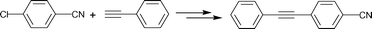One-pot Pd/C catalysed ‘domino’ HALEX and Sonogashira reactions: a ligand- and Cu-free alternative
Mehul B. Thathagar and Gadi Rothenberg*
van't Hoff Institute for Molecular Sciences, University of Amsterdam, Nieuwe Achtergracht 166, 1018 WV, Amsterdam, The Netherlands. E-mail: gadi@science.uva.nl; Fax: +31 20 525 5604
First published on 23rd November 2005
Abstract
The advantages of combining heterogeneous catalysis and aryl chloride substrates for cross-coupling are introduced. A heterogeneous Pd/C catalyst is used for activating aryl bromides and electron withdrawing aryl chlorides via a one-pot ‘domino’ HALEX–Sonogashira reaction. No ligand or co-catalyst is required, and the cross-coupling products are obtained in moderate to good yields. The influence of the solvent, base, iodide source and catalyst is evaluated. The catalyst is reusable for at least six consecutive reaction cycles. A variation on this reaction using catalytic amounts of KI is also proposed.
Introduction
The palladium and copper co-catalyzed coupling of terminal alkynes with aryl halides (the Sonogashira reaction) is one of the most widely used tools to form C–C bonds.1 It provides an efficient route to aryl alkyne derivatives, which are useful in diverse areas ranging from natural product chemistry2 to materials science.3Since its discovery, a variety of modifications were reported for this reaction, including microwave conditions4,5 and Pd-free,6–11 Cu-free,5,12–16 and ligand-free versions.8,17,18 However, in most studies aryl iodides are used as coupling partners to the alkynes. For practical purposes, the less reactive bromo- and chloro-aryls are attractive because of their lower cost and wider availability compared to iodides.19 The few efficient protocols reported for coupling of these substrates rely heavily on the use of electron-rich Pd complexes with or without CuI co-catalyst.20–26 These homogeneous Pd catalysts are laborious to synthesize, and their separation from the product mixture is often difficult and costly. Recently, we discovered ligand-free Cu and Cu/Pd nanoparticles as catalysts for Sonogashira11 and Suzuki coupling reactions.27,28 While they are highly active, the separation and recovery of these nanoparticles is tedious and may result in Pd-contaminated products. This problem can be overcome by using easily separable supported catalysts that are extensively studied for Heck and Suzuki reactions.29 Nevertheless, only a few reports describe the Sonogashira reaction catalyzed by these catalysts.15,18,30–32 Kotschy and co-workers31 have shown that the heterogeneous system (5 mol% Pd/C + 10 mol% CuI + 10 mol% PPh3) is active for coupling of aryl bromides and two activated chlorides with alkynes. However, its success remained linked to the use of CuI as co-catalyst, that also catalyzes the undesired oxidative homocoupling (Hay coupling) of terminal alkynes to the corresponding diyne.33,34 A Cu-free Pd-modified zeolite catalyst was also recently applied to couple non-activated bromides,18 but the yields were moderate (20–45%). Therefore, an efficient ‘Pd/C only’ catalyst system for coupling of aryl halides other than iodides would be of major interest for both industrial and academic applications.
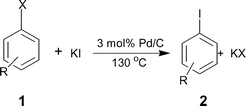
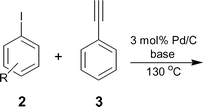
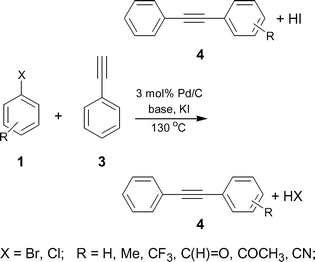
One of the possible ways of activating these halides is to use the halogen exchange (HALEX) reaction35,36 [eqn (1)] prior to Sonogashira coupling [eqn (2)]. In this two-step system, the aryl chloride is first converted to the reactive iodide that can then readily couple with the alkyne. However, for economical and environmental reasons it is essential to reduce the number of reaction steps for any synthesis. To shorten the synthetic procedure the promising strategy is to carry out a one pot sequential reaction, commonly referred to as a ‘multistep one-pot reaction’.37,38 Such reactions are attractive because they minimize waste and reduce processing times. We report here a ligand-free and Cu-free heterogeneous Pd/C catalyst to activate aryl bromides and chlorides using a ‘domino’ HALEX–Sonogashira reaction [eqn (3)].
 | (1) |
 | (2) |
 | (3) |
Results and discussion
In 1988, Bozell and Vogt39 reported an interesting two stage process where they activated chloroarenes using NaI for Heck reactions catalyzed by bimetallic Pd(dba)2–NiBr2. Inspired by this system, we tried the HALEX reaction to activate 4-chlorobenzonitrile 1 using KI as the iodide source in the presence of Pd/C catalyst. A mixture containing 1, 3 equiv. KI and 0.03 equiv. Pd/C (pellets) in DMF was heated at 130 °C under N2 for 48 h. The reaction progress was monitored by GC. We were excited to observe 64% conversion of 1 to 4-iodobenzonitrile 2 after 48 h. Further reaction for 24 h did not improve the yield. No conversion was observed at 110 °C, even after 3 d.In another experiment we carried out the Sonogashira coupling of 2 with phenylacetylene 3 using the same catalyst and solvent. In a typical reaction, 1.5 equiv. 3 and one equiv. aryl halide 2 were mixed in the presence of 2.5 equiv. KF and 0.03 equiv. Pd/C pellets at 130 °C under N2. A quantitative yield of 4-cyanodiphenylacetylene 4 was obtained within 8 h. After reaction completion, the catalyst was separated and the substituted diphenylacetylene 4 was isolated and analyzed by GC/MS.
These reactions can be considered as a sequential two-step procedure to obtain a Sonogashira coupling product. In principle, it should be possible to carry out a one pot reaction, where the in situ formed aryl iodide (via HALEX reaction) can readily couple with phenylacetylene to give the desired coupling product. We then examined this possibility using aryl halide 1, alkyne 3, KI, KF and Pd/C in DMF under similar reaction conditions and molar ratios of reagents as mentioned above. Note that no ligands or Cu co-catalysts were used as opposed to the Pd/C system described recently.31 After 48 h, we obtained a 34% yield of the substituted diphenylacetylene 4. Additionally, 2–3% dehalogenation of aryl halide and 5–6% homocoupling of phenylacetylene was observed. However, we did not observe any aryl fluoride, which can form via halogen exchange between aryl chlorides and KF in presence of phase transfer agents.40 Note that when the stirring rate was kept below 300 rpm the reaction failed, probably due to mass transfer limitations. Therefore, all further reactions were carried out at a sufficiently high stirring rate.
To optimize our system and find an efficient protocol for Sonogashira coupling, we studied the effect of various parameters such as the type of base, presence of water, iodide source and catalyst. The coupling of 4-chlorobenzonitrile 1 with phenylacetylene 3 to give diphenylacetylene 4 was used as a model reaction. Table 1 shows the results obtained for various bases, amount of water added and different iodide sources. Choosing the right base is crucial for a successful reaction. We obtained low yields with inorganic bases such as KOH, NaOH, acetate and carbonate, both in presence and absence of water (entries 1–5). Surprisingly, the most commonly used bases, tetrabutylammonium acetate (TBAA) and Et3N were not effective (entries 7 and 8). The best results were obtained with tetrabutylammonium fluoride (TBAF) and KF (entries 6 and 9). This indicates that the presence of fluorides is necessary to achieve good yields.
| ||||
|---|---|---|---|---|
| Entry | Iodide source | Base | Solvent | Yield of 4 (%)b |
| a Reaction conditions: 1.0 mmol 4-chlorobenzonitrile, 1.5 mmol phenylacetylene, 2.5 mmol base, 3.0 mmol iodide source, 3 mol% Pd/C, 5 mL solvent, N2 atmosphere, 130 °C, 48 h (the reaction time was not optimised).b GC yield of 4, corrected for the presence of an internal standard.c 5–8% dehalogenation of 1 was also observed.d Complete conversion of phenylacetylene to homocoupling product. | ||||
| 1 | KI | KOH | DMF | 15 |
| 2 | KI | KOH | DMF–H2O (1 : 1)c | 22 |
| 3 | KI | NaOH | DMF–H2O (1 : 1)c | 14 |
| 4 | KI | NaOAc | DMF–H2O (1 : 1)c | 8 |
| 5 | KI | K2CO3 | DMF–H2O (1 : 1)c | 10 |
| 6 | KI | TBAF | DMF | 51 |
| 7 | KI | TBAA | DMF | 6 |
| 8 | KI | Et3N | DMF | 3 |
| 9 | KI | KF | DMF | 34 |
| 10 | KI | KF | DMF–H2O (1 : 1)c | 41 |
| 11 | KI | KF | DMF–H2O (2 : 1)c | 49 |
| 12 | KI | KF | DMF–H2O (3 : 1)c | 58 |
| 13 | KI | KF | H2O | 0 |
| 14 | None | KF | DMF–H2O (3 : 1)c | <1 |
| 15 | NaI | KF | DMF–H2O (3 : 1)c | 43 |
| 16 | TBAI | KF | DMF | 2 |
| 17 | CuI | KF | DMF–H2O (3 : 1)c | 4d |
We used a DMF–H2O mixture to dissolve the bases that were not soluble in pure DMF, assuming that this might improve the yield. Indeed, in the case of KF the yield of 4 was nearly doubled when the DMF–H2O ratio was maintained at 3 : 1 (Table 1, entry 12). However, increasing the amount of water decreased the yield and the reaction in pure water failed completely (entries 10, 11 and 13). This agrees with the trend reported for Sonogashira coupling using DMF–H2O solvent in presence of heterogeneous Pd-modified zeolites.18 We also observed that the presence of water led to 5–8% dehalogenation, giving 4-cyanobenzene. Arai and Zhao41 observed a significant amount of dehalogenation of chlorobenzene in the Heck reaction using a Pd/C catalyst. They suggested that this reaction is likely to occur heterogeneously on the surface of Pd/C, whereas leached molecular Pd species are responsible for the desired cross-coupling product. This may also apply to our system, with the DMF–H2O combination acting as a source of formic acid and ultimately hydrogen.42–44
Next, we tested different iodide sources for this reaction. KI was the most efficient, followed by NaI, whereas Bu4NI and CuI gave less than 5% yield. In the case of CuI the starting alkyne 3 was completely consumed via the competitive Hay coupling pathway. Control experiments confirmed that no product was formed without iodide source, affirming that the cross-coupling proceeds via a HALEX reaction.
We also screened several commercially available catalysts and co-catalyst combinations (Table 2). Nevertheless, among the screened catalysts only 5 wt% Pd/C (pellets, from Ventron) A1 was effective (entry 1). It is known that a CuI co-catalyst can enhance the product yield for Sonogashira coupling of bromides and/or chlorides. In our case, however, we observed a deleterious effect on the yield in the presence of 5 mol% CuI, with formation of 45–50% of the homocoupling (Hay coupling) product (entry 2). A similar suppression of catalytic activity and oligomerisation/dimerisation of alkyne was also reported elsewhere.18,26,45 NiCl2 is generally used as a catalyst for activating chlorides towards the HALEX reaction,35 but using it along with A1 too did not improve the yield (entry 3). 5 wt% Pd/C (powder, from Fluka) A2 gave only moderate yield (entry 4). This was surprising because one imagines that powdered catalysts should offer less diffusion resistance for the reactants compared to pellets, leading to higher activity. We measured the surface areas of both the catalysts and found that it was higher for catalyst A1 (1039 m2 g−1) compared to A2 (824 m2 g−1), which explains our results. Other catalysts gave very low yields (entries 5–9).
| Entry | Catalyst (3 mol%) | Yield of 4 (%)b |
|---|---|---|
| a Reaction conditions: 1.0 mmol chlorobenzonitrile, 1.5 mmol phenylacetylene, 2.5 mmol KF, 3.0 mmol KI, 5 mL DMF–H2O (3 : 1), N2 atmosphere, 130 °C, 48 h (the reaction time was not optimised).b GC yield of 4, corrected for the presence of an internal standard.c Commercial catalyst (Alfa-Ventron).d 45–50% of homocoupling product was also observed.e Commercial catalysts (Fluka, Pd/Al2O3 # 76002; Pd/BaSO4 # 76022). | ||
| 1 | Pd/C (pellets) A1 | 58c |
| 2 | A1 + 5 mol% CuI | 20d |
| 3 | A1 + 5 mol% NiCl2 | 41 |
| 4 | Pd/C (powder) A2 | 18 |
| 5 | Pd(dba)2 + 5 mol% CuI | 12d |
| 6 | Pd/Al2O3 | 5e |
| 7 | Pd/BaSO4 | 0e |
| 8 | Pd(OAc)2 | 4 |
We then examined the scope and limitations of this ‘domino’ HALEX–Sonogashira protocol. As TBAF and KF were equally effective, various substrates were tested with both bases (Table 3). We were able to couple aryl chlorides with phenylacetylene in moderate to good yields and bromides in good to excellent yields. In the case of aryl chlorides, the highest yield (68%) was observed for p-CF3–C6H4–Cl (entry 2), reflecting the strong electron withdrawing effect. The decrease in the yields of aryl chlorides was in accordance with the decrease in the strength of the electron withdrawing group (entries 1–4). Our attempts to couple electron-neutral and electron-rich aryl chlorides failed even with 5 mol% Pd/C. For aryl bromides, a quantitative yield for the electron-neutral p-C6H5–Br (entry 7) and a good yield for the moderately electron-donating p-CH3–C6H4–Br (entry 9) were obtained. Little or no conversion was observed using aryl bromides with electron donating substituents. This shows that the HALEX step is crucial (cf. entries 4 and 8). Both bases gave similar yields for all the substrates. Control experiments carried out using chlorobenzene and KI in the presence of Pd/C at 130 °C confirmed that no HALEX product (iodobenzene) was formed. This is the reason for the failure of subsequent Sonogashira reaction when using deactivated aryl chlorides. Although the yields are not excellent, they are higher than any reported for heterogeneously catalyzed Sonogashira coupling of aryl chlorides. Moreover, this is the first demonstration of activating aryl chlorides using a ligand-free and Cu-free Pd/C catalyst.
| Entry | Substrate | Yield (%),b KF based | Yield (%),b TBAF basee |
|---|---|---|---|
| a Reaction conditions: 1.0 mmol substrate, 1.5 mmol phenylacetylene, 2.5 mmol base, 3.0 mmol KI, 3 mol% Pd/C, solvent 5 mL, N2 atmosphere, 130 °C, 48 h (time was not optimised).b GC yield of 4, corrected for the presence of an internal standard.c Isolated yields.d DMF–H2O (3 : 1) as solvent.e Pure DMF as solvent. | |||
| 1 | 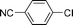 | 58 (43c) | 51 |
| 2 | 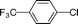 | 68 | 62 |
| 3 | 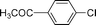 | 31 | 35 |
| 4 | 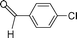 | 20 | 18 |
| 5 | 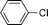 | 0 | 0 |
| 6 | 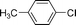 | 0 | 0 |
| 7 | 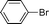 | >99 (82c) | 82 |
| 8 | 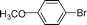 | 8 | 0 |
| 9 | 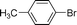 | 62 | 46 |
The reusability of the Pd/C A1 catalyst was also examined for the coupling of 1 with 3 using KF as the base in DMF–H2O (3 : 1). After the first run the catalyst was separated by simple vacuum filtration, washed with acetone and dried at 50 °C under vacuum for 4 h. The dried catalyst was then reused without any further activation. The same procedure was repeated for all further cycles (Fig. 1). The catalyst showed a slight decrease in the activity after the first cycle, but retained its activity in all further cycles.
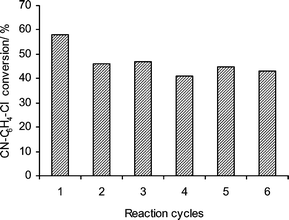 | ||
| Fig. 1 Recycling experiments for Pd/C in the HALEX–Sonogashira system. | ||
An attractive variation on this ‘domino’ process would be to use KI in catalytic amounts. If we can initiate the HALEX reaction using a catalytic amount of KI, the resulting iodide 2 would then readily react with 3 to give the Sonogashira product 4 plus HI. This acid could be neutralized by excess KOH releasing KI back to the system and closing the cycle (Scheme 1). This cycle can continue until the aryl chloride is consumed. We investigated this approach under various conditions but unfortunately we failed as yet to obtain any Sonogashira product. Although in theory the acid–base reaction step looks simple, it is possible that under our reaction conditions this step fails. One reason for this may be the low solubility of KOH and/or the reactants in the DMF–H2O (3 : 1) solvent. We could not find an optimum ratio of DMF–H2O where both KOH and the reactants were completely soluble. We are currently investigating this problem.
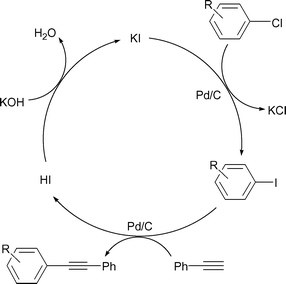 | ||
| Scheme 1 A variation on the one-pot HALEX–Sonogashira reaction using catalytic KI. | ||
In conclusion, we have demonstrated that it is possible to activate aryl chlorides and bromides via a one-pot HALEX–Sonogashira process, using heterogeneous Pd/C catalysts under ligand-free and copper-free conditions. The yields are good and the catalyst separation is simple and quantitative. This is an attractive alternative to the homogeneous Pd complexes generally used for activating aryl halides.
Experimental
Materials and instrumentation
1H NMR spectra were recorded in CD2Cl2 on a 300 MHz Varian Inova instrument. Chemical shift values are in ppm relative to Me4Si. Melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. GC analysis was performed using an Interscience GC-8000 gas chromatograph with a 100% dimethylpolysiloxane capillary column (DB-1, 30 m × 0.325 mm). GC/MS analysis was performed using a Hewlett-Packard 5890/5971 GC/MS equipped with a ZB-5 (zebron) column (15 m × 0.25 mm). All products are known compounds and were identified by comparison of their spectral properties to those of authentic samples. Samples for GC were washed with an equivalent amount of water, extracted with hexanes and filtered through an alumina plug prior to injection. GC conditions: isotherm at 105 °C (2 min); ramp at 30 °C min−1 to 280 °C; isotherm at 280 °C (5 min). All reactions were carried out under N2 atmosphere. 5 wt% Pd/C pellets A1 were obtained from Alfa-Ventron and 5 wt% Pd/C powder A2, 5 wt% Pd/Al2O3 and 10 wt% Pd/BaSO4 were purchased from Fluka. The textural analysis of the catalysts (A1 and A2) was performed by means of nitrogen adsorption at 77 K, using a Sorptomatic 1990 of CE Instruments. The isotherms were both of type I, indicating a microporous structure. The monolayer equivalent surface area was calculated using the Horvath Kawazoe equation.46 All other chemicals were purchased from commercial firms and were used as received.Halogen exchange (HALEX) reaction of 4-chlorobenzonitrile using KI
A Schlenk-type glass reactor equipped with a rubber septum, reflux condenser and a magnetic stirrer was evacuated and refilled with N2. The reactor was then charged with Pd/C (3.0 mol%) and KI (0.50 g, 3.0 mmol) in DMF. 4-Chlorobenzonitrile (1.0 mL, 1.00 M, 1 mmol) was added and the mixture was stirred at 130 °C for 48 h under a slight N2 overpressure. Reaction progress was monitored by GC.Pd/C catalysed Sonogashira coupling of phenylacetylene with iodobenzonitrile
The reactor was charged with Pd/C (3.0 mol% Pd relative to aryl halide), phenylacetylene (2.0 mL, 1.0 M, 1.5 mmol) and KF (0.14 g, 2.5 mmol) in DMF–H2O (1 : 1). 4-Iodobenzonitrile (1.0 mL, 1.00 M, 1 mmol) was added and the mixture was stirred at 130 °C for 8 h under a slight N2 overpressure. Reaction progress was monitored by GC.Parallel screening of catalysts and substrates for the one-pot HALEX–Sonogashira coupling reaction
Sets of 16 reactions were performed using a Chemspeed Smartstart 16-reactor block, modified in-house for efficient reflux and stirring. The reactors were charged with Pd catalysts (3.0 mol%), phenylacetylene (2.0 mL, 1.0 M, 1.5 mmol), base (2.5 mmol), iodide source (3.0 mmol) and aryl halide (1.0 mL, 1.00 M, 1 mmol). The reactors were evacuated and refilled with N2 three times and the mixture was stirred at 130 °C for 48 h under a slight N2 overpressure. Reaction progress was monitored by GC (pentadecane internal standard).Pd/C catalysed one pot HALEX–Sonogashira reaction
Catalyst reusability for coupling of phenylacetylene with 4-chlorobenzonitrile
For reusability studies the catalyst used in a preparative scale was separated using a vacuum filter, washed with acetone (3 × 10 mL) and reused after drying at 50 °C. All further runs were similar to that described for the fresh catalyst. Reaction samples were analysed by GC.Pd/C catalysed ‘domino’ HALEX–Sonogashira reaction using catalytic KI
The reaction set-up and the pre-reaction procedure were similar to that used in the one-pot HALEX and Sonogashira reaction performed on a preparative scale. The reactor was then charged with Pd/C (3.0 mol%), phenylacetylene (0.41 g, 4.0 mmol), KF (0.23 g, 4.0 mmol) and KI (0.02 g, 0.13 mmol, 5 mol%). 4-Chlorobenzonitrile (0.36 g, 2.6 mmol) was added and the mixture was stirred at 130 °C for 48 h under a slight overpressure of N2. Reaction progress was monitored by GC.Acknowledgements
We thank M. C. Mittelmeijer-Hazeleger for measuring the surface area of Pd/C catalysts and for useful discussions.References
- K. Sonogashira, in Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1997, p. 203 Search PubMed.
- (a) H. F. Chow, C. W. Wan, K. H. Low and Y. Y. Yeung, J. Org. Chem., 2001, 66, 1910 CrossRef CAS; (b) I. Paterson, R. D. M. Davies and R. Marquez, Angew. Chem., Int. Ed., 2001, 40, 603 CrossRef CAS; (c) M. Toyota, C. Komori and M. Ihara, J. Org. Chem., 2000, 65, 7110 CrossRef CAS.
- (a) U. H. F. Bunz, Chem. Rev., 2000, 100, 1605 CrossRef CAS; (b) R. L. Wu, J. S. Schumm, D. L. Pearson and J. M. Tour, J. Org. Chem., 1996, 61, 6906 CrossRef CAS.
- M. Erdélyi and A. Gogoll, J. Org. Chem., 2001, 66, 4165 CrossRef CAS.
- N. E. Leadbeater and B. J. Tominack, Tetrahedron Lett., 2003, 44, 8653 CrossRef CAS.
- K. Okuro, M. Furuune, M. Enna, M. Miura and M. Nomura, J. Org. Chem., 1993, 58, 4716 CrossRef CAS.
- R. K. Gujadhur, C. G. Bates and D. Venkataraman, Org. Lett., 2001, 3, 4315 CrossRef CAS.
- S. K. Kang, S. K. Yoon and Y. M. Kim, Org. Lett., 2001, 3, 2697 CrossRef CAS.
- S. Cacchi, G. Fabrizi and L. M. Parisi, Org. Lett., 2003, 5, 3843 CrossRef CAS.
- I. P. Beletskaya, G. V. Latyshev, A. V. Tsvetkov and N. V. Lukashev, Tetrahedron Lett., 2003, 44, 5011 CrossRef CAS.
- M. B. Thathagar, J. Beckers and G. Rothenberg, Green Chem., 2004, 6, 215 RSC.
- D. Méry, K. Héuze and D. Astruc, Chem. Commun., 2003, 1934 RSC.
- X. Fu, S. Zhang, J. Yin and D. P. Schumacher, Tetrahedron Lett., 2002, 43, 6673 CrossRef CAS.
- D. A. Alonso, C. Nájera and M. C. Pacheco, Tetrahedron Lett., 2002, 43, 9365 CrossRef CAS.
- B. M. Choudary, S. Madhi, N. S. Chowdari, M. L. Kantam and B. Sreedhar, J. Am. Chem. Soc., 2002, 124, 14127 CrossRef CAS.
- V. P. W. Böhm and W. A. Herrmann, Eur. J. Org. Chem., 2000, 3679 CrossRef CAS.
- S. Urgaonkar and J. G. Verkade, J. Org. Chem., 2004, 69, 5752 CrossRef CAS.
- L. Djakovitch and P. Rollet, Adv. Synth. Catal., 2004, 346, 1782 CrossRef CAS.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176 CrossRef CAS.
- M. Alami, B. Crousse and F. Ferri, J. Organomet. Chem., 2001, 624, 114 CrossRef CAS.
- M. Eckhardt and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 13642 CrossRef CAS.
- M. Feuerstein, H. Doucet and M. Santelli, Tetrahedron Lett., 2005, 46, 1717 CrossRef CAS.
- A. C. Hillier, G. A. Grasa, M. S. Viciu, H. M. Lee, C. L. Yang and S. P. Nolan, J. Organomet. Chem., 2002, 653, 69 CrossRef CAS.
- T. Hundertmark, A. F. Littke, S. L. Buchwald and G. C. Fu, Org. Lett., 2000, 2, 1729 CrossRef CAS.
- A. Köllhofer, T. Pullmann and H. Plenio, Angew. Chem., Int. Ed., 2003, 42, 1056 CrossRef CAS.
- D. Gelman and S. L. Buchwald, Angew. Chem., Int. Ed., 2003, 42, 5993 CrossRef CAS.
- M. B. Thathagar, J. Beckers and G. Rothenberg, J. Am. Chem. Soc., 2002, 124, 11858 CrossRef CAS.
- M. B. Thathagar, J. Beckers and G. Rothenberg, Adv. Synth. Catal., 2003, 345, 979 CrossRef CAS.
- (a) A. Papp, K. Miklos, M. Forgo and A. Molnar, J. Mol. Catal. A: Chem., 2005, 229, 107 CrossRef CAS; (b) J. Horniakova, T. Raja, Y. Kubota and Y. Sugi, J. Mol. Catal. A: Chem., 2004, 217, 73 CrossRef CAS; (c) A. Corma, H. Garcia, A. Leyva and A. Primo, Appl. Catal., A, 2004, 257, 77 CrossRef CAS; (d) Y. M. A. Yamada, K. Takeda, H. Takahashi and S. Ikegami, J. Org. Chem., 2003, 68, 7733 CrossRef CAS; (e) A. Biffis, M. Zecca and M. Basato, Eur. J. Inorg. Chem., 2001, 1131 CrossRef CAS; (f) R. L. Augustine and S. T. Oleary, J. Mol. Catal. A: Chem., 1995, 95, 277 CrossRef CAS; (g) D. A. Conlon, B. Pipik, S. Ferdinand, C. R. LeBlond, J. R. Sowa, Jr., B. Izzo, P. Collins, G. J. Ho, J. M. Williams, Y. J. Shi and Y. K. Sun, Adv. Synth. Catal., 2003, 345, 931 CrossRef CAS.
- R. G. Heidenreich, K. Köhler, J. G. E. Krauter and J. Pietsch, SYNLETT, 2002, 1118 CrossRef CAS.
- Z. Novak, A. Szabo, J. Repasi and A. Kotschy, J. Org. Chem., 2003, 68, 3327 CrossRef CAS.
- F. Quignard, S. Larbot, S. Goutodier and A. Choplin, J. Chem. Soc., Dalton Trans., 2002, 1147 RSC.
- A. S. Hay, J. Org. Chem., 1962, 27, 3320 CrossRef CAS.
- A. Elangovan, Y. H. Wang and T. I. Ho, Org. Lett., 2003, 5, 1841 CrossRef CAS.
- S. H. Yang, C. S. Li and C. H. Cheng, J. Org. Chem., 1987, 52, 691 CrossRef CAS.
- A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 14844 CrossRef CAS.
- I. Paterson, R. D. M. Davies and R. Marquez, Angew. Chem., Int. Ed., 2001, 40, 603 CrossRef CAS.
- R. A. Bunce, Tetrahedron, 1995, 51, 13103 CrossRef CAS.
- J. J. Bozell and C. E. Vogt, J. Am. Chem. Soc., 1988, 110, 2655 CrossRef CAS.
- Y. Sasson, S. Negussie, M. Royz and N. Mushkin, Chem. Commun., 1996, 297 RSC.
- F. Y. Zhao and M. Arai, React. Kinet. Catal. Lett., 2004, 81, 281 CrossRef CAS.
- S. Mukhopadhyay, G. Rothenberg, D. Gitis, H. Wiener and Y. Sasson, J. Chem. Soc., Perkin Trans. 2, 1999, 2481 RSC.
- H. Wiener, J. Blum and Y. Sasson, J. Org. Chem., 1991, 56, 6145 CrossRef.
- H. Wiener, J. Blum and Y. Sasson, J. Org. Chem., 1991, 56, 4481 CrossRef CAS.
- M. Feuerstein, H. Doucet and M. Santelli, Tetrahedron Lett., 2004, 45, 8443 CrossRef CAS.
- G. Horvath and K. Kawazoe, J. Chem. Eng. Jpn., 1983, 16, 470 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |