comparison of ground-based and Total Ozone Mapping Spectrometer erythemal UV doses at the island of Lampedusa in the period 1998–2003: Role of tropospheric aerosols, J. Geophys. Res. Atmos., 2005, 110, D01202 CrossRef.
- A. Arola, S. Kazadzis, N. Krotkov, A. F. Bais, J. Gröbner and J. R. Herman, Assessment of TOMS UV bias due to absorbing aerosols, J. Geophys. Res., 2005, in press Search PubMed.
- A. Lindfors and L. Vuilleumier, Erythemal UV at Davos (Switzerland), 1926–2003, estimated using total ozone, sunshine duration, and snow depth, J. Geophys. Res., 2005, 110, D02104 CrossRef.
- P. N. den Outer, H. Slaper and R. B. Tax, UV radiation in the Netherlands: Assessing long-term variability and trends in relation to ozone and clouds, J. Geophys. Res. Atmos., 2005, 110, D02203 CrossRef.
- A. V. Lindfors, A. Arola, J. Kaurola, P. Taalas and T. Svenoe, Long-term erythemal UV doses at Sodankyla estimated using total ozone, sunshine duration, and snow depth, J. Geophys. Res. Atmos., 2003, 108, 4518 CrossRef.
- O. Engelsen, G. H. Hansen and T. Svenoe, Long-term (1936–2003) ultraviolet and photosynthetically active radiation doses at a north Norwegian location in spring on the basis of total ozone and cloud cover, Geophys. Res. Lett., 2004, 31, L12103 CrossRef.
- K. Garane, F. Bais Alkiviadis, K. Tourpali, C. Meleti, C. S. Zerefos and S. Kazadzis, Variability of spectral UV irradiance at Thessaloniki, Greece, from 15 years measurements, in Ultraviolet Ground- and Space-based Measurements, Models, and Effects V, ed. G. Bernhard, J. R. Slusser, J. R. Herman and W. Gao, SPIE, San Diego, USA, 2005, vol. 5886 Search PubMed.
- N. Y. Chubarova, Y. I. Nezval, J. Verdebout, N. Krotkov and J. Herman, Long-term UV irradiance changes over Moscow and comparisons with UV estimates from TOMS and METEOSAT, in Ultraviolet Ground- and Space-based Measurements, Models, and Effects V, ed. G. Bernhard, J. R. Slusser, J. R. Herman and W. Gao, SPIE, San Diego, USA, 2005, vol. 5886, pp. 63–73 Search PubMed.
- M. Wild, H. Gilgen, A. Roesch, A. Ohmura, C. N. Long, E. G. Dutton, B. Forgan, A. Kallis, V. Russak and A. Tsvetkov, From dimming to brightening: Decadal changes in solar radiation at Earth's surface, Science, 2005, 308, 847–850 CrossRef CAS.
- G. Bernhard, C. R. Booth and J. C. Ehramjian, Version 2 data of the National Science Foundation's Ultraviolet Radiation Monitoring Network: South Pole, J. Geophys. Res. Atmos., 2004, 109, D21207 CrossRef.
- R. L. McKenzie, G. E. Bodeker, P. V. Johnston and M. Kotkamp, Long term changes in summertime UV radiation in New Zealand in response to ozone change, in Proceedings of the XX Quadrennial Ozone Symposium, ed. C. S. Zerefos, International Ozone Commission, Kos, Greece, 2004, vol. 1, pp. 257–258 Search PubMed.
- R. E. M. Griffin, Detection and measurement of total ozone from stellar spectra: Paper 2. Historic data from 1935–42, Atmos. Chem. Phys. Disc., 2005, 5, 10925–10946.
- R. E. M. Griffin, The detection and measurement of Telluric ozone from stellar spectra, Publ. Astron. Soc. Pac., 2005, 117, 885–894 CrossRef.
- R. E. M. Griffin, V. Fioletov and J. C. McConnel, Measurements of historical total ozone from the Chalogne-DIvan stellar spectrum programme: Paper 1. A reanalysis of the 1953–72 data and a comparison with the simultaneous Dobson Arosa measurements, J. Geophys. Res., 2005, in press Search PubMed.
- S. Huttunen, N. M. Lappalainen and J. Turunen, UV-absorbing compounds in subarctic herbarium bryophytes, Environ. Pollut., 2005, 133, 303–314 CrossRef CAS.
- S. Huttunen, T. Taipale, N. M. Lappalainen, E. Kubin, K. Lakkala and J. Kaurola, Environmental specimen bank samples of Pleurozium schreberi and Hylocomium splendens as indicators of the radiation environment at the surface, Environ. Pollut., 2005, 133, 315–326 CrossRef CAS.
- E. Verleyen, D. A. Hodgson, K. Sabbe and W. Vyverman, Late Holocene changes in ultraviolet raediation pentration recorded in an East Antarctic lake, J. Paleolimnol., 2005, 34, 191–202 Search PubMed.
- D. A. Hodgson, E. Verleyen, K. Sabbe, A. H. Squier, B. J. Keely, M. J. Leng, K. M. Saunders and W. Vyverman, Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core, Quat. Res., 2005, 64, 83–99 CrossRef.
- V. V. Zuev and S. L. Bondarenko, Reconstruction of multicentennial behavior of the total ozone content based on dendrochronological data, Dokl. Earth Sci., 2003, 393, 1120–1123 Search PubMed.
- K. R. Briffa, T. J. Osborn and F. H. Schweingruber, Large-scale temperature inferences from tree rings: a review, Glob. Planet Change, 2004, 40, 11–26 CrossRef.
- M. Bill, M. E. Conrad and A. H. Goldstein, Stable carbon isotope composition of atmospheric methyl bromide, J. Geophys. Res., 2004, 31, L04109 CrossRef.
- M. L. Cox, G. A. Sturrock, P. J. Fraser, S. T. Siems and P. B. Krummel, Pages: Identification of regional sources of methyl bromide and methyl iodide from AGAGE observations at Cape Grim, Tasmania, J. Atmos. Chem., 2005, 50, 59–77 CrossRef CAS.
- P. G. Simmonds, R. G. Derwent, A. J. Manning, P. J. Fraser, P. B. Krummel, S. O'Doherty, R. G. Prinn, D. M. Cunnold, B. R. Miller, H. J. Wang, D. B. Ryall, L. W. Porter, R. F. Weiss and P. K. Salameh, AGAGE observations of methyl bromide and methyl chloride at Mace Head, Ireland, and Cape Grim, Tasmania, 1998–2001, J. Atmos. Chem., 2005, 47, 243–269.
- K. R. Redeker, K. K. Treseder and M. F. Allen, Ectomycorrhizal fungi: A new source of atmospheric methyl halides?, Glob. Change Biol., 2004, 10, 1009–1016 Search PubMed.
- F. Keppler, R. M. Kalin, D. B. Harper, W. C. McRoberts and J. T. G. Hamilton, Carbon isotope anomaly in the major plant C1 pool and its global biogeochemical implications, Biogeoscience, 2004, 1, 123–131 Search PubMed.
- K. Kourtidis, Transfer of organic Br and Cl from the biosphere to the atmosphere during the Cretaceous/Tertiary Impact: Implications for the stratospheric ozone layer, Atmos. Chem. Phys. Disc., 2004, 4, 6769–6787.
- R. v. Glasow, R. v. Kuhlmann, M. G. Lawrence, U. Platt and P. J. Crutzen, Impact of reactive bromine chemistry in the troposphere, Atmos. Chem. Phys., 2004, 4, 2481–2497.
- O. W. Wingenter, K. B. Haase, P. Strutton, G. Friederich, S. Meinardi, D. R. Blake and F. S. Rowland, Changing concentrations of CO, CH4, C5H8, CH3Br, CH3I, and dimethyl sulfide during the southern ocean iron enrichment experiments, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 8537–8541 CrossRef CAS.
- K. R. Redeker, S. L. Manley, L. Brothers, K. McDuffee, M. Walser and R. J. Cicerone, Seasonal mass balance of halogens in simulated rice paddies, Geophys. Res. Lett., 2004, 31, L11504 CrossRef.
- E. De Vries, M. Louwman, M. Bastiaens, F. de Gruijl and J. Coebergh, Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973, J. Invest. Dermatol., 2004, 123, 634–638 CrossRef CAS.
- L. J. Christenson, T. A. Borrowman, C. M. Vachon, M. M. Tollefson, C. C. Otley, A. L. Weaver and R. K. Roenigk, Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years, J. Am. Med. Assoc., 2005, 294, 681–690 Search PubMed.
- B. L. Diffey, The future incidence of cutaneous melanoma within the UK, Br. J. Dermatol., 2004, 151, 868–872 CrossRef CAS.
- I. McDermid, Cancer incidence projections Australia 2002 to 2011, Australian Institute of Health and Welfare, Australasian Association of Cancer Registries and the National Cancer Strategies Group, Report No. 30, Canbera, Australia, August 2005, p. 166.
- E. De Vries, L. V. van de Poll-Franse, W. J. Louwman, F. R. de Gruijl and J. W. W. Coebergh, Prediction of skin cancer incidence in the Netherlands up to 2015, Br. J. Dermatol., 2005, 152, 481–488 CrossRef CAS.
- E. C. De Fabo, F. P. Noonan, T. Fears and G. Merlino, Ultraviolet B but not ultraviolet A initiates melanoma, Cancer Res., 2004, 64, 6372–6376 CAS.
- D. H. Sliney, Exposure geometry and spectral environment determine photobiological effects on the human eye, Photochem. Photobiol., 2005, 81, 483–489 CrossRef CAS.
- A. V. Parisi and N. Downs, Variation of the enhanced biologically damaging solar UV due to clouds, Photochem. Photobiol. Sci., 2004, 3, 643–647 RSC.
- A. V. Parisi and N. Downs, Cloud cover and horizontal plane eye damaging solar UV exposures, Int. J. Biometeorol., 2004, 49, 130–136 Search PubMed.
- N. Di Girolamo, M. Coroneo and D. Wakefield, Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation, Am. J. Pathol., 2005, 167, 489–503 Search PubMed.
- J. S. Paula, F. Thorn and A. A. Cruz, Prevalence of pterygium and cataract in indigenous populations of the Brazilian Amazon rain forest, Eye, 2005 DOI:10.1038/sj.eye.6701917.
- M. Al Bdour and M. M. Al Latayfeh, Risk factors for pterygium in an adult Jordanian population, Acta Opthalmol. Scand., 2004, 82, 64–67 Search PubMed.
- H. C. Kau, C. C. Tsai, W. M. Hsu, J. H. Liu and Y. H. Wei, Genetic polymorphism of hOGG1 and risk of pterygium in Chinese, Eye, 2004, 18, 635–639 Search PubMed.
- D. Reisman, J. W. McFadden and G. Lu, Loss of heterozygosity and p53 expression in pterygium, Cancer Lett., 2004, 206, 77–83 CrossRef CAS.
- L. Wang, W. Dai and L. Lu, Ultraviolet irradiation-induced K(+) channel activity involving p53 activation in corneal epithelial cells, Oncogene, 2005, 24, 3020–3027 CrossRef CAS.
- K. E. Smedby, H. Hjalgrim, M. Melbye, A. Torrang, K. Rostgaard, L. Munksgaard, J. Adami, M. Hansen, A. Porwit-MacDonald, B. A. Jensen, G. Roos, B. B. Pederson, C. Sundstrom, B. Glimelius and H. O. Adami, Ultraviolet radiation exposure and risk of malignant lymphoma, J. Nat. Cancer Inst., 2005, 97, 199–209 Search PubMed.
- A. M. Hughes, B. K. Armstrong, C. M. Vajdic, J. Turner, A. E. Grulich, L. Fritschi, S. Milliken, J. Kaldor, G. Benke and A. Kricker, Sun exposure may protect against non-Hodgkin lymphoma: a case-control study, Int. J. Cancer, 2004, 112, 865–871 CrossRef CAS.
- E. M. John, G. G. Schwartz, J. Koo, D. Van Den Berg and S. A. Ingles, Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer, Cancer Res., 2005, 65, 5470–5470 CrossRef CAS.
- M. Berwick, B. K. Armstrong, L. Ben-Porat, J. Fine, A. Kricker, C. Eberle and R. Barnhill, Sun exposure and mortality from melanoma, J. Nat. Cancer Inst., 2005, 97, 195–199 Search PubMed.
- E. A. Platz, M. F. Leitzmann, B. W. Hollis, W. C. Willett and E. Giovannucci, Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer, Cancer Causes Control, 2004, 152, 55–65.
- D. Bodiwala, C. J. Luscombe, M. E. French, S. Liu, M. F. Saxby, P. W. Jones, A. A. Fryer and R. C. Strange, Polymorphoisms in the vitamin D receptor gene, ultraviolet radiation, and susceptibility to prostate cancer, Environ. Mol. Mutagen., 2004, 43, 121–127 CrossRef CAS.
- M. Berwick and D. Kesler, Ultraviolet radiation exposure, vitamin D, and cancer, Photochem. Photobiol., 2005, in press Search PubMed.
- B. Dawson-Hughes, R. P. Heaney, M. F. Holick, P. Lips, P. J. Meunier and R. Vieth, Estimates of optimal vitamin D status, Osteoporosis Int., 2005, 16, 713–716 Search PubMed.
- O. Gillie, Sunlight robbery: Health benefits of sunlight are denied by current public health policy in the UK, Health Research Forum Occasional Reports 1, 1–42, www.healthresearchforum.org.uk/reports/sunlightrobbery.pdf, accessed September 20, 2005.
- W. B. Grant and M. F. Holick, Benefits and requirements of vitamin D for optimal health: A review, Alt. Med. Rev., 2005, 10, 94–111 Search PubMed.
- T. J. Green, C. M. Skeaff, J. E. Rockell, J. R. Taylor and S. J. Whiting, Serum 25-hydroxyvitamin D status New Zealand children, Asia Pac. J. Clin. Nutr., 2004, 13(Suppl), S46.
- H. Gronberg, Prostate cancer epidemiology, Lancet, 2003, 361, 859–864 CrossRef.
- F. Furukawa and T. Yoshimasu, Animal models of spontaneous and drug-induced cutaneous lupus erythematosus, Autoimmunol. Rev., 2005, 4, 345–350 Search PubMed.
- F. J. Burns, A. N. Uddin, F. Wu, A. Nadas and T. G. Rossman, Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study, Environ. Health Perspect., 2004, 112, 599–603 CAS.
- J. Parker, S. L. Klein, M. K. McClintock, W. L. Morison, X. Ye, C. J. Conti, N. Peterson, C. H. Nousari and F. A. Tausk, Chronic stress accelerates ultraviolet-induced cutaneous carcinogenesis, J. Am. Acad. Dermatol., 2004, 519, 19–22.
- T. Koda, T. Umezu, R. Kamata, K. Morohoshi, T. Ohta and M. Morita, Uterotrophic effects of benzophenone derivatives and a p-hydroxybenzoate used in ultraviolet screens, Environ. Res., 2005, 98, 40–45 CrossRef CAS.
- L. J. Mills and C. Chichester, Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations?, Sci. Total Environ., 2005, 134, 31–34.
- K. Morohoshi, H. Yamamoto, R. Kamata, F. Shiraishi, T. Koda and M. Morita, Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays, Toxicol. in Vitro, 2005, 19, 457–469 CrossRef CAS.
- E. Gomez, A. Pillon, H. Fenet, D. Rosain, M. J. Duchesne, J. C. Nicolas, P. Balaguer and C. Casellas, Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV screens, and musks, J. Toxicol. Environ. Health A, 2005, 68, 239–251 Search PubMed.
- S. Durrer, K. Maerkel, M. Schlumpf and W. Lichtensteiger, Estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor, Endocrinology, 2005, 462, 130–139.
- T. Suzuki, S. Kitamura, R. Khota, K. Sugihara, N. Fujimoto and S. Ohta, Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens, Toxicol. Appl. Pharmacol., 2005, 203, 9–17 CrossRef CAS.
- A. Klann, G. Levy, I. Lutz, C. Muller, W. Kloas and J. P. Hildebrandt, Estrogen-like effects of ultraviolet screen 3-(4-methylbenzylidene)-camphor (Eusolex 6300) on cell proliferation and gene induction in mammalian and amphibian cells, Environ. Res., 2005, 97, 274–281 CrossRef CAS.
- R. H. Schreurs, E. Sonneveld, J. H. Jansen, W. Seinen and B. van der Burg, Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays, Toxicol. Sci., 2005, 83, 264–272 CAS.
- M. Schlumpf, P. Schmid, S. Durrer, M. Conscience, K. Maerkel, M. Henseler, M. Gruetter, I. Herzog, S. Reolon, R. Ceccatelli, O. Faass, E. Stutz, H. Jarry, W. Wuttke and W. Lichtensteiger, Endocrine activity and developmental toxicity of cosmetic UV filters-an update, Toxicology, 2004, 205, 113–122 CrossRef CAS.
- T. M. Robson, V. A. Pancotto, C. L. Ballaré, O. E. Sala, A. L. Scopel and M. M. Caldwell, Reduction of solar UV-B mediates changes in the Sphagnum capitulum microenvironment and the peatland microfungal community, Oecologia, 2004, 140, 480–490 Search PubMed.
- S. A. Robinson, J. D. Turnbull and C. E. Lovelock, Impact of changes in natural ultraviolet radiation on pigment composition, physiological and morphological characteristics of the Antarctic moss, Grimmia antarctici, Glob. Change Biol., 2005, 11, 476–489 Search PubMed.
- D. G. Milchunas, J. Y. King, A. R. Mosier, J. C. Moore, J. A. Morgan, M. H. Quirk and J. R. Slusser, UV radiation effects on plant growth and forage quality in a shortgrass steppe ecosystem, Photochem. Photobiol., 2004, 79, 404–410 CrossRef CAS.
- S. B. M. Chimphango, C. F. Musil and F. D. Dakora, Responses to ultraviolet-B radiation by purely symbiotic and NO3-fed nodulated tree and shrub legumes indigenous to southern Africa, Tree Physiol., 2004, 24, 181–192 Search PubMed.
- C. M. Correia, J. M. Moutinho Pereira, J. F. Countinho, L. O. Björn and J. M. G. Torres-Pereira, Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a Mediterranean field study, Europ. J. Agronom., 2005, 22, 337–347 Search PubMed.
- S. Koti, K. R. Reddy, V. R. Reddy, V. G. Kakani and D. Zhao, Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths, J. Exp. Bot., 2004, 56, 725–736 Search PubMed.
- D. Zhao, K. R. Reddy, V. G. Kakani, A. R. Mohammed, J. J. Read and W. Gao, Leaf and canopy photosynthetic characteristics of cotton (Gossypium hirsutum) under elevated CO2 concentration and UV-B radiation, J. Plant Physiol., 2004, 161, 581–590 CrossRef CAS.
- T. Teklemariam and T. J. Blake, Effects of UVB preconditioning on heat tolerance of cucumber (Cucumis sativus L.), Environ. Exp. Bot., 2003, 50, 169–182 CrossRef CAS.
- T. Teklemariam and T. J. Blake, Phenylalanine ammonia-lyase-induced freezing tolerance in jack pine (Pinus banksiana) seedlings treated with low, ambient levels of ultraviolet-B radiation, Physiol. Plant., 2004, 122, 244–253 CrossRef CAS.
- L. Chalker-Scott and J. D. Scott, Elevated ultraviolet-B radiation induces cross-protection to cold in leaves of Rhododendron under field conditions., Photochem. Photobiol., 2004, 79, 199–204 CrossRef CAS.
- J. E. Blum, P. Casati, V. Walbot and A. E. Stapleton, Split-plot microarray design allows sensitive detection of expression differences after ultraviolet radiation in the inbred parental lines of a key maize mapping population, Plant, Cell Environ., 2004, 27, 1374–1386 CrossRef CAS.
- P. Casati and V. Walbot, Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues, Genome Biol., 2004, 5, R16 Search PubMed.
- A. J. Richardson and D. S. Schoeman, Climate impact on plankton ecosystems in the northeast Atlantic, Science, 2004, 305, 1609–1612 CrossRef CAS.
- C. S. Cockell and C. Cordoba-Jabonero, Coupling of climate change and biotic UV exposure through changing snow-ice covers in terrestrial habitats, Photochem. Photobiol., 2004, 79, 26–31 CrossRef CAS.
- A. M. Piotrowski, S. L. Goldstein, S. R. Hemming and R. G. Fairbanks, Temporal relationships of carbon cycling and ocean circulation at glacial boundaries, Science, 2005, 307, 1933–1938 CrossRef CAS.
- E. J. MacFadyen, C. E. Williamson, G. Grad, M. Lowery, W. H. Jeffrey and D. L. Mitchell, Molecular response to climate change: temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria, Glob. Change Biol., 2004, 10, 408–416 Search PubMed.
- W. F. Vincent, M. Rautio and R. Pientiz, Climate control of biological UV exposure in polar and alpine aquatic ecosystems, in Arctic Environmental Change, ed. J. B. Orbaek, Springer, Berlin, 2005, in press Search PubMed.
- W. F. Vincent and C. Belzile, Biological UV exposure in the polar oceans: Arctic-Antarctic comparisons, in Antarctic Biology in a Global Context ed. A. H. L. Huiskes, W. W. C. Gieskes, J. Rozema, R. M. L. Schorno, S. M. van der Vies and W. J. Wolff, Backhuys Publ, Leiden, NL, 2003, pp. 176–181 Search PubMed.
- A. J. Cook, A. J. Fox, D. G. Vaughan and J. G. Ferringno, Retreating glacier fronts on the Antarctic peninsula over the past half-century, Science, 2005, 308, 541–544 CrossRef CAS.
- J. E. Frederick and Y. Liao, Photosynthetically active sunlight at high southern latitudes, Photochem. Photobiol., 2005, 81, 603–608 CrossRef CAS.
- A. D. Persaud and C. E. Williamson, Ultraviolet and temperature effects on plankton rotifers and crustaceans in northern temperate lakes, Freshwater Biol., 2005, 50, 467–476 CrossRef.
- R. Przeslawski, A. R. Davis and K. Benkendorff, Synergistic effects associated with climate change and the development of rocky shore molluscs, Glob. Change Biol., 2005, 11, 515–522 Search PubMed.
- G. T. Ankley, S. J. Degitz, S. A. Diamond and J. E. Tietge, Assessment of environmental stressors potentially responsible for malformations in North American anuran amphibians, Ecotoxicol. Environ. Safety, 2004, 58, 7–16 CrossRef CAS.
- J. M. Kiesecker, L. K. Belden, K. Shea and M. J. Rubbo, Amphibian decline and emerging disease, Am. Sci., 2004, 92, 138–147 Search PubMed.
- W. J. Palen, C. E. Williamson, A. A. Clauser and D. E. Schindler, Impact of UV-B exposure on amphibian embryos: linking species physiology and oviposition behaviour, Proc. R. Soc. London, Ser. B, 2005, 272, 1227–1234 CrossRef.
- V. E. Villafañe, M. A. Marcoval and E. W. Helbling, Photosynthesis versus irradiance characteristics in phytoplankton assemblages off Patagonia (Argentina): Temporal variability and solar UVR effects, Mar. Ecol.: Prog. Ser., 2004, 284, 23–34 Search PubMed.
- J. Cadet, E. E. Sage and T. Douki, Ultraviolet radiation-mediated damage to cellular DNA, Mutat. Res., 2005, 571, 3–17 CrossRef CAS.
- A. Kumar, M. B. Tyagi and P. N. Jha, Evidences showing ultraviolet-B radiation-induced damage of DNA in cyanobacteria and its detection by PCR assay, Biochem. Biophys. Res. Commun., 2004, 318, 1025–1030 CrossRef CAS.
- D.-P. Häder and R. P. Sinha, Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact, Mutat. Res., 2005, 571, 221–233 CrossRef.
- G. P. Pfeifer, Y.-H. You and A. Besaratinia, Mutations induced by ultraviolet light, Mutat. Res., 2005, 71, 19–31 CrossRef.
- S. Braatsch and G. Klug, ORF90, a gene required for photoreactivation in Rhodobacter capsulatus SB 1103 encodes a cyclobutane pyrimidine dimer photolyase, Photosynth. Res., 2004, 79, 167–177 CrossRef CAS.
- S. Y. Yun and Y. N. Lee, Purification and some properties of superoxide dismutase from Deinococcus radiophilus, the UV-resistant bacterium, Extremophiles, 2004, 8, 237–242 Search PubMed.
- J. P. Smol, A. P. Wolfe, H. J. B. Birks, M. S. V. Douglas, V. J. Jones, A. Korhola, R. Pienitz, K. Rühland, S. Sorvari, D. Antoniades, S. J. Brooks, M.-A. Fallu, M. Hughes, B. E. Keatley, T. E. Laing, N. Michelutti, L. Nazarova, M. Nyman, A. M. Paterson, B. Perren, R. Quinlan, M. Rautio, É. Saulnier-Talbot, S. Siitonen, N. Solovieva and J. Weckström, Climate-driven regime shifts in the biological communities of arctic lakes, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 4397–4402 CrossRef CAS.
- D. O. Hessen, P. Blomqvist, G. Dahl-Hansen, S. Drakare and E. S. Lindstroem, Production and food web interactions of Arctic freshwater plankton and responses to increased DO, Arch. Hydrobiol., 2004, 159, 289–307 Search PubMed.
- A. Schmittner, Decline of the marine ecosystem caused by a reduction in the Atlantic overturning circulation, Nature, 2005, 434, 628–633 CrossRef CAS.
- J. P. Sachs and R. F. Anderson, Increased productivity in the subantarctic ocean during Heinrich events, Nature, 2005, 434, 1118–1121 CrossRef CAS.
- I. Obernosterer and R. Benner, Competition between biological and photochemical processes in the mineralization of dissolved organic carbon, Limnol. Oceanogr., 2004, 49, 117–124 CAS.
- H. X. Xie, O. C. Zafiriou, W. J. Cai, R. G. Zepp and Y. C. Wang, Photooxidation and its effects on the carboxyl content of dissolved organic matter in two coastal rivers in the Southeastern United States, Environ. Sci. Technol., 2004, 38, 4113–4119 CrossRef CAS.
- M. J. Pullin, S. Bertilsson, J. V. Goldstone and B. M. Voelker, Effects of sunlight and hydroxyl radical on dissolved organic matter: Bacterial growth efficiency and production of carboxylic acids and other substrates, Limnol. Oceanogr., 2004, 49, 2011–2022 CAS.
- R. Del Vecchio and N. V. Blough, Spatial and seasonal distribution of chromophoric dissolved organic matter and dissolved organic carbon in the Middle Atlantic Bight, Mar. Chem., 2004, 89, 169–187 CrossRef CAS.
- N. A. Hines and P. L. Brezonik, Mercury dynamics in a small Northern Minnesota lake: water to air exchange and photoreactions of mercury, Mar. Chem., 2004, 90, 137–149 CrossRef CAS.
- J. D. Lalonde, M. Amyot, J. Orvoine, F. M. M. Morel, J. C. Auclair and P. A. Ariya, Photoinduced oxidation of Hg0(aq) in the waters from the St. Lawrence estuary, Environ. Sci. Technol., 2004, 38, 508–514 CrossRef CAS.
- S. D. Siciliano, N. J. O'Driscoll, R. Tordon, J. Hill, S. Beauchamp and D. R. S. Lean, Abiotic production of methylmercury by solar radiation, Environ. Sci. Technol., 2005, 39, 1071–1077 CrossRef CAS.
- M. Kainz and A. Mazumder, Effect of algal and bacterial diet on methyl mercury concentrations in zooplankton, Environ. Sci. Technol., 2005, 39, 1666–1672 CrossRef CAS.
- C. D. Deal, D. J. Kieber, D. A. Toole, K. Stamnes, S. Jiang and N. Uzuka, Dimethylsulfide Photolysis Rates and Apparent Quantum Yields in Bering Sea Seawater, Cont. Shelf Res., 2005, 25, 1825–1835 CrossRef.
- G. A. Cutter, L. S. Cutter and K. C. Filippino, Sources and cycling of carbonyl sulfide in the Sargasso Sea, Limnol. Oceanogr., 2004, 49, 555–565 CAS.
- A. A. P. Pszenny, J. Moldanov, W. C. Keene, R. Sander, J. R. Maben, M. Martinez, P. J. Crutzen, D. Perner and R. G. Prinn, Halogen cycling and aerosol pH in the Hawaiian marine boundary layer, Atmos. Chem. Phys., 2004, 4, 147–168 CAS.
- R. Sander, W. C. Keene, A. A. P. Pszenny, R. Arimoto, G. P. Ayers, E. Baboukas, J. M. Cainey, P. J. Crutzen, R. A. Duce, G. Honninger, B. J. Huebert, W. Maenhaut, N. Mihalopoulos, V. C. Turekian and R. Van Dingenen, Inorganic bromine in the marine boundary layer: a critical review, Atmos. Chem. Phys., 2003, 3, 1301–1336 CAS.
- J. Lee-Taylor and K. R. Redeker, Reevaluation of global emissions from rice paddies of methyl iodide and other species, Geophys. Res. Lett., 2005, 32, L15801 CrossRef.
- Y. Du, X. G. Guan, W. M. Kwok, L. M. Chu and D. L. Phillips, Comparison of the dehalogenation of dihalomethanes (CH2XI, where X = Cl, Br, I) following ultraviolet photolysis in aqueous and NaCl saltwater environments, J. Phys. Chem. A, 2005, 109, 5872–5882 CrossRef CAS.
- X. G. Guan, Y. Du, Y. L. Li, W. M. Kwok and D. L. Phillips, Comparison of the dehalogenation of polyhalomethanes and production of strong acids in aqueous and salt (NaCl) water environments: Ultraviolet photolysis of CH2I2, J. Chem. Phys., 2004, 121, 8399–8409 CrossRef CAS.
- L. Meunier, H.-U. Laubscher, S. J. Hug and B. Sulzberger, Effects of size and origin of natural dissolved organic matter compounds on the redox cycling of iron in sunlit surface waters, Aquat. Sci., 2005, 67, in press Search PubMed.
- M. W. Lam, C. J. Young, R. A. Brain, D. J. Johnson, M. A. Hanson, C. J. Wilson, S. M. Richards, K. R. Solomon and S. A. Mabury, Aquatic persistence of eight pharmaceuticals in a microcosm study, Environ. Toxicol. Chem., 2004, 23, 1431–1440 CrossRef CAS.
- M. W. Lam and S. A. Mabury, Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters, Aquat. Sci., 2005, 67, 177–188 Search PubMed.
- A. L. Boreen, W. A. Arnold and K. McNeill, Triplet-sensitized photodegradation of sulfa drugs containing six-membered heterocyclic groups: Identification of an SO2 extrusion photoproduct, Environ. Sci. Technol., 2005, 39, 3630–3638 CrossRef CAS.
- P. L. Miller and Y. P. Chin, Indirect photolysis promoted by natural and engineered wetland water constituents: Processes leading to alachlor degradation, Environ. Sci. Technol., 2005, 39, 4454–4462 CrossRef CAS.
- Y. P. Chin, P. L. Miller, L. K. Zeng, K. Cawley and L. K. Weavers, Photosensitized degradation of bisphenol a by dissolved organic matter, Environ. Sci. Technol., 2004, 38, 5888–5894 CrossRef CAS.
- A. M. McNally, E. C. Moody and K. McNeill, Kinetics and mechanism of the sensitized photodegradation of lignin model compounds, Photochem. Photobiol. Sci., 2005, 4, 268–274 RSC.
- L. A. Molot, J. J. Hudson, P. J. Dillon and S. A. Miller, Effect of pH on photo-oxidation of dissolved organic carbon by hydroxyl radicals in a coloured, softwater stream, Aquat. Sci., 2005, 67, 189–195 Search PubMed.
- L. M. Avery, R. I. L. Smith and H. M. West, Response of rhizosphere microbial communities associated with Antarctic hairgrass (Deschampsia antarctica) to UV radiation, Polar Biol., 2003, 26, 525–529 Search PubMed.
- L. M. Avery, P. C. Thorpe, K. Thompson, N. D. Paul, J. P. Grime and H. M. West, Physical disturbance of an upland grassland influences the impact of elevated UV-B radiation on metabolic profiles of below-ground micro-organisms, Glob. Change Biol., 2004, 10, 1146–1154 Search PubMed.
- I. S. A. Isaksen, C. Zerefos, K. Kourtidis, C. Meleti, S. B. Dalsoren, J. K. Sundet, A. Grini, P. Zanis and D. Balis, Tropospheric ozone changes at unpolluted and semipolluted regions induced by stratospheric ozone changes, J. Geophys. Res. Atmos., 2005, 111, DO2302.
- R. G. Prinn, J. Huang, R. F. Weiss, D. M. Cunnold, P. J. Fraser, P. G. Simmonds, A. McCulloch, C. Harth, S. Reimann, P. Salameh, O. D. S., R. H. J. Wang, L. W. Porter, B. R. Miller and K. P. B., Evidence for variability of atmospheric hydroxyl radicals over the past quarter century, Geophys. Res. Lett., 2005, 32, L07809 CrossRef.
- J. L. Li, D. M. Cunnold, H. J. Wang, R. F. Weiss, B. R. Miller, C. Harth, P. Salameh and J. M. Harris, Halocarbon emissions estimated from advanced global atmospheric gases experiment measured pollution events at Trinidad Head, California, J. Geophys. Res. Atmos., 2005, 110, D05739.
- P. O. Wennberg, S. Peacock, J. T. Randerson and R. Bleck, Recent changes in the air-sea gas exchange of methyl chloroform, Geophys. Res. Lett., 2004, 31, L16112 CrossRef.
- M. R. Manning, D. C. Lowe, R. C. Moss, G. E. Bodeker and W. Allan, Short-term variations in the oxidizing power of the atmosphere, Nature, 2005, 436, 1001–1004 CrossRef CAS.
- C. J. Young, M. D. Hurley, T. J. Wallington and S. A. Mabury, Atmospheric lifetime and global warming potential of a perfluoropolyether, in FLUOROS Meeting, Toronto, ON, 2005 Search PubMed.
- UNEP, Case Study #16. Preparation of perfluoropolyether diols with high functionality (difunctional molecules content 99%), UNEP, Process Agents Task Force, http://www.unep.org/ozone/teap/Reports/PATF/PACS16R0.pdf, accessed September 19, 2005.
- H. Bösch, C. Camy-Peyret, M. P. Chipperfield, R. Fitzenberger, H. Harder, U. Platt and K. Pfeilsticker, Upper limits of stratospheric IO and OIO inferred from center-to-limb-darkening-corrected balloon-borne solar occultation visible spectra: Implications for total gaseous iodine and stratospheric ozone, J. Geophys. Res. Atmos., 2003, 108, D003078.
- J. G. Calvert and S. E. Lindberg, Potential influence of iodine-containing compounds on the chemistry of the troposphere in the polar spring. I. Ozone depletion, Atmos. Environ., 2004, 38, 5087–5104 CrossRef CAS.
- L. Matuana, M and N. M. Stark, Surface chemistry and mechanical property changes of wood-fiber/high-density polyethylene composites after accelerated weathering, J. Appl. Polymer. Sci., 2004, 94, 2263–2273 CrossRef.
- L. Cang, D. H. Kim and A. J. Ragauskas, Brightness reversion of mechanical pulps XIX. Photostabilization of mechanical pulps by UV absorbers: Surface photochemical studies using diffuse reflectance techniques, J. Wood Chem. Technol., 2004, 20, 30–53 Search PubMed.
- K. Antos and J. Sadlar, Influence of aromatic brominated flame retardants on alkane photo-oxidation: A model and polymer study, Polym. Degrad. Stab., 2005, 90, 180–187 CrossRef CAS.
- I. H. Craig, J. R. White and P. C. Kin, Crystallization and chemi-crystallization of recycled photo-degraded polypropyrene, Polymer, 2005, 46, 505–512 CrossRef CAS.
- X. Chen, J. Wang and J. Shen, Effect of UV-irradiation on poly(vinyl chrolide) modified by methyl methacrylate, Polym. Degrad. Stab., 2005, 87, 527–533 CrossRef CAS.
- Y. Taguchi, Y. Ishida, S. Tsuge, H. Ohtani, K. Kimura, T. Yoshikawa and T. Matsubara, Structural change of a polymeric hindered amine light stabilizer in polypropylene during UV-irradiation studied by reactive thermal desorption-gas chromatography, Polym. Degrad. Stab., 2004, 83, 221–227 CrossRef CAS.
- S. Scierka, P. L. Drzal, A. L. Foster, L. Amanda and S. Svetik, Nanomechanical properties of UV-degraded TiO2/epoxy nanocomposites, in Fundamentals of Nanoindentation and Nanotribology III, ed. K. J. Wahl, N. Huber, A. B. Mann, D. F. Bahr and Y.-T. Cheng, (Mater. Res. Soc. Symp. Proc. 841, Warrendale, PA, 2005), R5.10 pp. 217–222 Search PubMed.
- T. Mikami, M. Watanabe and T. Furuya, Effect of temperature on weatherability test of ABS and PC, in 16th Conference of Material Life Society, Society of Materials Science, Kyoto, Japan, 2005, pp. 29–30 Search PubMed.
- J. F. Power, S. W. Fu and O. V. Nepotchatykh, Depth profiling of the optical absorption coefficient in ultraviolet-degraded poly(vinylchloride) films by dual beam laser light profile, Appl. Spectrosc., 2005, 59, 511–518 CrossRef CAS.
Footnote |
| † List of contributing authors in alphabetical order: Anthony Andrady, Pieter J. Aucamp, Alkiviadis F. Bais, Carlos L. Ballaré, Lars Olof Björn, Janet F. Bornman (Co-Chair), Martyn Caldwell, Anthony P. Cullen, David J. Erickson, Frank R. de Gruijl, Donat-P. Häder, Mohammad Ilyas, G. Kulandaivelu, H. D. Kumar, Janice Longstreth, Richard L. McKenzie, Mary Norval, Halim Hamid Redhwi, Raymond C. Smith, Keith R. Solomon (Secretary), Barbara Sulzberger, Yukio Takizawa, Xiaoyan Tang (Co-Chair), Alan H. Teramura, Ayako Torikai, Jan C. van der Leun (Co-Chair), Stephen R. Wilson, Robert C. Worrest and Richard G. Zepp. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2006 |