Iminophosphorane-mediated efficient synthesis of new tricyclic 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones
Received
27th September 2005
, Accepted 8th November 2005
First published on 28th November 2005
Abstract
The carbodiimides 2, obtained from aza-Wittig reactions of iminophosphorane 1 with aromatic isocyanates, reacted with hydrazine to give selectively 6-amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-ones 5. Compounds 5 were further transformed to iminophosphoranes 6 by reaction with triphenylphosphine, hexachloroethane and triethylamine. A tandem aza-Wittig reaction of iminophosphorane 6 with isocyanate or acyl chloride generated previously unreported 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones 10 or 12 in satisfactory yield. X-ray structure analysis of 10g verified the proposed structure and the reaction selectivity.
Introduction
7H-1,2,3-Triazolo[4,5-d]pyrimidin-7-ones (azaguanines) are of great importance because of their structural similarity with guanines. Some derivatives of them have shown remarkable biological (antiguanine) properties such as antitumor, antiviral, and anti-HIV activities,1–5 whereas others exhibited good fungicidal activities.6 On the other hand, heterocycles containing the 1,2,4-triazole nucleus also exhibit various biological activities; several of them have been used as fungicidal, bactericidal, insecticidal, antitumor and anti-inflammatory agents.7–13 The introduction of a triazole ring to the triazolo[4,5-d]pyrimidin-7-one system is expected to influence the biological activities significantly. However, this tricyclic system has been much less investigated and there is no report on synthesis of 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones.
Recently we have been interested in the synthesis of quinazolinones, thienopyrimidinones and imidazolinones via aza-Wittig reaction of α or β ethoxycarbonyl iminophosphorane with aromatic isocyanate and subsequent reaction with various nucleophiles under mild conditions.14–18 Here we wish to report an efficient approach to the synthesis of previously unreported 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones by tandem iminophosphorane-mediated annulation of easily accessible N-(5-arylamino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-on-6-yl)iminotriphenylphosphorane with isocyanates or acyl chloride.
Results and discussion
Iminophosphorane 119 reacted with aromatic isocyanates to give carbodiimides 2, which were allowed to react with hydrazine to give selectively 6-amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-ones 5 in 72–81% yields (Scheme 1, Table 1). No formation of the potential regioisomer 4 was observed. It is worth noting that the reaction of β ethoxycarbonyl carbodiimide with hydrazine seems to be controversial. Our recent result on reaction of β-ethoxycarbonyl phenylcarbodiimide with hydrazine gave selectively 3-aminoquinazolin-4(3H)-one,20 consistent with formation of 5. However, an early report on a similar reaction using carbodiimide 7 resulted in the formation of 2-hydrazinopyrimidinone 8, another possible regioisomer (Scheme 2).19 The selective formation of 5 can be rationalized in terms of an initial nucleophilic addition of hydrazine to give the guanidine intermediate 3 which directly cyclizes to give 5 across the strong nucleophilic hydrazine group rather than the arylamine one.21
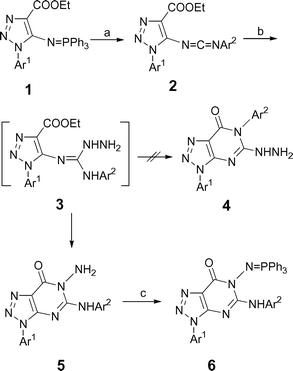 |
| | Scheme 1 Synthesis of compounds 5 and 6. (a) Ar2NCO, CH2Cl2, 0–5 °C, 24–30 h; (b) NH2NH2·H2O, CH3CN, rt, 10–20 min; (c) PPh3, C2Cl6, NEt3, CH2Cl2, rt, 4–6 h. | |
Table 1 Preparation of compounds 5 or 6
| |
Ar1 |
Ar2 |
Yield (%)a |
|
Isolated yields based on iminophosphorane 1.
|
|
5a
|
Ph |
Ph |
91 |
|
5b
|
Ph |
4-ClC6H4 |
86 |
|
5c
|
Ph |
4-MeC6H4 |
84 |
|
6a
|
Ph |
Ph |
88 |
|
6b
|
Ph |
4-ClC6H4 |
90 |
|
6c
|
Ph |
4-MeC6H4 |
90 |
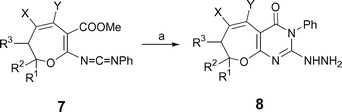 |
| | Scheme 2 Literature synthesis of compounds 8 from carbodiimide 7. (a) NH2NH2·H2O, EtOH, rt, 3 h. | |
Compounds 5 were easily converted to novel functionalized iminophosphoranes 6via reaction with triphenylphosphine, hexachloroethane and triethylamine in good yields (88–90%, Scheme 1, Table 1). When solutions of iminophosphoranes 6 in dry methylene chloride were treated with aromatic isocyanate at refluxing temperature, the previously unreported 6-arylamino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones 10 were isolated as crystalline solids in good yields (78–91%, Table 2, Scheme 3). Presumably, the conversion of 6 into 10 involves initial aza-Wittig reaction between the iminophosphorane 6 and the isocyanate to give a carbodiimide 9 as highly reactive intermediate, which easily undergoes ring closure across the arylamino group to give the otherwise not readily available 6-arylamino substituted 1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones 10. It is noteworthy that the reaction can be easily carried out at refluxing temperature (CH2Cl2 as solvent) under mild neutral condition and the separation of 10 from the reaction mixture was also easily carried out by simple filtration.
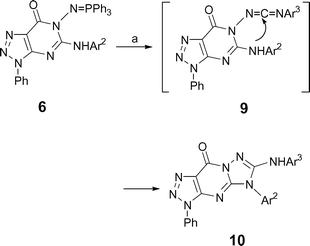 |
| | Scheme 3 Preparation of tricyclic compound 10. (a) Ar3NCO, CH2Cl2, 40 °C, 1–2 h. | |
Table 2 Preparation of compounds 10a–h or 12a–f
| |
Ar2 |
Ar3 |
R |
Yield (%)a |
|
Isolated yields based on iminophosphorane 6.
|
|
10a
|
Ph |
Ph |
|
91 |
|
10b
|
Ph |
4-ClC6H4 |
|
84 |
|
10c
|
4-ClC6H4 |
Ph |
|
78 |
|
10d
|
4-ClC6H4 |
4-ClC6H4 |
|
90 |
|
10e
|
4-ClC6H4 |
4-MeC6H4 |
|
83 |
|
10f
|
4-ClC6H4 |
3-MeC6H4 |
|
84 |
|
10g
|
4-MeC6H4 |
Ph |
|
82 |
|
10h
|
4-MeC6H4 |
4-MeC6H4 |
|
87 |
|
12a
|
Ph |
|
Ph |
78 |
|
12b
|
Ph |
|
Me |
84 |
|
12c
|
4-ClC6H4 |
|
Ph |
86 |
|
12d
|
4-ClC6H4 |
|
Me |
73 |
|
12e
|
4-MeC6H4 |
|
Ph |
75 |
|
12f
|
4-MeC6H4 |
|
Me |
85 |
Iminophosphoranes 6 reacted with acyl chlorides in the presence of triethylamine in methylene chloride at refluxing temperature to give 6-substituted 1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones 12 in good yields (73–86%, Table 2, Scheme 4). The formation of 12 can be viewed as an initial aza-Wittig reaction between the iminophosphorane 6 and acyl chloride in presence of triethylamine affording the intermediate imidoyl chloride 11 which undergoes cyclization to give 12.
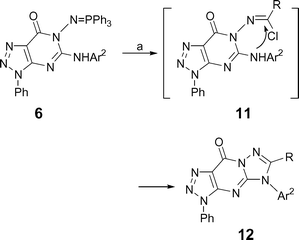 |
| | Scheme 4 Synthesis of compounds 12 (a) RCOCl, CH2Cl2, NEt3, 40 °C, 2–4 h. | |
The structure of 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones 10 and 12 was confirmed by their spectral data. Furthermore a single crystal of 10g was obtained from a DMF solution of 10g. X-ray structure analysis verified again the proposed structure, and showed that all ring atoms in the tricyclic moiety are essentially planar, with the maxim deviation of 0.0392 Å for N(2) from the heterocyclic plane (Fig. 1). The bond lengths of C(4)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(4), C(5)
N(4), C(5)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(6), and C(1)
N(6), and C(1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(2) are 1.307(3) Å, 1.311(3) Å and 1.386(3) Å, are longer than that of the typical C
C(2) are 1.307(3) Å, 1.311(3) Å and 1.386(3) Å, are longer than that of the typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1.28 Å) and C
N (1.28 Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1.34 Å) bonds respectively, while the single bond lengths of C(1)–N(1), C(2)–N(3), C(1)–N(4), C(4)–N(5), C(4)–N(7), N(7)–C(5) and C(2)–C(3) are 1.354(3) Å, 1.371(3) Å, 1.361(3) Å, 1.370(3) Å, 1.368(3) Å, 1.389(3) Å and 1.435(3) Å respectively, are significantly shorter than the typical C(sp2)–N(1.426 Å) and C–C(1.53 Å), showing a degree of delocalization.
C (1.34 Å) bonds respectively, while the single bond lengths of C(1)–N(1), C(2)–N(3), C(1)–N(4), C(4)–N(5), C(4)–N(7), N(7)–C(5) and C(2)–C(3) are 1.354(3) Å, 1.371(3) Å, 1.361(3) Å, 1.370(3) Å, 1.368(3) Å, 1.389(3) Å and 1.435(3) Å respectively, are significantly shorter than the typical C(sp2)–N(1.426 Å) and C–C(1.53 Å), showing a degree of delocalization.
![ORTEP diagram of the crystal structure of tricyclic compound 10g (50% thermal ellipsoids). For clarity, solvent molecular (DMF) and all hydrogen atoms have been omitted. Select bond lengths [Å]: C(1)–N(1) 1.354(3), N(1)–N(2) 1.377(3), N(2)–N(3) 1.304(3), C(2)–N(3) 1.371(3), C(1)–C(2) 1.386(3), C(2)–C(3) 1.435(3), C(3)–N(5) 1.420(3), C(4)–N(5) 1.370(3), C(4)–N(4) 1.307(3), C(1)–N(4) 1.361(3), N(5)–N(6) 1.403(3), C(5)–N(6) 1.311(3), C(5)–N(7) 1.389(3), C(4)–N(7) 1.368(3), C(3)–O(1) 1.211(3), C(5)–N(8) 1.347(3), C(6)–N(8) 1.414(3). Selected bond angles [deg]: N(3)–N(2)–N(1) 108.3(2), C(1)–N(1)–N(2) 109.8(2), N(1)–C(1)–C(2) 104.5(2), N(2)–N(1)–C(19) 120.9(2), C(1)–C(2)–C(3) 121.7(2), N(5)–C(3)–C(2) 107.5(2), C(4)–N(5)–C(3) 124.9(2), N(4)–C(4)–N(5) 128.5(2), C(4)–N(4)–C(1) 108.3(2), N(4)–C(1)–C(2) 129.0(2), C(4)–N(5)–N(6) 112.23(18), C(5)–N(6)–N(5) 103.03(19), N(6)–C(5)–N(7) 112.9(2), C(4)–N(7)–C(5) 107.0(2), N(7)–C(4)–N(5) 104.8(2), C(4)–N(7)–C(12) 123.9(2).CCDC reference number 279775. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513715b](/image/article/2006/OB/b513715b/b513715b-f1.gif) |
| | Fig. 1 ORTEP diagram of the crystal structure of tricyclic compound 10g (50% thermal ellipsoids). For clarity, solvent molecular (DMF) and all hydrogen atoms have been omitted. Select bond lengths [Å]: C(1)–N(1) 1.354(3), N(1)–N(2) 1.377(3), N(2)–N(3) 1.304(3), C(2)–N(3) 1.371(3), C(1)–C(2) 1.386(3), C(2)–C(3) 1.435(3), C(3)–N(5) 1.420(3), C(4)–N(5) 1.370(3), C(4)–N(4) 1.307(3), C(1)–N(4) 1.361(3), N(5)–N(6) 1.403(3), C(5)–N(6) 1.311(3), C(5)–N(7) 1.389(3), C(4)–N(7) 1.368(3), C(3)–O(1) 1.211(3), C(5)–N(8) 1.347(3), C(6)–N(8) 1.414(3). Selected bond angles [deg]: N(3)–N(2)–N(1) 108.3(2), C(1)–N(1)–N(2) 109.8(2), N(1)–C(1)–C(2) 104.5(2), N(2)–N(1)–C(19) 120.9(2), C(1)–C(2)–C(3) 121.7(2), N(5)–C(3)–C(2) 107.5(2), C(4)–N(5)–C(3) 124.9(2), N(4)–C(4)–N(5) 128.5(2), C(4)–N(4)–C(1) 108.3(2), N(4)–C(1)–C(2) 129.0(2), C(4)–N(5)–N(6) 112.23(18), C(5)–N(6)–N(5) 103.03(19), N(6)–C(5)–N(7) 112.9(2), C(4)–N(7)–C(5) 107.0(2), N(7)–C(4)–N(5) 104.8(2), C(4)–N(7)–C(12) 123.9(2).† | |
In conclusion, we have developed an efficient iminophosphorane-mediated synthesis of previously unreported 3,5-dihydro-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-ones via aza-Wittig reactions. The spectral and X-ray analysis of the product verified the proposed structure and the reaction selectivity.
Experimental
General materials and methods
Reagents and chemicals were obtained from Acros, Aldrich, Shanghai or Beijing chemicals and were used without further purification. All solvents were freshly distilled. Melting points are uncorrected. MS were measured on a Finnigan Trace MS spectrometer. IR were recorded on a PE-983 infrared spectrometer as KBr pellets with absorption in cm−1. NMR were recorded in CDCl3 or DMSO-d6 on a Varian Mercury 400 spectrometer and resonances are given in ppm (δ) relative to TMS. Elementary analyses were taken on a Vario EL III elementary analysis instrument.
5-Arylamino-6-amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-ones (5)
To a solution of iminophosphorane 16 (2 mmol) in dry methylene chloride (15 mL) was added aromatic isocyanate (2 mmol) under nitrogen at room temperature. After the reaction mixture was stood for 24–30 hours at 0–5 °C, the solvent was removed off under reduced pressure and ether–petroleum ether (1:2, 20 mL) was added to precipitate triphenylphosphine oxide. After filtration the solvent was removed to give carbodiimide 2, which was used directly without further purification. To the solution of 2 prepared above in CH3CN (15 ml) was added hydrazine hydrate (0.24 g, 4 mmol, 85%) in EtOH (5 mL). The mixture was stirred for 10 min at room temperature and filtered to give 5-arylamino-6-amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-ones 5.
6-Amino-3,6-dihydro-3-phenyl-5-phenylamino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (5a).
White crystals (580 mg, 91% yield), mp: 253–255 °C; IR (KBr) cm−1 3329, 3262 (N–H), 1718 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1542, 1203; 1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 7.19–8.15 (m, 10H), 8.94 (s, 1H); MS m/z (%) 319 (M+, 9), 291 (2), 274 (2), 118 (18), 77 (100); Anal. Calcd for C16H13N7O: C, 60.18; H, 4.10; N, 30.70. Found: C, 60.35; H, 4.03; N, 30.93%.
O), 1542, 1203; 1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 7.19–8.15 (m, 10H), 8.94 (s, 1H); MS m/z (%) 319 (M+, 9), 291 (2), 274 (2), 118 (18), 77 (100); Anal. Calcd for C16H13N7O: C, 60.18; H, 4.10; N, 30.70. Found: C, 60.35; H, 4.03; N, 30.93%.
6-Amino-5-(4-chlorophenyl)amino-3,6-dihydro-3-phenyl-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (5b).
White crystals (607 mg, 86% yield), mp: >300 °C; IR (KBr) cm−1 3309, 3263 (N–H), 1716 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1559, 1204; 1H NMR (400 MHz, DMSO-d6) δ 5.75 (s, 2H), 7.43–8.04 (m, 9H), 10.01 (s, 1H); MS m/z (%) 355/353 (M+, 15/45), 325 (12), 309 (5), 111 (23), 77 (100); Anal. Calcd for C16H12ClN7O: C, 54.32; H, 3.42; N, 27.71. Found: C, 54.47; H, 3.64; N, 27.57%.
O), 1559, 1204; 1H NMR (400 MHz, DMSO-d6) δ 5.75 (s, 2H), 7.43–8.04 (m, 9H), 10.01 (s, 1H); MS m/z (%) 355/353 (M+, 15/45), 325 (12), 309 (5), 111 (23), 77 (100); Anal. Calcd for C16H12ClN7O: C, 54.32; H, 3.42; N, 27.71. Found: C, 54.47; H, 3.64; N, 27.57%.
6-Amino-3,6-dihydro-5-(4-methylphenyl)amino-3-phenyl-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (5c).
White crystals (560 mg, 84% yield), mp: 253–255 °C; IR (KBr) cm−1 3314, 3260 (N–H), 1701 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556, 1205; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 5.74 (s, 2H), 7.18–8.07 (m, 9H), 9.80 (s, 1H); MS m/z (%) 333 (M+, 57), 305 (19), 289 (15), 131 (26), 77 (100); Anal. Calcd for C17H15N7O: C, 61.25; H, 4.54; N, 29.41. Found: C, 61.08; H, 4.69; N, 29.64%.
O), 1556, 1205; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 5.74 (s, 2H), 7.18–8.07 (m, 9H), 9.80 (s, 1H); MS m/z (%) 333 (M+, 57), 305 (19), 289 (15), 131 (26), 77 (100); Anal. Calcd for C17H15N7O: C, 61.25; H, 4.54; N, 29.41. Found: C, 61.08; H, 4.69; N, 29.64%.
5-Arylamino-6-(triphenylphosphoranylidene)amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-ones (6)
To a mixture of 5 (8 mmol), PPh3 (3.14 g, 12 mmol) and C2Cl6 (2.84 g, 12 mmol) in dry CH2Cl2 (40 mL), was added dropwise NEt3 (2.42 g, 24 mmol) at room temperature. The colour of the reaction mixture quickly turned yellow. After being stirred for 4–6 h, the solvent was removed under reduced pressure and the residue was recrystallized from EtOH to give iminophosphorane 6.
3,6-Dihydro-3-phenyl-5-phenylamino-6-(triphenylphosphoranylidene)amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (6a).
White crystals (4.07 g, yield 88%), mp: 278–280 °C; IR (KBr) cm−1: 3264 (N–H), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1550, 1110; 1H NMR (CDCl3, 400 MHz) δ: 7.12–8.18 (m, 25H), 9.90 (s, 1H); MS m/z (%) 579 (M+, 62), 551 (8), 262 (74), 183 (83), 77 (100); Anal. Calcd for C34H26N7OP: C, 70.46; H, 4.52; N, 16.92. Found: C, 70.68; H, 4.37; N, 16.75%.
O), 1550, 1110; 1H NMR (CDCl3, 400 MHz) δ: 7.12–8.18 (m, 25H), 9.90 (s, 1H); MS m/z (%) 579 (M+, 62), 551 (8), 262 (74), 183 (83), 77 (100); Anal. Calcd for C34H26N7OP: C, 70.46; H, 4.52; N, 16.92. Found: C, 70.68; H, 4.37; N, 16.75%.
5-(4-Chlorophenyl)amino-3,6-dihydro-3-phenyl-6-(triphenylphosphoranylidene)amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (6b).
White crystals (4.42 g, yield 90%), mp: 287–288 °C; IR (KBr) cm−1: 3305 (N–H), 1701 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1549, 1107; 1H NMR (CDCl3, 400 MHz) δ: 7.26–8.14 (m, 24H), 9.93 (s, 1H); MS m/z (%) 615/613 (M+, 3/9), 276 (16), 262 (24), 183 (78), 77 (100); Anal. Calcd for C34H25ClN7OP: C, 66.51; H, 4.10; N, 15.97. Found: C, 66.35; H, 4.35; N, 15.84%.
O), 1549, 1107; 1H NMR (CDCl3, 400 MHz) δ: 7.26–8.14 (m, 24H), 9.93 (s, 1H); MS m/z (%) 615/613 (M+, 3/9), 276 (16), 262 (24), 183 (78), 77 (100); Anal. Calcd for C34H25ClN7OP: C, 66.51; H, 4.10; N, 15.97. Found: C, 66.35; H, 4.35; N, 15.84%.
3,6-Dihydro-5-(4-methylphenyl)amino-3-phenyl-6-(triphenylphosphoranylidene)amino-7H-1,2,3-triazolo[4,5-d]pyrimidin-7-one (6c).
White crystals (4.26 g, yield 90%), mp: >300 °C; IR (KBr) cm−1: 3276 (N–H), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1557, 1107; 1H NMR (CDCl3, 400 MHz) δ: 2.36 (s, 3H), 7.17–8.19 (m, 24H), 9.79 (s, 1H); MS m/z (%) 593 (M+, 28), 304 (40), 262 (60), 183 (100), 77 (95); Anal. Calcd for C35H28N7OP: C, 70.82; H, 4.75; N, 16.52. Found: C, 70.75; H, 4.76; N, 16.68%.
O), 1557, 1107; 1H NMR (CDCl3, 400 MHz) δ: 2.36 (s, 3H), 7.17–8.19 (m, 24H), 9.79 (s, 1H); MS m/z (%) 593 (M+, 28), 304 (40), 262 (60), 183 (100), 77 (95); Anal. Calcd for C35H28N7OP: C, 70.82; H, 4.75; N, 16.52. Found: C, 70.75; H, 4.76; N, 16.68%.
6-Arylamino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10)
To a solution of iminophosphorane 6 (1 mmol) in dry methylene chloride (10 mL) was added aromatic isocyanate (1 mmol) under nitrogen at room temperature. After the solution is stirred at refluxing temperature for 1–2 h, the white precipitated solid is collected by filtration and recrystallized from CH2Cl2–ethanol to give 10 as crystalline solids.
3,5-Dihydro-3,5-diphenyl-6-phenylamino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10a).
White crystals (384 mg, yield 91%), mp: >300 °C; IR (KBr) cm−1: 3413 (N–H), 1720 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1399; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.95 (m, 15H), 9.16 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ 119.8, 123.5 (2), 124.6, 125.7 (2), 127.8 (2), 129.5 (2), 129.8 (2), 130.2, 130.4, 132.3 (2), 134.1, 134.9, 135.8, 147.7, 148.3, 150.2, 150.4; MS m/z (%) 420 (M+, 26), 392 (10), 260 (14), 245 (16), 77 (100); Anal. Calcd for C23H16N8O: C, 65.71; H, 3.84; N, 26.65. Found: C, 65.94; H, 3.97; N, 26.58%.
O), 1544, 1399; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.95 (m, 15H), 9.16 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ 119.8, 123.5 (2), 124.6, 125.7 (2), 127.8 (2), 129.5 (2), 129.8 (2), 130.2, 130.4, 132.3 (2), 134.1, 134.9, 135.8, 147.7, 148.3, 150.2, 150.4; MS m/z (%) 420 (M+, 26), 392 (10), 260 (14), 245 (16), 77 (100); Anal. Calcd for C23H16N8O: C, 65.71; H, 3.84; N, 26.65. Found: C, 65.94; H, 3.97; N, 26.58%.
6-(4-Chlorophenyl)amino-3,5-dihydro-3,5-diphenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10b).
White crystals (381 mg, yield 84%), mp: >300 °C; IR (KBr) cm−1: 3403 (N–H), 1721 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 7.42–7.94 (m, 14H), 9.30 (s, 1H); MS m/z (%) 456/454 (M+, 3/9), 426 (6), 274 (13), 258 (10), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.65; H, 3.35; N, 24.52%.
O), 1544, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 7.42–7.94 (m, 14H), 9.30 (s, 1H); MS m/z (%) 456/454 (M+, 3/9), 426 (6), 274 (13), 258 (10), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.65; H, 3.35; N, 24.52%.
5-(4-Chlorophenyl)-3,5-dihydro-3-phenyl-6-phenylamino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10c).
White crystals (355 mg, yield 78%), mp: >300 °C; IR (KBr) cm−1: 3402 (N–H), 1721 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1540, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.94 (m, 14H), 9.16 (s, 1H); MS m/z (%) 456/454 (M+, 15/44), 426 (17), 294 (15), 273 (38), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.87; H, 3.41; N, 24.47%.
O), 1540, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.94 (m, 14H), 9.16 (s, 1H); MS m/z (%) 456/454 (M+, 15/44), 426 (17), 294 (15), 273 (38), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.87; H, 3.41; N, 24.47%.
5-(4-Chlorophenyl)-6-(4-chlorophenyl)amino-3,5-dihydro-3-phenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10d).
White crystals (440 mg, yield 90%), mp: >300 °C; IR (KBr) cm−1: 3407 (N–H), 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.44–7.93 (m, 13H), 9.30 (s, 1H); MS m/z (%) 492/490/488 (M+, 3/16/25), 460 (12), 308 (15), 273 (43), 77 (100); Anal. Calcd for C23H14Cl2N8O: C, 56.46; H, 2.88; N, 22.90. Found: C, 56.31; H, 2.90; N, 22.81%.
O), 1544, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.44–7.93 (m, 13H), 9.30 (s, 1H); MS m/z (%) 492/490/488 (M+, 3/16/25), 460 (12), 308 (15), 273 (43), 77 (100); Anal. Calcd for C23H14Cl2N8O: C, 56.46; H, 2.88; N, 22.90. Found: C, 56.31; H, 2.90; N, 22.81%.
5-(4-Chlorophenyl)-3,5-dihydro-6-(4-methylphenyl)amino-3-phenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10e).
White crystals (388 mg, yield 83%), mp: >300 °C; IR (KBr) cm−1: 3364 (N–H), 1731 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1541, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.27 (s, 3H), 7.16–7.93 (m, 13H), 9.04 (s, 1H); MS m/z (%) 470/468 (M+, 11/35), 440 (14), 308 (28), 273 (42), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.35; H, 3.52; N, 23.97%.
O), 1541, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.27 (s, 3H), 7.16–7.93 (m, 13H), 9.04 (s, 1H); MS m/z (%) 470/468 (M+, 11/35), 440 (14), 308 (28), 273 (42), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.35; H, 3.52; N, 23.97%.
5-(4-Chlorophenyl)-3,5-dihydro-6-(3-methylphenyl)amino-3-phenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10f).
White crystals (393 mg, yield 84%), mp: >300 °C; IR (KBr) cm−1: 3359 (N–H), 1733 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.29 (s, 3H), 6.87–7.94 (m, 13H), 9.07 (s, 1H); MS m/z (%) 470/468 (M+, 9/28), 440 (10), 308 (17), 273 (26), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.41; H, 3.78; N, 23.84%.
O), 1543, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.29 (s, 3H), 6.87–7.94 (m, 13H), 9.07 (s, 1H); MS m/z (%) 470/468 (M+, 9/28), 440 (10), 308 (17), 273 (26), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.41; H, 3.78; N, 23.84%.
3,5-Dihydro-5-(4-methylphenyl)-3-phenyl-6-phenylamino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10g).
White crystals (355 mg, yield 82%), mp: >300 °C; IR (KBr) cm−1: 3402 (N–H), 1719 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1457; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.54 (s, 3H), 6.88–7.82 (m, 14H); 13C NMR (CDCl3–TFA, 100 MHz) δ 21.1, 119.4, 123.0 (2), 124.4, 125.4 (2), 127.7 (2), 129.5 (2), 129.9 (2), 130.0, 130.4, 132.1 (2), 134.3, 135.6, 143.7, 147.8, 148.4, 150.3, 150.6; MS m/z (%) 434 (M+, 42), 406 (19), 288 (22), 274 (17), 77 (100); Anal. Calcd for C24H18N8O: C, 66.35; H, 4.18; N, 25.79. Found: C, 66.15; H, 4.10; N, 25.88%.
O), 1543, 1457; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.54 (s, 3H), 6.88–7.82 (m, 14H); 13C NMR (CDCl3–TFA, 100 MHz) δ 21.1, 119.4, 123.0 (2), 124.4, 125.4 (2), 127.7 (2), 129.5 (2), 129.9 (2), 130.0, 130.4, 132.1 (2), 134.3, 135.6, 143.7, 147.8, 148.4, 150.3, 150.6; MS m/z (%) 434 (M+, 42), 406 (19), 288 (22), 274 (17), 77 (100); Anal. Calcd for C24H18N8O: C, 66.35; H, 4.18; N, 25.79. Found: C, 66.15; H, 4.10; N, 25.88%.
3,5-Dihydro-5-(4-methylphenyl)-3-phenyl-6-(4-methylphenyl)amino-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (10h).
White crystals (390 mg, yield 87%), mp: >300 °C; IR (KBr) cm−1: 3411 (N–H), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1545, 1510; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.15 (s, 3H), 2.54 (s, 3H), 6.70–7.82 (m, 13H); 13C NMR (CDCl3–TFA, 100 MHz) δ 20.6, 21.1, 122.8 (2), 124.1, 125.3 (2), 127.5 (2), 128.4, 129.2 (2), 129.7 (2), 129.8, 130.2, 132.0 (2), 134.0, 135.5, 143.8, 147.7, 148.2, 150.1, 150.4; MS m/z (%) 448 (M+, 42), 420 (19), 288 (40), 273 (16), 91 (100). Anal. Calcd for C25H20N8O: C, 66.95; H, 4.49; N, 24.98; Found: C, 66.84; H, 4.57; N, 25.05%.
O), 1545, 1510; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.15 (s, 3H), 2.54 (s, 3H), 6.70–7.82 (m, 13H); 13C NMR (CDCl3–TFA, 100 MHz) δ 20.6, 21.1, 122.8 (2), 124.1, 125.3 (2), 127.5 (2), 128.4, 129.2 (2), 129.7 (2), 129.8, 130.2, 132.0 (2), 134.0, 135.5, 143.8, 147.7, 148.2, 150.1, 150.4; MS m/z (%) 448 (M+, 42), 420 (19), 288 (40), 273 (16), 91 (100). Anal. Calcd for C25H20N8O: C, 66.95; H, 4.49; N, 24.98; Found: C, 66.84; H, 4.57; N, 25.05%.
1,2,3-Triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12)
To a solution of iminophosphorane 6 (1 mmol) in dry CH2Cl2 (10 mL) was added acyl chloride (1 mmol) and triethylamine (0.10 g, 1 mmol) under nitrogen at room temperature. The solution was stirred at refluxing temperature for 2–4 h. The white precipitated ammonium salt was separated by filtration and the filtrate was concentrated to dryness. The residue was recrystallized from CH2Cl2–ethanol to give 12 as crystalline solids.
3,5-Dihydro-3,5,6-triphenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12a).
White crystals (316 mg, 78% yield), mp: >300 °C; IR (KBr) cm−1: 1736 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1548, 1510; 1H NMR (400 MHz, CDCl3) δ 7.36–7.59 (m, 13H), 8.02 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 121.4, 123.0, 124.2, 126.7 (2), 128.4 (2), 128.6 (2), 128.8 (2), 129.2, 129.5 (2), 130.7 (2), 132.0, 132.4, 134.4, 141.0, 148.6, 150.2, 150.4, 153.2; MS m/z (%) 405 (M+, 6), 377 (5), 245 (7), 103 (30), 77 (100); Anal. Calcd for C23H15N7O: C, 68.14; H, 3.73; N, 24.18. Found: C, 68.25; H, 3.81; N, 24.05%.
O), 1548, 1510; 1H NMR (400 MHz, CDCl3) δ 7.36–7.59 (m, 13H), 8.02 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 121.4, 123.0, 124.2, 126.7 (2), 128.4 (2), 128.6 (2), 128.8 (2), 129.2, 129.5 (2), 130.7 (2), 132.0, 132.4, 134.4, 141.0, 148.6, 150.2, 150.4, 153.2; MS m/z (%) 405 (M+, 6), 377 (5), 245 (7), 103 (30), 77 (100); Anal. Calcd for C23H15N7O: C, 68.14; H, 3.73; N, 24.18. Found: C, 68.25; H, 3.81; N, 24.05%.
3,5-Dihydro-3,5-diphenyl-6-methyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12b).
White crystals (289 mg, 84% yield), mp: >300 °C; IR (KBr) cm−1: 1743 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1557, 1498; 1H NMR (400 MHz, CDCl3–TFA) δ 2.52 (s, 3H), 7.46–7.89 (m, 10H); 13C NMR (CDCl3–TFA, 100 MHz) δ 11.9, 121.7 (2), 125.0, 127.0 (2), 129.0, 129.4 (2), 130.4 (2), 130.5, 131.0, 135.0, 148.4, 149.6, 150.1, 153.0; MS m/z (%) 343 (M+, 23), 315 (16), 286 (16), 245 (9), 77 (100); Anal. Calcd for C18H13N7O: C, 62.97; H, 3.82; N, 28.56. Found: C, 62.75; H, 3.91; N, 28.36%.
O), 1557, 1498; 1H NMR (400 MHz, CDCl3–TFA) δ 2.52 (s, 3H), 7.46–7.89 (m, 10H); 13C NMR (CDCl3–TFA, 100 MHz) δ 11.9, 121.7 (2), 125.0, 127.0 (2), 129.0, 129.4 (2), 130.4 (2), 130.5, 131.0, 135.0, 148.4, 149.6, 150.1, 153.0; MS m/z (%) 343 (M+, 23), 315 (16), 286 (16), 245 (9), 77 (100); Anal. Calcd for C18H13N7O: C, 62.97; H, 3.82; N, 28.56. Found: C, 62.75; H, 3.91; N, 28.36%.
5-(4-Chlorophenyl)-3,5-dihydro-3,6-diphenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12c).
White crystals (377 mg, 86% yield), mp: 287–288 °C; IR (KBr) cm−1 1731 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1535, 1494; 1H NMR (400 MHz, CDCl3–TFA) δ 7.25–7.59 (m, 12H), 7.96 (d, J = 8.4 Hz, 2H); MS m/z (%) 441/439 (M+, 6/20), 411 (16), 279 (22), 111 (69), 77 (100); Anal. Calcd for C23H14ClN7O: C, 62.80; H, 3.21; N, 22.29. Found: C, 62.73; H, 3.15; N, 22.33%.
O), 1535, 1494; 1H NMR (400 MHz, CDCl3–TFA) δ 7.25–7.59 (m, 12H), 7.96 (d, J = 8.4 Hz, 2H); MS m/z (%) 441/439 (M+, 6/20), 411 (16), 279 (22), 111 (69), 77 (100); Anal. Calcd for C23H14ClN7O: C, 62.80; H, 3.21; N, 22.29. Found: C, 62.73; H, 3.15; N, 22.33%.
5-(4-Chlorophenyl)-3,5-dihydro-6-methyl-3-phenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12d).
White crystals (275 mg, 73% yield), mp: >300 °C; IR (KBr) cm−1 1740 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1554, 1509; 1H NMR (400 MHz, CDCl3/TFA) δ 2.49 (s, 3H), 7.42–7.92 (m, 10H); MS m/z (%) 379/377 (M+, 8/27), 349 (19), 320 (19), 273 (21), 111 (100); Anal. Calcd for C18H12ClN7O: C, 57.23; H, 3.20; N, 25.95. Found: C, 57.47; H, 3.15; N, 25.87%.
O), 1554, 1509; 1H NMR (400 MHz, CDCl3/TFA) δ 2.49 (s, 3H), 7.42–7.92 (m, 10H); MS m/z (%) 379/377 (M+, 8/27), 349 (19), 320 (19), 273 (21), 111 (100); Anal. Calcd for C18H12ClN7O: C, 57.23; H, 3.20; N, 25.95. Found: C, 57.47; H, 3.15; N, 25.87%.
3,5-Dihydro-3,6-diphenyl-5-(4-methylphenyl)-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12e).
White crystals (314 mg, 75% yield), mp: >300 °C; IR (KBr) cm−1 1732 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1510; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.26–8.09 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ 21.1, 121.8, 123.2, 124.8, 127.0 (2), 128.8 (2), 128.9 (2), 129.0 (2), 129.3, 129.5 (2), 130.9 (2), 132.3, 134.9, 141.4, 148.5, 150.2, 150.5, 153.3; MS m/z (%) 419 (M+, 90), 391 (61), 262 (24), 259 (80), 91 (100); Anal. Calcd for C24H17N7O: C, 68.73; H, 4.09; N, 23.38. Found: C, 68.80; H, 4.16; N, 22.36%.
O), 1543, 1510; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.26–8.09 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ 21.1, 121.8, 123.2, 124.8, 127.0 (2), 128.8 (2), 128.9 (2), 129.0 (2), 129.3, 129.5 (2), 130.9 (2), 132.3, 134.9, 141.4, 148.5, 150.2, 150.5, 153.3; MS m/z (%) 419 (M+, 90), 391 (61), 262 (24), 259 (80), 91 (100); Anal. Calcd for C24H17N7O: C, 68.73; H, 4.09; N, 23.38. Found: C, 68.80; H, 4.16; N, 22.36%.
3,5-Dihydro-6-methyl-5-(4-methylphenyl)-3-phenyl-1,2,3-triazolo[4,5-d]-1,2,4-triazolo[1,5-a]pyrimidin-9-one (12f).
White crystals (304 mg, 85% yield), mp: >300 °C; IR (KBr) cm−1 1737 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1558, 1517; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 2.51 (s, 3H), 7.34–8.05 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ 11.9, 21.2, 122.2 (2), 124.7, 126.7 (2), 127.6, 129.5 (2), 129.6, 131.1 (2), 134.7, 141.9, 148.5, 149.7, 150.2, 153.6; MS m/z (%) 357 (M+, 70), 329 (49), 288 (21), 259 (26), 91 (100). Anal. Calcd for C19H15N7O: C, 63.86; H, 4.23; N, 27.44. Found: C, 63.73; H, 4.36; N, 27.31%.
O), 1558, 1517; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 2.51 (s, 3H), 7.34–8.05 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ 11.9, 21.2, 122.2 (2), 124.7, 126.7 (2), 127.6, 129.5 (2), 129.6, 131.1 (2), 134.7, 141.9, 148.5, 149.7, 150.2, 153.6; MS m/z (%) 357 (M+, 70), 329 (49), 288 (21), 259 (26), 91 (100). Anal. Calcd for C19H15N7O: C, 63.86; H, 4.23; N, 27.44. Found: C, 63.73; H, 4.36; N, 27.31%.
Acknowledgements
We gratefully acknowledge financial support of this work by the National Basic Research Program of China (2004CCA00100) and the National Natural Science Foundation of China (Project No. 20102001).
References
- L. Santana, M. Teijeira, E. Uriarte, J. Balzarini and E. De Clercq, Eur. J. Med. Chem., 2002, 37, 755 CrossRef CAS.
- J. M. Blanco, O. Caamano, F. Fernandez, X. Garcia-Mera, A. R. Hergueta, C. Lopez, J. E. Rodriguez-Borges, J. Balzarini and E. De Clerco, Chem. Pharm. Bull., 1999, 47, 1314 CAS.
- M. I. Nieto, O. Caamano, F. Fernandez, M. Gomez, J. Balzarini and E. De Clercq, Nucleosides Nucleotides Nucleic Acids, 2002, 21, 243 CrossRef CAS.
-
W. A. Slusarchyk and R. Zahler, US 5723609, 1988; W. A. Slusarchyk and R. Zahler, Chem. Abstr., 1998, 128, 230636c Search PubMed.
-
E. Matthes, M. Von Janta-Lipinski, K. Reimer, W. Mueller, H. Meisel, C. Lehmann and J. Schildt, EP 409227, 1991; E. Matthes, M. Von Janta-Lipinski, K. Reimer, W. Mueller, H. Meisel, C. Lehmann and J. Schildt, Chem. Abstr., 1991, 115, 159680m Search PubMed.
- F. E. Nielsen, E. B. Pedersen and M. Begtrup, Liebigs Ann. Chem., 1984, 1848–1859 CrossRef CAS.
-
J. Onodera, S. Sato, S. Kumazawa, A. Ito, S. Saishoji and Y. Niizeki, JP 96127568, 1996; J. Onodera, S. Sato, S. Kumazawa, A. Ito, S. Saishoji and Y. Niizeki, Chem. Abstr., 1996, 125, 142713h Search PubMed.
- H. Mikamo, X. H. Yin, Y. Hayasaki, M. Satoh and T. Tamaya, Chemotherapy, 2001, 47, 377 Search PubMed.
- R. P. Dickinson, A. W. Bell, C. A. Hitchcock, S. Narayana-Swami, S. J. Ray, K. Richardson and P. F. Troke, Bioorg. Med. Chem. Lett., 1996, 6, 2031 CrossRef CAS.
- N. Ulusoy, A. Gursoy and G. Otuk, Farmaco, 2001, 56, 947 CrossRef CAS.
-
V. B. Hegde, S. J. Bis, E. C. Heo, C. T. Hamilton, P. L. Johnson, L. L. Karr, T. P. Martin, P. A. Neese, N. Orr, F. E. Tisdell, M. C. H. Yap and Y. Zhu, US 20020019370; V. B. Hegde, S. J. Bis, E. C. Heo, C. T. Hamilton, P. L. Johnson, L. L. Karr, T. P. Martin, P. A. Neese, N. Orr, F. E. Tisdell, M. C. H. Yap and Y. Zhu, Chem. Abstr., 2002, 136, 146541 Search PubMed.
- S.-I. Nagai, S. Takemoto, T. Ueda, K. Mizutani, Y. Uozumi and H. Tokuda, J. Heterocycl. Chem., 2001, 38, 1097 Search PubMed.
- E. Palaska, G. Sahin, P. Kelicen, N. T. Durlu and G. Altinok, Farmaco, 2002, 57, 101 CrossRef CAS.
- M. W. Ding, S. Z. Xu and J. F. Zhao, J. Org. Chem., 2004, 69, 8366 CrossRef CAS.
- M. W. Ding, S. J. Yang and J. Zhu, Synthesis, 2004, 75 CrossRef CAS.
- Y. Liang, M. W. Ding, Z. J. Liu and X. P. Liu, Synth. Commun., 2003, 33, 2843 CrossRef CAS.
- M. W. Ding, Y. Sun, S. J. Yang, X. P. Liu and Z. J. Liu, Synth. Commun., 2003, 33, 1651 CrossRef CAS.
- M. W. Ding, Y. Sun, X. P. Liu and Z. J. Liu, Org. Prep. Proced. Int., 2003, 35, 391 CrossRef CAS.
- H. Wamhoff and G. Haffmanns, Chem. Ber., 1984, 117, 585 CrossRef CAS.
- M. W. Ding, Y. F. Chen and N. Y. Huang, Eur. J. Org. Chem., 2004, 3872 CrossRef CAS.
- J. F. Zhao, C. Xie, M. W. Ding and H. W. He, Chem. Lett., 2005, 34, 1022 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2006 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(4), C(5)
N(4), C(5)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(6), and C(1)
N(6), and C(1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(2) are 1.307(3) Å, 1.311(3) Å and 1.386(3) Å, are longer than that of the typical C
C(2) are 1.307(3) Å, 1.311(3) Å and 1.386(3) Å, are longer than that of the typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1.28 Å) and C
N (1.28 Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1.34 Å) bonds respectively, while the single bond lengths of C(1)–N(1), C(2)–N(3), C(1)–N(4), C(4)–N(5), C(4)–N(7), N(7)–C(5) and C(2)–C(3) are 1.354(3) Å, 1.371(3) Å, 1.361(3) Å, 1.370(3) Å, 1.368(3) Å, 1.389(3) Å and 1.435(3) Å respectively, are significantly shorter than the typical C(sp2)–N(1.426 Å) and C–C(1.53 Å), showing a degree of delocalization.
C (1.34 Å) bonds respectively, while the single bond lengths of C(1)–N(1), C(2)–N(3), C(1)–N(4), C(4)–N(5), C(4)–N(7), N(7)–C(5) and C(2)–C(3) are 1.354(3) Å, 1.371(3) Å, 1.361(3) Å, 1.370(3) Å, 1.368(3) Å, 1.389(3) Å and 1.435(3) Å respectively, are significantly shorter than the typical C(sp2)–N(1.426 Å) and C–C(1.53 Å), showing a degree of delocalization.![ORTEP diagram of the crystal structure of tricyclic compound 10g (50% thermal ellipsoids). For clarity, solvent molecular (DMF) and all hydrogen atoms have been omitted. Select bond lengths [Å]: C(1)–N(1) 1.354(3), N(1)–N(2) 1.377(3), N(2)–N(3) 1.304(3), C(2)–N(3) 1.371(3), C(1)–C(2) 1.386(3), C(2)–C(3) 1.435(3), C(3)–N(5) 1.420(3), C(4)–N(5) 1.370(3), C(4)–N(4) 1.307(3), C(1)–N(4) 1.361(3), N(5)–N(6) 1.403(3), C(5)–N(6) 1.311(3), C(5)–N(7) 1.389(3), C(4)–N(7) 1.368(3), C(3)–O(1) 1.211(3), C(5)–N(8) 1.347(3), C(6)–N(8) 1.414(3). Selected bond angles [deg]: N(3)–N(2)–N(1) 108.3(2), C(1)–N(1)–N(2) 109.8(2), N(1)–C(1)–C(2) 104.5(2), N(2)–N(1)–C(19) 120.9(2), C(1)–C(2)–C(3) 121.7(2), N(5)–C(3)–C(2) 107.5(2), C(4)–N(5)–C(3) 124.9(2), N(4)–C(4)–N(5) 128.5(2), C(4)–N(4)–C(1) 108.3(2), N(4)–C(1)–C(2) 129.0(2), C(4)–N(5)–N(6) 112.23(18), C(5)–N(6)–N(5) 103.03(19), N(6)–C(5)–N(7) 112.9(2), C(4)–N(7)–C(5) 107.0(2), N(7)–C(4)–N(5) 104.8(2), C(4)–N(7)–C(12) 123.9(2).CCDC reference number 279775. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513715b](/image/article/2006/OB/b513715b/b513715b-f1.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1542, 1203; 1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 7.19–8.15 (m, 10H), 8.94 (s, 1H); MS m/z (%) 319 (M+, 9), 291 (2), 274 (2), 118 (18), 77 (100); Anal. Calcd for C16H13N7O: C, 60.18; H, 4.10; N, 30.70. Found: C, 60.35; H, 4.03; N, 30.93%.
O), 1542, 1203; 1H NMR (400 MHz, CDCl3) δ 4.75 (s, 2H), 7.19–8.15 (m, 10H), 8.94 (s, 1H); MS m/z (%) 319 (M+, 9), 291 (2), 274 (2), 118 (18), 77 (100); Anal. Calcd for C16H13N7O: C, 60.18; H, 4.10; N, 30.70. Found: C, 60.35; H, 4.03; N, 30.93%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1559, 1204; 1H NMR (400 MHz, DMSO-d6) δ 5.75 (s, 2H), 7.43–8.04 (m, 9H), 10.01 (s, 1H); MS m/z (%) 355/353 (M+, 15/45), 325 (12), 309 (5), 111 (23), 77 (100); Anal. Calcd for C16H12ClN7O: C, 54.32; H, 3.42; N, 27.71. Found: C, 54.47; H, 3.64; N, 27.57%.
O), 1559, 1204; 1H NMR (400 MHz, DMSO-d6) δ 5.75 (s, 2H), 7.43–8.04 (m, 9H), 10.01 (s, 1H); MS m/z (%) 355/353 (M+, 15/45), 325 (12), 309 (5), 111 (23), 77 (100); Anal. Calcd for C16H12ClN7O: C, 54.32; H, 3.42; N, 27.71. Found: C, 54.47; H, 3.64; N, 27.57%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1556, 1205; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 5.74 (s, 2H), 7.18–8.07 (m, 9H), 9.80 (s, 1H); MS m/z (%) 333 (M+, 57), 305 (19), 289 (15), 131 (26), 77 (100); Anal. Calcd for C17H15N7O: C, 61.25; H, 4.54; N, 29.41. Found: C, 61.08; H, 4.69; N, 29.64%.
O), 1556, 1205; 1H NMR (400 MHz, DMSO-d6) δ 2.30 (s, 3H), 5.74 (s, 2H), 7.18–8.07 (m, 9H), 9.80 (s, 1H); MS m/z (%) 333 (M+, 57), 305 (19), 289 (15), 131 (26), 77 (100); Anal. Calcd for C17H15N7O: C, 61.25; H, 4.54; N, 29.41. Found: C, 61.08; H, 4.69; N, 29.64%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1550, 1110; 1H NMR (CDCl3, 400 MHz) δ: 7.12–8.18 (m, 25H), 9.90 (s, 1H); MS m/z (%) 579 (M+, 62), 551 (8), 262 (74), 183 (83), 77 (100); Anal. Calcd for C34H26N7OP: C, 70.46; H, 4.52; N, 16.92. Found: C, 70.68; H, 4.37; N, 16.75%.
O), 1550, 1110; 1H NMR (CDCl3, 400 MHz) δ: 7.12–8.18 (m, 25H), 9.90 (s, 1H); MS m/z (%) 579 (M+, 62), 551 (8), 262 (74), 183 (83), 77 (100); Anal. Calcd for C34H26N7OP: C, 70.46; H, 4.52; N, 16.92. Found: C, 70.68; H, 4.37; N, 16.75%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1549, 1107; 1H NMR (CDCl3, 400 MHz) δ: 7.26–8.14 (m, 24H), 9.93 (s, 1H); MS m/z (%) 615/613 (M+, 3/9), 276 (16), 262 (24), 183 (78), 77 (100); Anal. Calcd for C34H25ClN7OP: C, 66.51; H, 4.10; N, 15.97. Found: C, 66.35; H, 4.35; N, 15.84%.
O), 1549, 1107; 1H NMR (CDCl3, 400 MHz) δ: 7.26–8.14 (m, 24H), 9.93 (s, 1H); MS m/z (%) 615/613 (M+, 3/9), 276 (16), 262 (24), 183 (78), 77 (100); Anal. Calcd for C34H25ClN7OP: C, 66.51; H, 4.10; N, 15.97. Found: C, 66.35; H, 4.35; N, 15.84%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1557, 1107; 1H NMR (CDCl3, 400 MHz) δ: 2.36 (s, 3H), 7.17–8.19 (m, 24H), 9.79 (s, 1H); MS m/z (%) 593 (M+, 28), 304 (40), 262 (60), 183 (100), 77 (95); Anal. Calcd for C35H28N7OP: C, 70.82; H, 4.75; N, 16.52. Found: C, 70.75; H, 4.76; N, 16.68%.
O), 1557, 1107; 1H NMR (CDCl3, 400 MHz) δ: 2.36 (s, 3H), 7.17–8.19 (m, 24H), 9.79 (s, 1H); MS m/z (%) 593 (M+, 28), 304 (40), 262 (60), 183 (100), 77 (95); Anal. Calcd for C35H28N7OP: C, 70.82; H, 4.75; N, 16.52. Found: C, 70.75; H, 4.76; N, 16.68%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1399; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.95 (m, 15H), 9.16 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ 119.8, 123.5 (2), 124.6, 125.7 (2), 127.8 (2), 129.5 (2), 129.8 (2), 130.2, 130.4, 132.3 (2), 134.1, 134.9, 135.8, 147.7, 148.3, 150.2, 150.4; MS m/z (%) 420 (M+, 26), 392 (10), 260 (14), 245 (16), 77 (100); Anal. Calcd for C23H16N8O: C, 65.71; H, 3.84; N, 26.65. Found: C, 65.94; H, 3.97; N, 26.58%.
O), 1544, 1399; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.95 (m, 15H), 9.16 (s, 1H); 13C NMR (DMSO-d6, 100 MHz) δ 119.8, 123.5 (2), 124.6, 125.7 (2), 127.8 (2), 129.5 (2), 129.8 (2), 130.2, 130.4, 132.3 (2), 134.1, 134.9, 135.8, 147.7, 148.3, 150.2, 150.4; MS m/z (%) 420 (M+, 26), 392 (10), 260 (14), 245 (16), 77 (100); Anal. Calcd for C23H16N8O: C, 65.71; H, 3.84; N, 26.65. Found: C, 65.94; H, 3.97; N, 26.58%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 7.42–7.94 (m, 14H), 9.30 (s, 1H); MS m/z (%) 456/454 (M+, 3/9), 426 (6), 274 (13), 258 (10), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.65; H, 3.35; N, 24.52%.
O), 1544, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 7.42–7.94 (m, 14H), 9.30 (s, 1H); MS m/z (%) 456/454 (M+, 3/9), 426 (6), 274 (13), 258 (10), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.65; H, 3.35; N, 24.52%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1540, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.94 (m, 14H), 9.16 (s, 1H); MS m/z (%) 456/454 (M+, 15/44), 426 (17), 294 (15), 273 (38), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.87; H, 3.41; N, 24.47%.
O), 1540, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.08–7.94 (m, 14H), 9.16 (s, 1H); MS m/z (%) 456/454 (M+, 15/44), 426 (17), 294 (15), 273 (38), 77 (100); Anal. Calcd for C23H15ClN8O: C, 60.73; H, 3.32; N, 24.63. Found: C, 60.87; H, 3.41; N, 24.47%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1544, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.44–7.93 (m, 13H), 9.30 (s, 1H); MS m/z (%) 492/490/488 (M+, 3/16/25), 460 (12), 308 (15), 273 (43), 77 (100); Anal. Calcd for C23H14Cl2N8O: C, 56.46; H, 2.88; N, 22.90. Found: C, 56.31; H, 2.90; N, 22.81%.
O), 1544, 1492; 1H NMR (DMSO-d6, 400 MHz) δ: 7.44–7.93 (m, 13H), 9.30 (s, 1H); MS m/z (%) 492/490/488 (M+, 3/16/25), 460 (12), 308 (15), 273 (43), 77 (100); Anal. Calcd for C23H14Cl2N8O: C, 56.46; H, 2.88; N, 22.90. Found: C, 56.31; H, 2.90; N, 22.81%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1541, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.27 (s, 3H), 7.16–7.93 (m, 13H), 9.04 (s, 1H); MS m/z (%) 470/468 (M+, 11/35), 440 (14), 308 (28), 273 (42), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.35; H, 3.52; N, 23.97%.
O), 1541, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.27 (s, 3H), 7.16–7.93 (m, 13H), 9.04 (s, 1H); MS m/z (%) 470/468 (M+, 11/35), 440 (14), 308 (28), 273 (42), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.35; H, 3.52; N, 23.97%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.29 (s, 3H), 6.87–7.94 (m, 13H), 9.07 (s, 1H); MS m/z (%) 470/468 (M+, 9/28), 440 (10), 308 (17), 273 (26), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.41; H, 3.78; N, 23.84%.
O), 1543, 1493; 1H NMR (DMSO-d6, 400 MHz) δ: 2.29 (s, 3H), 6.87–7.94 (m, 13H), 9.07 (s, 1H); MS m/z (%) 470/468 (M+, 9/28), 440 (10), 308 (17), 273 (26), 77 (100); Anal. Calcd for C24H17ClN8O: C, 61.48; H, 3.65; N, 23.90. Found: C, 61.41; H, 3.78; N, 23.84%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1457; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.54 (s, 3H), 6.88–7.82 (m, 14H); 13C NMR (CDCl3–TFA, 100 MHz) δ 21.1, 119.4, 123.0 (2), 124.4, 125.4 (2), 127.7 (2), 129.5 (2), 129.9 (2), 130.0, 130.4, 132.1 (2), 134.3, 135.6, 143.7, 147.8, 148.4, 150.3, 150.6; MS m/z (%) 434 (M+, 42), 406 (19), 288 (22), 274 (17), 77 (100); Anal. Calcd for C24H18N8O: C, 66.35; H, 4.18; N, 25.79. Found: C, 66.15; H, 4.10; N, 25.88%.
O), 1543, 1457; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.54 (s, 3H), 6.88–7.82 (m, 14H); 13C NMR (CDCl3–TFA, 100 MHz) δ 21.1, 119.4, 123.0 (2), 124.4, 125.4 (2), 127.7 (2), 129.5 (2), 129.9 (2), 130.0, 130.4, 132.1 (2), 134.3, 135.6, 143.7, 147.8, 148.4, 150.3, 150.6; MS m/z (%) 434 (M+, 42), 406 (19), 288 (22), 274 (17), 77 (100); Anal. Calcd for C24H18N8O: C, 66.35; H, 4.18; N, 25.79. Found: C, 66.15; H, 4.10; N, 25.88%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1545, 1510; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.15 (s, 3H), 2.54 (s, 3H), 6.70–7.82 (m, 13H); 13C NMR (CDCl3–TFA, 100 MHz) δ 20.6, 21.1, 122.8 (2), 124.1, 125.3 (2), 127.5 (2), 128.4, 129.2 (2), 129.7 (2), 129.8, 130.2, 132.0 (2), 134.0, 135.5, 143.8, 147.7, 148.2, 150.1, 150.4; MS m/z (%) 448 (M+, 42), 420 (19), 288 (40), 273 (16), 91 (100). Anal. Calcd for C25H20N8O: C, 66.95; H, 4.49; N, 24.98; Found: C, 66.84; H, 4.57; N, 25.05%.
O), 1545, 1510; 1H NMR (CDCl3–TFA, 400 MHz) δ: 2.15 (s, 3H), 2.54 (s, 3H), 6.70–7.82 (m, 13H); 13C NMR (CDCl3–TFA, 100 MHz) δ 20.6, 21.1, 122.8 (2), 124.1, 125.3 (2), 127.5 (2), 128.4, 129.2 (2), 129.7 (2), 129.8, 130.2, 132.0 (2), 134.0, 135.5, 143.8, 147.7, 148.2, 150.1, 150.4; MS m/z (%) 448 (M+, 42), 420 (19), 288 (40), 273 (16), 91 (100). Anal. Calcd for C25H20N8O: C, 66.95; H, 4.49; N, 24.98; Found: C, 66.84; H, 4.57; N, 25.05%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1548, 1510; 1H NMR (400 MHz, CDCl3) δ 7.36–7.59 (m, 13H), 8.02 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 121.4, 123.0, 124.2, 126.7 (2), 128.4 (2), 128.6 (2), 128.8 (2), 129.2, 129.5 (2), 130.7 (2), 132.0, 132.4, 134.4, 141.0, 148.6, 150.2, 150.4, 153.2; MS m/z (%) 405 (M+, 6), 377 (5), 245 (7), 103 (30), 77 (100); Anal. Calcd for C23H15N7O: C, 68.14; H, 3.73; N, 24.18. Found: C, 68.25; H, 3.81; N, 24.05%.
O), 1548, 1510; 1H NMR (400 MHz, CDCl3) δ 7.36–7.59 (m, 13H), 8.02 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 121.4, 123.0, 124.2, 126.7 (2), 128.4 (2), 128.6 (2), 128.8 (2), 129.2, 129.5 (2), 130.7 (2), 132.0, 132.4, 134.4, 141.0, 148.6, 150.2, 150.4, 153.2; MS m/z (%) 405 (M+, 6), 377 (5), 245 (7), 103 (30), 77 (100); Anal. Calcd for C23H15N7O: C, 68.14; H, 3.73; N, 24.18. Found: C, 68.25; H, 3.81; N, 24.05%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1557, 1498; 1H NMR (400 MHz, CDCl3–TFA) δ 2.52 (s, 3H), 7.46–7.89 (m, 10H); 13C NMR (CDCl3–TFA, 100 MHz) δ 11.9, 121.7 (2), 125.0, 127.0 (2), 129.0, 129.4 (2), 130.4 (2), 130.5, 131.0, 135.0, 148.4, 149.6, 150.1, 153.0; MS m/z (%) 343 (M+, 23), 315 (16), 286 (16), 245 (9), 77 (100); Anal. Calcd for C18H13N7O: C, 62.97; H, 3.82; N, 28.56. Found: C, 62.75; H, 3.91; N, 28.36%.
O), 1557, 1498; 1H NMR (400 MHz, CDCl3–TFA) δ 2.52 (s, 3H), 7.46–7.89 (m, 10H); 13C NMR (CDCl3–TFA, 100 MHz) δ 11.9, 121.7 (2), 125.0, 127.0 (2), 129.0, 129.4 (2), 130.4 (2), 130.5, 131.0, 135.0, 148.4, 149.6, 150.1, 153.0; MS m/z (%) 343 (M+, 23), 315 (16), 286 (16), 245 (9), 77 (100); Anal. Calcd for C18H13N7O: C, 62.97; H, 3.82; N, 28.56. Found: C, 62.75; H, 3.91; N, 28.36%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1535, 1494; 1H NMR (400 MHz, CDCl3–TFA) δ 7.25–7.59 (m, 12H), 7.96 (d, J = 8.4 Hz, 2H); MS m/z (%) 441/439 (M+, 6/20), 411 (16), 279 (22), 111 (69), 77 (100); Anal. Calcd for C23H14ClN7O: C, 62.80; H, 3.21; N, 22.29. Found: C, 62.73; H, 3.15; N, 22.33%.
O), 1535, 1494; 1H NMR (400 MHz, CDCl3–TFA) δ 7.25–7.59 (m, 12H), 7.96 (d, J = 8.4 Hz, 2H); MS m/z (%) 441/439 (M+, 6/20), 411 (16), 279 (22), 111 (69), 77 (100); Anal. Calcd for C23H14ClN7O: C, 62.80; H, 3.21; N, 22.29. Found: C, 62.73; H, 3.15; N, 22.33%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1554, 1509; 1H NMR (400 MHz, CDCl3/TFA) δ 2.49 (s, 3H), 7.42–7.92 (m, 10H); MS m/z (%) 379/377 (M+, 8/27), 349 (19), 320 (19), 273 (21), 111 (100); Anal. Calcd for C18H12ClN7O: C, 57.23; H, 3.20; N, 25.95. Found: C, 57.47; H, 3.15; N, 25.87%.
O), 1554, 1509; 1H NMR (400 MHz, CDCl3/TFA) δ 2.49 (s, 3H), 7.42–7.92 (m, 10H); MS m/z (%) 379/377 (M+, 8/27), 349 (19), 320 (19), 273 (21), 111 (100); Anal. Calcd for C18H12ClN7O: C, 57.23; H, 3.20; N, 25.95. Found: C, 57.47; H, 3.15; N, 25.87%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1543, 1510; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.26–8.09 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ 21.1, 121.8, 123.2, 124.8, 127.0 (2), 128.8 (2), 128.9 (2), 129.0 (2), 129.3, 129.5 (2), 130.9 (2), 132.3, 134.9, 141.4, 148.5, 150.2, 150.5, 153.3; MS m/z (%) 419 (M+, 90), 391 (61), 262 (24), 259 (80), 91 (100); Anal. Calcd for C24H17N7O: C, 68.73; H, 4.09; N, 23.38. Found: C, 68.80; H, 4.16; N, 22.36%.
O), 1543, 1510; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.26–8.09 (m, 14H); 13C NMR (CDCl3, 100 MHz) δ 21.1, 121.8, 123.2, 124.8, 127.0 (2), 128.8 (2), 128.9 (2), 129.0 (2), 129.3, 129.5 (2), 130.9 (2), 132.3, 134.9, 141.4, 148.5, 150.2, 150.5, 153.3; MS m/z (%) 419 (M+, 90), 391 (61), 262 (24), 259 (80), 91 (100); Anal. Calcd for C24H17N7O: C, 68.73; H, 4.09; N, 23.38. Found: C, 68.80; H, 4.16; N, 22.36%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1558, 1517; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 2.51 (s, 3H), 7.34–8.05 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ 11.9, 21.2, 122.2 (2), 124.7, 126.7 (2), 127.6, 129.5 (2), 129.6, 131.1 (2), 134.7, 141.9, 148.5, 149.7, 150.2, 153.6; MS m/z (%) 357 (M+, 70), 329 (49), 288 (21), 259 (26), 91 (100). Anal. Calcd for C19H15N7O: C, 63.86; H, 4.23; N, 27.44. Found: C, 63.73; H, 4.36; N, 27.31%.
O), 1558, 1517; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 2.51 (s, 3H), 7.34–8.05 (m, 9H); 13C NMR (CDCl3, 100 MHz) δ 11.9, 21.2, 122.2 (2), 124.7, 126.7 (2), 127.6, 129.5 (2), 129.6, 131.1 (2), 134.7, 141.9, 148.5, 149.7, 150.2, 153.6; MS m/z (%) 357 (M+, 70), 329 (49), 288 (21), 259 (26), 91 (100). Anal. Calcd for C19H15N7O: C, 63.86; H, 4.23; N, 27.44. Found: C, 63.73; H, 4.36; N, 27.31%.
