Metabolites from Eutypa species that are pathogens on grapes
Daniel Jiménez-Teja, Rosario Hernández-Galán and Isidro González Collado*
Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Cádiz, Apdo. 40, 11510 Puerto Real, Cádiz, Spain
First published on 28th November 2005
Abstract
Covering: up to the present
Structural and synthetic studies of the metabolites isolated from Eutypa lata are reviewed. This fungus is the causative agent of Eutypa dieback disease, also known as eutyposis or “dying-arm disease”, a perennial canker that affects grapevines and many other woody fruit plants. The review, which encompasses all the literature in this field up to the present and in which 76 references are cited, also includes a detailed study of the biological activity of the metabolites, especially the role of toxins in the development of the plant disease. Some aspects of the synthesis and biosynthesis of these metabolites and related compounds are discussed.
 Daniel Jiménez-Teja | Daniel Jiménez-Teja studied chemistry at the University of Cadiz. He is carrying out his PhD at Cadiz University with Professor I. G. Collado and Dr R. H. Galán, where he is working on the isolation of secondary metabolites from the phytopathogenic fungi Botrytis, Colletotrichum and Eutypa. He is currently completing his PhD at Reading University, UK, where he is working on the synthesis of secondary metabolites and analogues of the fungus Colletotrichum with Professor Laurence M. Harwood. |
 Rosario Hernández-Galán | Dr R. Hernández-Galán is a Titular Professor of Organic Chemistry at the University of Cadiz, Andalucia, Spain. She studied chemistry at the University of Cádiz and obtained a DPhil in 1991 for her work on the synthesis of 3-(1,1-dimethylallyl)coumarins under the tutelage of Professor F. R. Luis. She completed her PhD studies at the Dyson Perrins Laboratory, Oxford, with Professor Laurence M. Harwood. Her current research interest includes the biosynthesis and synthesis of toxins from phytopathogenic fungi. |
 Isidro G. Collado | Dr Isidro G. Collado is a Professor of Organic Chemistry at the University of Cadiz, Andalucia, Spain. He received a BSc in Chemistry from the University of Seville in 1978. After working for one year at the Facultad de Ciencias of Cadiz University on the synthesis of pyrrole derivatives, he completed his PhD on natural product chemistry. He then worked as a postdoctoral fellow with Professors B. M. Fraga and J. R. Hanson on gibberellin chemistry at the Consejo Superior de Investigaciones Científicas (Tenerife) and Sussex University, respectively. His current research interests include the synthesis of bioactive molecules and design of selective fungicides. |
1 Introduction
Eutypa dieback, also known as eutyposis or “dying-arm disease”, is a perennial canker disease that affects grapevines (Vitis vinifera L. (Vitaceae)) and many other woody fruit plants, such as apricots.1 The causative agent, the ascomycete fungus Eutypa lata (Pers:Fr.) Tul. & C. Tul. (anamorph Libertella blepharis A. L. Smith), (initially named E. armeniacae),2 enters the plant primarily through pruning wounds, leading to necrosis of woody tissues in the vicinity of the point of infection and to stunting of new shoots. This results in small, deformed, chlorotic leaves and scrawny fruit clusters. The disease is progressive over many years, and failure to control it leads to severe economic losses, primarily as a consequence of decreased yields and reduced longevity of the grapevines.3The disease affects all major grape-producing areas around the world. It is equally prevalent in the United States, Australia, and Europe, and is very difficult to control.4,5 In Australia, yield losses of at least 850 and 740 kg ha−1 have been reported for Shiraz and Cabernet Sauvignon vines, respectively. In addition, E. lata has also infected other important agricultural crops in the Central Valley of California, including almonds, apricots, cherries, olives, peaches, and walnuts.3 The fungus is found throughout California, with the economic impact being a result of decreased yields, increased vineyard management costs, and the shortened lifespan of the vines. In fact, not only do infected vines have a shortened lifespan, but they also produce fewer and smaller grapes than unaffected vines. All infected plants die eventually, since no curative treatments are available to date. The cost to wine grape production alone in California has been estimated to be in excess of $260 million a year,3 and it is estimated that as much as 20% of the total vineyard acreage (750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 acres) is infected. Yield decreases in California for infected Chenin Blanc or French Columbard grapes are estimated to range from 30% to over 60%, while vineyards older than 20 years had up to 83% yield reduction relative to their peak production at about 10–12 years of age.6
000 acres) is infected. Yield decreases in California for infected Chenin Blanc or French Columbard grapes are estimated to range from 30% to over 60%, while vineyards older than 20 years had up to 83% yield reduction relative to their peak production at about 10–12 years of age.6
2 Metabolites isolated from Eutypa lata
Disease severity is commonly associated with the degree of toxin production, permitting either colonization or systemic invasion by the pathogen. This is the case for Eutypa lata, which produces a number of structurally related secondary metabolites, mainly acetylenic phenols and heterocyclic analogues (Fig. 1). Thus, the phytotoxicity of E. lata probably results from a suite of structurally related compounds, with each compound probably having a different level of toxicity and different molecular targets within the plant cell. | ||
| Fig. 1 Metabolites isolated from Eutypa lata and analogues. | ||
Eutypine 1, 4-hydroxy-3-(3-methylbut-3-ene-1-ynyl)benzaldehyde, is a toxic compound secreted by E. lata which possesses an unusual five-carbon acetylenic side chain. This toxin was isolated and identified from fungal cultures of an unspecified strain of E. lata, together with structurally related metabolites,7,8 and was determined to be the principal phytotoxin on the basis of assays performed on excised leaves and leaf protoplasts.9
The biological action of eutypine 1 has been observed extensively in grapevines. Eutypine, which is synthesized by the fungus in the trunk, is believed to be transported by the ascending sap to the herbaceous parts of the plant, where it spreads throughout leaves and inflorescences to participate actively in the expression of disease symptoms.10 Eutypine itself exhibits weak acid properties and a marked lipophilic character. It has also been demonstrated that the toxin penetrates cells through a passive diffusion mechanism and tends to accumulate in the cytoplasm due to an ion-trapping mechanism related to the ionization state of the molecule.10
Moreover, the possibility that mitochondria may be a biological target for this toxin is suggested by the fact that it modifies the rate of respiration and the energy balance of the grapevine cells.11–13 Research focusing on the molecular mode of action of eutypine at the mitochondrial level has been carried out with methyleutypine, the non-deprotonatable derivative of 1.11 The effects of both molecules on mitochondrial respiration and membrane potential were compared using isolated mitochondria from grapevine cells in suspension cultures. Eutypine 1 was found to induce marked stimulation of oxygen consumption and had a depolarizing effect, while methyleutypine exhibited a very small effect on both the rate of oxygen uptake and membrane potential. For high eutypine concentrations, a mixed effect corresponding to a direct inhibition of electron transport and uncoupling was observed. Moreover, at concentrations below 200 mM, eutypine displayed a linear relationship between oxidation rate and membrane potential similar to that of the classical protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). However, unlike CCCP, eutypine induces a potential-dependent proton conductance that may be due to the potential-dependent migration of the dissociated form of the toxin across the membrane. It has thus been concluded that eutypine uncouples mitochondrial oxidative phosphorylation and decreases the ADP/O ratio in grapevine cells by increasing proton leaks, which it accomplishes by means of a cyclic protonophore mechanism.12,13
The toxin behaves in an unspecific fashion and appears to be an important virulence factor involved in symptom development of the disease.12 However, the absence of toxin-deficient mutants of the fungus as well as its long incubation period in the trunk before symptom development has prevented much-needed critical study of the toxin in vines.
Nevertheless, researchers have been able to establish that in grapevine cells, eutypine 1 is metabolized into another compound identified as 4-hydroxy-3-(3-methylbut-3-en-1-ynyl)benzyl alcohol, otherwise known as eutypinol 2 (Fig. 2). This compound does not exhibit toxic effects towards the grapevine and has no protonophoric activity. Thus, Colrat et al.14 found that detoxification occurred in grapevine cells through the enzymatic reduction of eutypine into its corresponding alcohol, eutypinol 2, and that the reduction of 2 is an NADPH-dependent enzymatic reaction.15 The finding that eutypine reductase enzyme (ERE) exhibited a high affinity for eutypine indicates that this enzyme may also play an important role in the detoxification of eutypine 1. Other findings included the fact that eutypinol 2 did not exhibit any uncoupling activity in mitochondria.16 Comparison of the detoxification activity of leaf tissues from the two genotypes of Vitis vinifera also seems to indicate that there is a relationship between the susceptibility of the cultivars to Eutypa dieback and the ability of tissues to deactivate eutypine 1. In addition, novel NADPH-dependent aldehyde reductase genes from Vigna radiate, which confer resistance to eutypine, have been reported.17,18 These data suggest that detoxification contributes to the defence reactions of the grapevine, allowing the infected plant to combat the toxin.16
 | ||
| Fig. 2 Reduction of eutypine by eutypine reductase. | ||
Examination of a laboratory culture of a strain of the fungus by Renaud et al.8,19 led to the identification of several phenolic compounds bearing a pentenyne side chain ortho to the hydroxyl group, including eutypinol 2, eutypine 1, its cyclization product 2-isopropenyl-5-formylbenzofuran 4, and the epoxidized chromanones 11 and 12 (Fig. 1).19 A general method for the analysis and identification of these metabolites by means of gas chromatography/mass spectrometry of their trimethylsilyl ether derivatives has been developed, and compounds have been quantified by means of HPLC.20
A comparative study of the metabolites produced by strains of E. lata obtained from grapevines in California and Italy on malt yeast broth (MYB) and potato dextrose broth (PDB) showed that 1 was not the primary constituent, and led to the isolation and identification of 6-hydroxymethyl-2,2-dimethyl-2H-chromene 5, eulatachromene, from an Italian Eutypa culture. In addition, siccayne 6, together with its O-methyl analogue eulatinol 7 and the acid analogue of eutypine, 3, were isolated as further constituents of E. lata.20 Interestingly, eutypinol 2 was the major metabolite produced under most culture conditions while eutypine 1 was not produced by the Californian strain even though it has been found to be pathogenic to grapevines. These results suggest that the phytotoxicity of E. lata may be due to a suite of structurally related compounds, each exhibiting a different degree of activity. Thus, rather than attributing phytotoxicity to compound 1 alone, 2, 4, and 5 all seem to play a role. To examine this possibility further, metabolites 1, 2, 4, 5 and 7, along with chromene analogues 8–10, were synthesized in sufficient amounts for evaluation, and a rapid, quantitative bioassay involving topical application of individual compounds to culture disks of grape leaves with subsequent measurement of chlorophyll loss was carried out to provide a relative measure of tissue damage.21
The metabolite eulatachromene 5 was found to be more phytotoxic than eutypine 1. The cyclization product 4 also showed significant activity, whereas the reduction product eutypinol 2 was inactive, as was the quinol siccayne 7.21 Comparison of eulatachromene 5 with the three synthetic analogues 8–10 showed that only compound 8 exhibited analogous activity, while 9 and 10 were inactive in the bioassay. These results led to the conclusion that the active chromenes 5 and 8, along with the benzofuran 4, exhibited greater activity than the E. lata metabolites 1 and 2.21
Recently, it has also been reported that the secondary metabolites 6 and 7 inhibit mitochondrial respiration, based on the results of a model bioassay using the yeast Saccharomyces cerevisiae.22 Eulatinol 7, a methoxyquinol, exhibited necrotic effects in the bioassay. The analogue desmethyleulatinol siccayne 6 was first isolated from Helminthosporium siccans and subsequently reported to occur in the marine basidiomycete Halocyphina villosa. Both compounds inhibit mitochondrial respiration.22
The variety of metabolites biosynthesized by the fungus, together with the conflicting information as to those responsible for dieback, required that more fundamental information was necessary before metabolite detection in grapevines could be considered a useful diagnostic tool for the disease. A study using a number of various strains was therefore designed to establish which fungal growth conditions were best suited to metabolite production. The results indicated that metabolite production on grapevine extract was much greater than that on artificial media, thereby suggesting that this native substrate provides optimal production conditions. In addition, a more representative profile of the metabolites produced in the natural disease state was obtained.23
Two highly oxygenated cyclohexene oxides have also been isolated from E. lata, namely eutypoxide B 1324 and the novel allenic epoxycyclohexane 14.25 Both compounds feature a highly oxygenated cyclohexene oxide with several stereogenic centers, which are important characteristics of various biologically active compounds. For example, crotepoxide, isolated from the fruit of Croton macrostachys,26,27 cyclophellitol, obtained from a Phellinus mushroom,28 and eupenoxide,29 produced by a fungus of the genus Eupenicillium, are typical naturally occurring cyclohexene oxides that display various strong biological activities including anti-tumoral, anti-fungal, anti-bacterial, and enzyme inhibitory activities.
The metabolites of the mangrove fungus Eutypa sp. (#424) from southern China have also been studied, and a new compound, eutypoid A 15, which is a derivative of an α,β-unsaturated-γ-lactone, has been isolated from the mycelium. Similar compounds have been found to possess diverse pharmacological activities, including inhibition of cyclin-dependent kinases.30
Finally, in order to gain insight into the biochemistry of E. lata, both the sterol composition and the total sterol content of this pathogen have been investigated.31 The major sterol was identified as ergosterol; this 4-desmethylsterol accounted for 88% of the total sterols in solid medium and 78% in liquid medium. In addition to ergosterol, four minor 4-desmethylsterols, ergosta-5,7,9(11),22-tetraen-3β-ol, ergosta-7,22-dien-3β-ol, fecosterol and episterol were present, each accounting for 1–4% of the total sterol in solid medium and 1.8–13% in liquid medium. These results suggest that the sterol biosynthesis pathway of E. lata is very similar to that of other pathogenic filamentous fungi.31
3 Synthesis of Eutypa toxins
A number of syntheses of eutypine 1 and several of its congeners have been developed.32–35 Of the various methods used, the most convenient for preparing compounds 1 and 2, in satisfactory yields, proved to be a modification21 of the method of Bates et al.,34 summarized in Scheme 1. In addition, the syntheses of compounds 5–7 (Schemes 2 and 3) and 8–10 have been improved, although to date, the yields have generally been poor.21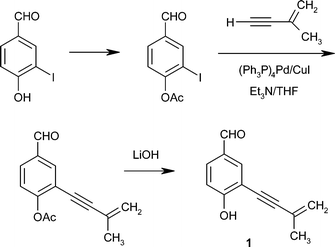 | ||
| Scheme 1 Synthesis of eutypine 1.21,34 | ||
Eulatachromene 5 and eutypinol 2 are the two most widely distributed and abundant products found in the cultures of several strains of Eutypa.23 The fact that eutypinol 2 is present in much higher levels than any of the other metabolites suggests that inactive compound 2 may serve as a precursor for transformation into active eulatachromene 5 in the plant tissue through a simple reduction of the acetylenic bond in the side chain and subsequent cyclization. Unfortunately, although the synthesis of 2,2-dimethyl-6-hydroxymethylchromene 5 as an intermediate in the preparation of chromane derivatives (evaluated for their effects on platelet aggregation) had previously been reported,36 details of the method of preparation and the physical properties of the compounds were not described.
Compound 5 was therefore synthesized de novo by methods based on those used for other chromene derivatives, as shown in Scheme 2.21 The success of this synthetic route allowed for the preparation of quantities that were sufficient to carry out biological evaluations of additional chromenes that correspond structurally to Eutypa metabolites. Thus, following the same procedure, but starting from 4-hydroxybenzaldehide, chromene-6-carboxaldehyde 8 and its oxidized derivative 9 were obtained via hydrolysis of the ester 10 in overall yields of 74% and 69%, respectively. Compound 5 exhibited necrotic effects in bioassays and inhibited photophosphorylation and electron transport in chloroplasts, as well as mitochondrial respiration.21
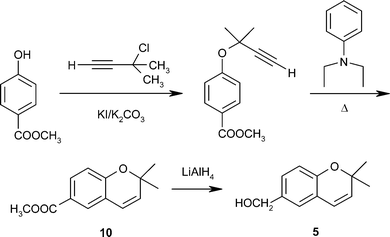 | ||
| Scheme 2 Synthesis of eulatachromene 5.21 | ||
Eutypine 1 was transformed into siccayne 7 by two five-step variations, with overall yields from 4-hydroxybenzaldehyde of 26% and 10%, respectively.32 Siccayne was therefore prepared from commercially available 3-iodophenol, by a five-step synthesis, with overall yield of 16.7%. The route is summarized in Scheme 3.21
 | ||
| Scheme 3 Synthesis of siccayne 7.21,32 | ||
The isolation19 and first racemic syntheses of eutypoxide B 13 were reported in 1992 by Tabacchi et al., who employed the Diels–Alder reaction as the key step.24
The first enantiodivergent synthesis of eutypoxide B was reported by Ogasawara et al. in 1993 (Scheme 5).37 Alternative methods to that described in the first paper were subsequently patented.38–40 Because the synthesis of 13 could formally be achieved by regio- and enantioselective addition of a C5 subunit and the epoxide oxygen to meso-(Z)-1,4-dihydroxycyclohexa-2,5-diene 16 from the face anti to the hydroxyl groups, the authors began the synthesis using the optically pure 17, obtained by the lipase-mediated asymmetrization of a meso-precursor, as a chiral equivalent of 16 (Scheme 4).
 | ||
| Scheme 4 Retrosynthetic analysis of eutypoxide B 13 | ||
 | ||
| Scheme 5 Ogasawara’s synthesis of eutypoxide B 13.37 | ||
Thus, the synthesis of (−)- and (+)-eutypoxide B 13, shown in Scheme 5, was accomplished in a stereo- and regio-controlled manner by using the single chiral building block 17 as a chiral equivalent of 16. First, compound 17 was oxidized to the ketone 18 to carry out regiospecific 1,4-addition of vinylmagnesium. Reduction of 19 with sodium borohydride, followed by thermolysis and epoxidation, yielded 23. Reaction with prop-2-enylmagnesium bromide in the presence of copper(I) iodide, with subsequent oxidation and isomerization, afforded eutypoxide B 13 in 63% yield. Moreover, this enantiodivergent approach was found to be compatible with the synthesis of both enantiomers of eutypoxide B 13.
An efficient enantioselective synthesis of (+)-eutypoxide B, the enantiomer corresponding to the natural product, has also been reported.41 The advantage of this particular synthetic route is that it requires neither drastic reaction conditions nor expensive reagents and equipment. The starting material chosen was (−)-quinic acid, a cyclohexane-based natural substance isolated from the plant species Cinchona. Two highly stereoselective and efficient sequential 1,4-additions to enone systems derived from D-(−)-quinic acid were the key steps in the synthesis (Scheme 6).41 The synthesis of a diastereoisomer of (+)-eutypoxide B was also carried out successfully.
 | ||
| Scheme 6 Synthesis of (+)-eutypoxide B 13.41 | ||
Other quick and efficient syntheses of each enantiomer were achieved through base-catalyzed asymmetric Diels–Alder reactions with 3-hydroxy-2-pyrone and chiral acrylates, as shown in Scheme 7.42 In these syntheses the authors used this reaction to form the bridged bicyclolactones 24a and 24b. These compounds were then used as the chiral starting materials, as they have the appropriate functional groups in the correct positions. Then, with the help of an appropriate chiral auxiliary, (−)- and (+)-eutypoxide B were formed.
 | ||
| Scheme 7 Asymmetric synthesis of (+)- and (−)-eutypoxide B 13.42 | ||
The stereospecific synthesis of the novel allenic cyclohexanoid epoxide 14 has also been reported by Gordon and Tabacchi, Scheme 8.25,43 Key steps in this synthesis include a Diels–Alder reaction between a 1,4-dioxygenated buta-l,3-diene and a ketene equivalent, and the coupling of a mixed isopropenyl cuprate with propargylic sulfinate. The stereospecific synthesis of the allene was achieved in eight steps with a 7.4% overall yield. Investigations are now underway to make the synthesis enantiospecific by using a chiral ketene equivalent.43
 | ||
| Scheme 8 Stereospecific synthesis of allene epoxide 14.25,43 | ||
Phytotoxins related to eutypoxide 13 and the allenic cyclohexanoid epoxide 14 have been isolated from Pestalotiopsis theae44 and Tricholoma acerbum.45 Several strategies have been described to synthesize these phytotoxins. These methodologies could be applied to the syntheses of those compounds from Eutypa lata with oxygenated cyclohexane oxide structures.46–50
4 Biosynthesis of metabolites isolated from Eutypa and related compounds
Although the synthesis of carbon-14-labeled eutypine, via a Wittig reaction using [14C]methyl iodide as a precursor, has been reported, the biosynthesis of metabolites isolated from Eutypa has not been studied.51 However, the biosynthesis of these metabolites or analogous compounds isolated from other organisms can facilitate these studies. For example, chromenes and isopropenylbenzofurans, structurally related to eulatachromene 5 and 2-isoproprenyl-5-formylbenzofuran 4, have been isolated from the genus Encelia (Asteraceae)52 and from Ageratum houstonianum.53 Studies on the biosynthesis of the aromatic ring of chromenes were carried out by feeding 14C-labelled compounds and stable isotopes.53 Although earlier studies suggesting a biogenetic origin from acetate were reported,54 the results obtained indicated that the aromatic ring of these products originates from the shikimic acid pathway.53 The studies were carried out by feeding of [14C]-phenylalanine and L-[2-13C]-phenylalanine; the incorporation was analysed by GC-MS and 13C NMR. Two pathways from phenylalanine yielding the aromatic ring of the chromenes were possible, one involving cinnamic acid and the other involving phenylethylamine. The use of the phenylalanine inhibitors aminooxyacetic acid (AOA) and aminooxyphenylpropionic acid (AOPP) proved that the chromenes and benzofurans originate from the shikimic acid pathway, and that the cinnamic acid is the precursor.53Other metabolites structurally related to eutypine – eulatinol, eutypinic acid and siccayne – have been studied with regard to their biosynthesis in other organisms. These compounds have been found in Triglochin maritime, a salt-tolerant plant, in the fungi Trametes suaveolens and Bjerkandera adusta, and in Streptomyces xanthochromogenus. In all the cases the studies concluded that these products originate from the shikimic acid pathway.55–58
5 Considerations about the control of Eutypa
According to the papers reviewed in this work, Eutypa dieback of grapevines (Vitis vinifera) is considered one of the most destructive diseases of the woody tissues of this plant. The disease, which is equally prevalent in the United States, Australia, and Europe, occurs when the pruning wounds of grapevines become infected with spores from E. lata. It is difficult to control, and as it can be lethal, replanting becomes the only course of action.Since the foliar symptoms, which are probably due to toxins produced by the fungus in the wood and translocated to the shoots, do not become visible until several years after infection, non-destructive procedures for early detection and removal of infected vines are urgently needed. Unfortunately, although a PCR-based assay for detecting the fungus in the grapevine wood has been developed,59 it does not distinguish between pathogenic and non-pathogenic strains, nor does it provide a measure of the degree of virulence.
Recently, success in obtaining transgenic plants from grapevine rootstock 110 Richter Vitis berlandieri × V. rupestris that express the Vr-ERE gene has been reported. These transgenic plants possess a gene encoded for the eutypine reductase isolate of mung beans, and were found to be more resistant to Eutypa dieback than natural plants.18 These results suggest that the Vr-ERE gene may be a good candidate for conferring resistence to the toxin, and open up new opportunities to study the role of the toxin in the development of the disease. A patent for these plants has thus been developed.60
For the past 30 years, benomyl, a broad-spectrum fungicide, has been the sole means of controlling E. lata. However, this fungicide presents several disadvantages. For example, while benomyl is effective for short-term control of the disease, more long-term strategies to control the pathogen are needed, since wounds are susceptible for as long as 4 weeks after pruning. Additionally, to achieve effective control, it is necessary to make multiple applications of the fungicide over a short period of time. In effect, then, no post-infection eradication method currently exists.61,62
Several studies on fungicides against Eutypa have also been reported.63–65 The fungicide flusilazole, for example, has been found to inhibit the ascospore germination, while other fungicides such as fenarimol, iprodione, myclobutanil, and vinclozolin inhibit mycelial growth.63 Bonnemain et al.64 studied a series of derivatives of the phenylpyrrole fungicide fenpiclonil and found that one of these derivatives, N-carboxymethyl-3-cyano-4-(2,3-dichlorophenyl)pyrrole, exhibited fungicidal activity against E. latain vitro. A method using an injection into the heartwood of the tree of a triazole fungicide such as cyproconazole or phosphoric acid has also been developed.65
In addition, attempts to control the fungus by rational methods have been reported.66–71 For example, Van der Gehynst et al. studied the possibility of using benomyl-resistant Fusarium lateritium strains for biocontrol of E. lata, since F. lateritium had been identified as an agent involved in the development of biological control against this pathogen.66–68 Recently, F. lateritium and Trichoderma sp. were screened for their ability to consume eutypine 1.69 Transformation–time courses were performed, and an HPLC method was developed to analyze toxin metabolism and to identify and quantify the converted products. The results showed that the aldehyde function of eutypine 1 was reduced to eutypinol 2, as by V. vinifera cv. Merlot. A supplementary detoxification pathway, where the aldehyde function was oxidized to eutypinic acid 3, was revealed. Biological assays on cells of V. vinifera cv. Chasselas displayed a lower toxicity than the toxins. The possibility of selecting new biocontrol agents against trunk diseases of grapevines based on microbial detoxification has been discussed.69
Moreover, several compounds have shown fungicidal activity against this pathogen, and have been found to stimulate plant growth and enhance plant defences.70–72
In summary, methods based on the detection in grapevine tissues of the fungal metabolites responsible for toxicity appear to be the best approach for rapid, early diagnosis of the disease. However, conflicting information concerning the toxins responsible for dieback and the wide range of bioactivity displayed by metabolites biosynthesized by the fungus point to the need for more fundamental information before metabolite detection in grapevines can be used as an efficient diagnostic tool for the disease. In this vein, attempts are currently underway to correlate the presence of individual metabolites with DNA probes specific for pathogenic and non-pathogenic E. lata strains developed using PCR techniques.73
Recently, a second species of Eutypa, E. leptoplaca, has been identified on grapevines in California and its pathogenicity has been established. The importance of E. leptoplaca in relation to Eutypa dieback and its role as a necrotrophic pathogen have also been discussed.74,75 The characterization of metabolites from this new pathogenic species should shed more light on the identification of Eutypa species as a whole.
6 Conclusions
In summary, the literature on the species Eutypa lata has been reviewed with an emphasis on the secondary metabolites produced by this fungus and the biological activities displayed for these toxins. The characterization of these metabolites not only facilitates the identification of different species of this genus, but also sheds light on the role of these toxins in the infection mechanism of this organism. During the preparation of this manuscript a method to diagnose Eutypa lata infection in grape by metabolite analysis has been reported.767 Acknowledgements
This research was supported by grant from MCYT. AGL2003-06480-C02-01, Spain. We thank Dr J. R. Hanson, Sussex University, for manuscript revision.8 References
- M. V. Carter, A. Bolay and F. Rappaz, Rev. Plant Pathol., 1983, 62, 251–258 Search PubMed.
- W. J. Moller and A. N. Kasimatis, Plant Dis. Rep., 1978, 62, 254–258 Search PubMed.
- T. Wicks and K. Davis, Aust. Grapegrower Winemaker, Annu. Tech. Issue, 1999, 15–16 Search PubMed.
- G. P. Munkvold, J. A. Duthie and J. J. Marois, Phytopathology, 1994, 84, 186–192 Search PubMed.
- G. P. Munkvold and J. J. Marois, Phytopathology, 1995, 85, 249–256 Search PubMed.
- G. P. Munkvold and J. J. Marois, Plant Dis., 1994, 78, 200–207 Search PubMed.
- G. Tsoupras, P. De Angelis, T. Zesiger, J. M. Renoud and R. Tabacchi, Bioact. Mol., 1988, 93–100 Search PubMed.
- J. M. Renoud, G. Tsoupras and R. Tabacchi, Helv. Chim. Acta, 1989, 72, 929–932 CrossRef.
- P. Tey-Rulh, I. Philippe, J. M. Renaud, G. Tsoupras, P. De Angelis, J. Fallot and R. Tabacchi, Phytochemistry, 1991, 30(2), 471–473 CrossRef CAS.
- B. E. Amborabe, P. Fleurat-Lessard, J. Bonmort, J. P. Roustan and G. Roblin, Plant Physiol. Biochem. (Paris), 2001, 39(1), 51–58 CrossRef CAS.
- C. Deswarte, J. Eychenne, J. Davy de Virville, J. Roustan, F. Moreau and J. Fallot, Arch. Biochem. Biophys., 1996, 334, 200–205 CrossRef CAS.
- C. Deswarte, H. Canut, A. Klaebe, J. P. Roustain and J. Fallot, J. Plant Pathol., 1996, 149(3/4), 336–342 Search PubMed.
- J. Fallot, C. Deswarte, S. Dalmayrac, S. Colrat and J. P. Roustan, Plant Biol. Pathol., 1997, 320, 149–158 Search PubMed.
- S. Colrat, C. Deswarte, A. Latche, A. Klaebe, M. Bouzayen, J. Fallot and J. P. Roustan, Planta, 1999, 207(4), 544–550 CrossRef CAS.
- M. Afifi, M. C. Monje, V. Legrand and J. P. Roustan, Anal. Chim. Acta, 2004, 513(1), 21–27 CrossRef CAS.
- S. Colrat, A. Latché, M. Guis, J. C. Pech, M. Bouzayen, J. Fallot and J. P. Roustan, Plant Physiol., 1999, 119, 621–626 CrossRef CAS.
- P. Guillen, M. Guis, G. Martinez-Reina, S. Colrat, S. Dalmayrac, C. Deswarte, M. Bouzayen, J. P. Roustan, J. Fallot, J. C. Pech and A. Latche, Plant J., 1998, 16, 335–343 CrossRef CAS.
- V. Legrand, S. Dalmayrac, A. Latche, J. C. Pech, M. Bouzayen, J. Fallota, L. Torregrosa, A. Bouquet and J. P. Roustan, Plant Sci., 2003, 164, 809–814.
- J. M. Renoud, G. Tsoupras, H. Stoeckli-Evans and R. Tabacchi, Helv. Chim. Acta, 1989, 72, 1262–1267 CAS.
- R. J. Molyneux, N. Mahoney, P. Bayman, R. Y. Wong, K. Meyer and N. Irelan, J. Agric. Food Chem., 2002, 50, 1393–1399 CrossRef CAS.
- L. R. Smith, N. Mahoney and R. J. Molyneux, J. Nat. Prod., 2003, 66, 169–176 CrossRef CAS.
- J. H. Kim, N. Mahoney, K. L. Chan, R. J. Molyneux and B. C. Campbell, Curr. Microbiol., 2004, 49, 282–287 CrossRef CAS.
- N. Mahoney, R. Lardner, R. J. Molyneux, E. S. Scott, L. R. Smith and T. K. Schoch, Phytochemistry, 2003, 64, 475–484 CrossRef CAS.
- E. Defrancq, J. Gordon, A. Brodard and R. Tabacchi, Helv. Chim. Acta, 1992, 75(1), 276–81 CAS.
- M. Jean, G. Tsoupras, H. Stoceckli-Evans and R. Tabacchi, Helv. Chim. Acta, 1989, 72(6), 1262–1267 CAS.
- S. M. Kupchan, R. J. Hemingway, P. Coggon, A. T. McPhail and G. A. Sim, J. Am. Chem. Soc., 1968, 90(11), 2982–3 CrossRef CAS.
- S. M. Kupchan, R. J. Hemingway and R. M. Smith, J. Org. Chem., 1969, 34(12), 3898–902 CrossRef CAS.
- S. Atsumi, K. Umezawa, H. Iinuma, H. Naganawa, H. Nakamura, Y. Iitaka and T. Takeuchi, J. Antibiot., 1990, 43(1), 49–53 CAS.
- R. K. Duke and R. W. Rickards, J. Org. Chem., 1984, 49(11), 1898–904 CrossRef CAS.
- Y. Lin, H. Li, G. Jiang, S. Zhou, L. L. P. Vrijmoed and E. G. B. Jones, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 41(7), 1542–1544.
- L. Chapuis, M. F. Corio-Costet and C. Malosse, Phytochemistry, 1996, 42, 1599–1601 CrossRef CAS.
- E. Defranq, T. Zesiger and R. Tabacchi, Helv. Chim. Acta, 1993, 76(1), 425–430 CrossRef CAS.
- S. Perrin-Cherioux, E. Abou-Mansour and R. Tabacchi, Phytopathol. Mediterr., 2004, 43(1), 83–86 Search PubMed.
- R. W. Bates, C. J. Gabel, J. Ji and T. Rama-Devi, Tetrahedron, 1995, 51(30), 8199–212 CrossRef CAS.
- C. Amatore, E. Blart, J. P. Genet, A. Jutand, S. Lemaire-Audoire and M. Savignac, J. Org. Chem., 1995, 60(21), 6829–39 CrossRef CAS.
- Y. Aida, T. Kasama, H. Fukaya, Takeuchi, K. Sugiyama and S. Tobinaga, Clin. Exp. Pharmacol. Physiol., 1988, 25, 939–944.
- S. Takano, M. Moriya and K. Ogasawara, J. Chem. Soc., Chem. Commun., 1993, 614–615 RSC.
- S. Takano and K. Ogasawara, US Pat. 101
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765, 1994 Search PubMed.
765, 1994 Search PubMed. - S. Takano and K. Ogasawara, Eur. Pat. Appl., EP0627396, 1994 Search PubMed.
- K. Ogasawara, J. Synth. Org. Chem., Jpn., 1999, 57, 957–968 CAS.
- M. T. Barros, C. D. Maycock and M. R. Ventura, J. Org. Chem., 1997, 62, 3984–3988 CrossRef CAS.
- H. Shimizu, H. Okamura, T. Iwagawa and M. Nakatani, Tetrahedron, 2001, 57, 1903–1908 CrossRef CAS.
- J. Gordon and R. Tabacchi, J. Org. Chem., 1992, 57, 4728–4731 CrossRef CAS.
- T. Nagata, Y. Ando and A. Hirota, Biosci., Biotechnol., Biochem., 1992, 56, 810–811 CrossRef CAS.
- L. Garlaschelli, E. Magistrali, G. Vidari and O. Zuffardi, Tetrahedron Lett., 1995, 36, 5633–5636 CrossRef CAS.
- A. E. Graham, D. McKerrecher, D. H. Davies and R. J. K. Taylor, Tetrahedron Lett., 1996, 37(41), 7445–7448 CrossRef CAS.
- M. W. Miller and C. R. Johnson, J. Org. Chem., 1997, 62(6), 1582–1583 CrossRef CAS.
- T. Tachihara and T. Kitahara, Tetrahedron, 2003, 59(10), 1773–1780 CrossRef CAS.
- E.-I. Negishi, Z. Tan, S.-Y. Liou and B. Liao, Tetrahedron, 2000, 56(52), 10197–10207 CrossRef CAS.
- M. T. Barros, C. D. Maycock and M. R. Ventura, Chem. Eur. J., 2000, 6(21), 3991–3996 CrossRef CAS.
- E. Defrancq and R. Tabacchi, J. Labelled Compd. Radiopharm., 1992, 31(12), 1057–63 CrossRef CAS.
- P. Proksch and F. Rodriguez, J. Chromatogr., 1982, 240(2), 543–6 CrossRef CAS.
- R. Siebertz, P. Proksch and L. Witte, Phytochemistry, 1990, 29(7), 2135–2138 CrossRef CAS.
- A. V. Vyas and N. B. Mulchandani, Phytochemistry, 1980, 19, 2597–8 CrossRef CAS.
- A. Lomascolo, M. Asther, D. Navarro, C. Antona, M. Delattre and L. Lesage-Meessen, Lett. Appl. Microbiol., 2001, 32, 262–267 CrossRef CAS.
- J. Strikart Nielsen and B. Lindberg Moller, Arch. Biochem. Biophys., 1999, 368, 121–130 CrossRef.
- H. Cho, J. M. Beale, G. Graff, U. Mocek, A. Nakagawa, S. Omura and H. G. Floss, J. Am. Chem. Soc., 1993, 115, 12296–12304 CrossRef CAS.
- P. J. Silk and J. B. Macaulay, Chemosphere, 2003, 52, 503–512 CrossRef CAS.
- P. Lecomte, J. P. Peros, D. Blancard, N. Bastien and C. Délye, Appl. Environ. Microbiol., 2000, 66, 4475–4480 CrossRef CAS.
- A. Latche, J. P. Roustan, M. Bouzayen, J. C. Pech and J. Fallot, Eur. Pat. Appl. EP0856584, 1998 Search PubMed.
- R. C. Pearson, Am. J. Enol. Vitic., 1982, 33(1), 51–52 Search PubMed.
- W. J. Moller, D. E. Ramos and R. R. Sanborn, Plant Dis. Rep., 1977, 61(7), 600–604 Search PubMed.
- G. P. Munkvold and J. J. Marois, Plant Dis., 1993, 77(1), 50–55 Search PubMed.
- J. F. Chollet, F. Rocher, C. Jousse, C. Delétage-Grandon, G. Bashiardes and J. L. Bonnemain, Pest Manage. Sci., 2004, 60, 1063–1072 Search PubMed.
- A. G. Spiers and B. G. Ward, PCT Int. Appl. WO9530332, 1995 Search PubMed.
- G. McMahan, W. Yeh, M. N. Marshall, M. Olsen, S. Sananikone, J. Y. Wu, D. E. Block and J. S. Van der Gehynst, J. Ind. Microbiol. Biotechnol., 2001, 26, 151–155 CrossRef CAS.
- M. V. Carter and T. V. Price, Aust. J. Agric. Res., 1974, 25(1), 105–119 Search PubMed.
- Y. S. Tsantrizos, X. J. Xu, F. Sauriol and R. C. Hynes, Can. J. Chem., 1993, 71(9), 1362–1367 CAS.
- D. Christen, M. Tharin, S. Perrin-Cherioux, E. Abou-Mansour, R. Tabacchi and G. Défago, J. Agric. Food Chem., 2005, 53, 7043–7051 CrossRef CAS.
- A. Aziz, B. Poinssot, X. Daire, M. Adrian, A. Bezier, B. Lambert, J. M. Joubert and A. Pugin, Mol. Plant-Microbe Interact., 2003, 16(12), 1118–1128 Search PubMed.
- Fr. Pat. FR2780972, 2000 Search PubMed.
- B. E. Amborabé, P. Fleurat-Lessard, J. F. Chollet and G. Roblin, Plant Physiol. Biochem., 2002, 40, 1051–1060 CrossRef CAS.
- R. Lardner, N. Mahoney, R. J. Molyneux and E. Scott, Aust. Grapegrower Winemaker, Annu. Tech. Issue, 2002, 78–79 Search PubMed.
- P. E. Rolshausen, F. Trouillas and W. D. Gubler, Plant Dis., 2004, 88(9), 925–929 Search PubMed.
- F. P. Trouillas and W. Gubler, Mycol. Res., 2004, 108(10), 1195–1204 Search PubMed.
- N. Mahoney, R. J. Molyneux, L. R. Smith, T. K. Schoch, P. E. Rolshausen and W. D. Gubler, J. Agric. Food Chem., 2005, 53(21), 8148–8155 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
