Natural products from medicinal plants: non-alkaloidal natural constituents of the Thalictrum species
Elena A.
Khamidullina
a,
Alexandra S.
Gromova
b,
Vladislav I.
Lutsky
b and
Noel L.
Owen
*c
aIrkutsk A. E. Favorsky Institute of Chemistry Siberian Branch of RAS, Russia 664033
bIrkutsk State Technical University, Russia 664074
cDept of Chemistry and Biochemistry, Brigham Young University, Provo, Utah, USA 84602
First published on 6th December 2005
Abstract
Covering: 1959 to 2005
Thalictrum is an important plant genus that is widely used in traditional medicine. In this review considerable attention has been given to triterpenoid saponins in connection with their specific distribution in the Thalictrum genus and with their biological activity. All other non-alkaloid compounds isolated from the Thalictrum genus are also reviewed; these metabolites are discussed in relation to their structural features and to their role in the plants.
 Elena A. Khamidullina | Dr Elena A. Khamidullina studied chemistry at Irkutsk State University, Russia. She completed her PhD in the A. E. Favorsky Institute of Chemistry, Siberian Division of RAS and her work dealt with triterpenoids and saponins from Siberian plants. Her current research interests include biologically active compounds derived from plants, and the design of biologically active compounds based on natural products. |
 Alexandra Gromova | Dr Alexandra Gromova studied chemistry at the Irkutsk State University, Russia. She obtained her PhD degree from the Novosibirsk Institute of Organic Chemistry RAS. She then worked in the A. E. Favorsky Institute of Chemistry Siberian Division of RAS as a Senior Research Fellow. She is currently working at the Irkutsk State Technical University as a Senior Researcher. She has authored more than 110 scientific publications in international journals. Her research field is the isolation and elucidation of natural products with biological properties: e.g. phenols, flavonoids, saponins and alkaloids. |
 Vladislav Lutsky | Dr Vladislav Lutsky graduated from the Irkutsk State University. He received his PhD degree from the Novosibirsk Institute of Organic Chemistry RAS and carried out his postdoctoral work in the A. E. Favorsky Institute of Chemistry, Siberian Division of RAS (until 2001). In 1999 he was invited to Brigham Young University (Provo, Utah, USA) and worked for a year at the Department of Chemistry and Biochemistry as a visiting scientist. Currently he is a senior lecturer at the Irkutsk State Technical University. He has authored more than 130 scientific publications and his main research interests involve the isolation and structure elucidation of biologically active natural products from plants. |
 Noel Owen | Dr Noel Owen is a graduate of the University of Wales, with a PhD from Cambridge University and postdoctoral experience at Harvard University. He is a spectroscopist having worked in the area of vibrational, rotational and nuclear magnetic resonance spectroscopy. His current research interests involve the vibrational spectra of wood and wood products and the structure determination of natural products extracted from plants and plant endophytes. |
1 Introduction
For centuries plants of the Thalictrum genus have been used extensively as herbal medicines—in Russia (Siberian region) to treat gastric-intestinal diseases, female disorders, various neoplasms and pulmonary tuberculosis,1–4 and in Japan (Nagano Prefecture) to treat stomach disorders.5 In China Thalictrum plants have a long history as folk medicines in the treatment of many kinds of diseases. At least 43 species of Thalictrum have been used because of their special and proven therapeutic effects.6 Until recently, studies on the chemical constituents of the Thalictrum genus have been mainly confined to the alkaloids. More than two hundred alkaloids have been isolated from this genus; this information has been summarized elsewhere.7–11 Authors6 have attempted to relate pharmacological activities of these plants to the composition of the alkaloids and have shown that species containing alkaloids of different types possess different therapeutic effects. Our research12 has revealed that the biological activity of these plants is not restricted to the alkaloid-containing species. We have investigated selected species, which did not contain alkaloids and have found interesting and potentially important biological activities.In this review considerable attention has been given to triterpenoid saponins in connection with their specific distribution in the Thalictrum genus and with their biological activity. For the present instance the term “specific distribution” implies a dependence of chemical composition of the plants on ecological factors and geographical conditions. The original discovery of triterpenoid saponins in plants of the Thalictrum genus was made by some of the authors of this review, and the first few articles13–17 describing these compounds were published in 1981–1983. Further investigations by Japanese, Chinese and Korean research groups have shown a great diversity of triterpenoid saponins in plants of this genus, caused both by changes in the genin structure as well as in the composition of the carbohydrate chain. To date more than 60 triterpenoids and their glycosides have been discovered in the genus, and most of them are new compounds. Those saponins, which have been isolated from plants of the Thalictrum genus, have considerable biological activity. This review includes the original results regarding anti-cancer activity and the effect of these compounds on mammalian reproductive systems.
In addition to triterpenoids and their glycosides, all other non-alkaloid compounds isolated from the Thalictrum genus are reviewed; these include flavonoids, cyanogenic glycosides, hydrocarbons, high molecular weight alcohols, fatty acids, phenolic compounds and sterols. These metabolites are discussed in relation to their structural features and to their role in the plants.
The relevant literature published since 1959 to the present is reviewed, with 120 cited references.
2 Brief botanical characteristics of the Thalictrum genus
The genus Thalictrum belongs to the order Ranunculales, and the family Ranunculaceae. The order Ranunculales consists of 10 families, among which Ranunculaceae is the largest (with ca. 50 genera and more than 2000 species).18–20 As the family Ranunculaceae is considered one of the most primitive of the angiosperms, it is of significant biological interest, since some of the species are characterized by progressive morphological indications both of vegetative and generative organs.21,22 The Ranunculaceae family is divided to four subfamilies, according to the structure of the flowers and fruits and cytology data:22 Helleboroideae, Ranunculoideae, Thalictroideae and Coptidoideae.19 The Thalictroideae subfamily includes the Thalictrum genus. This particular genus is distributed over many parts of the Earth, mainly in the temperate and cold zones of the both hemispheres. It is extremely polymorphous due to the breadth of its ecological–geographical diversity. A careful study of the systematic location of the Thalictrum genus has been given by Bochanceva.23 At the end of the 1960's Tamura24 suggested intraspecific systematization of the Thalictrum genus, which takes into account the existing genus system. Tamura's systematization is currently considered to be the most advanced.21,25The Thalictrum genus consists of about 110 species of herbaceous perennials.26 It should be noted that the independence of some Thalictrum species is not generally accepted and confirmation is needed in specific cases, but on the other hand, some species (e.g. T. minus) have been divided into subspecies.1
3 Secondary metabolites of the Thalictrum genus
3.1 Triterpenoid glycosides and their genins
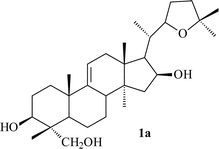
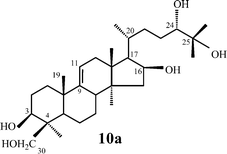
The first reports on triterpenoids from the Thalictrum genus appeared in 1981–1983 and involved the isolation of triterpenoid saponins from Thalictrum minus and T. foetidum collected in Eastern Siberia.13–17Plants of the Thalictrum genus produce both tetracyclic (lanostane type) and pentacyclic (oleanane type) triterpenoids. Fifty-three saponins have been isolated from 9 species of the Thalictrum genus: 38 new tetracyclic and 15 pentacyclic saponins. The tetracyclic saponins contain 21 new genins. Some of them (2, 11, 12, 15, 28–31, 43) have been characterized as novel compounds. In certain cases artifacts have been obtained during isolation procedures (1a,1610a,2719a,504428). The other genins have been characterized as part of their respective glycosides.
From the 15 pentacyclic saponins, twelve are new compounds. The novelty of these saponins is determined by the structures of the carbohydrate chains.
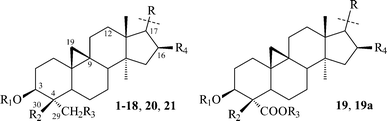
Cycloartanes with an acyclic side chain (on atom C-17). This type of compound is represented by structures 1–21, and includes saponins and triterpenoids derived from their respective saponins (Table 1). Thalicoside A (1) was the first glycoside isolated from the aerial part of Thalictrum minus.29 Enzymatic hydrolysis (Helix pomatia) of 1 failed to produce any genin, but an acid hydrolysis led to an artifact (1a), which not only did not contain a cyclopropane ring but also had alterations in the side chain. The structure of 1a was elucidated by X-ray analyses.14,16 The aglycon-thalicogenin (2) was obtained by periodate oxidation of 1 followed by treatment with alkali. The 9,11-cyclopropane ring and the primary hydroxyl group on C-4 were found to be arranged on different sides of the molecular plane on the basis of 13C NMR spectral data of 2.30,31 The configuration of the C-22 asymmetric center of 2 was established as S on the basis of X-ray analysis of 1a and by comparison of the 13C NMR chemical shifts of 2 with those of 22(S) and 22(R)-hydroxycholestanols.31
| R | No. | R1 | R2 | R3 | R4 | Ref. |
|---|---|---|---|---|---|---|
|
2 | H | CH3 | OH | OH | 16,29 |
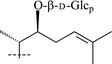
| 1 | β-D-Galp | CH3 | O-β-D-Glcp | OH | 30 | |
| 4 | α-L-Arap | CH3 | O-β-D-Glcp | OH | 34 | |
|
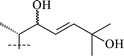
3 | β-D-Galp | CH3 | O-β-D-Glcp | OH | 33 |
|
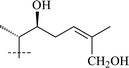
5 | β-D-Galp | CH3 | O-β-D-Glcp | OH | 35 |
|
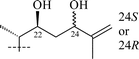
6 | β-D-Galp | CH3 | O-β-D-Glcp | OH | 36 |
| 15 | H | CH3 | H | H | 45,47 | |
| 14 | β-D-Quip-(1→2)-α-L-Rhap-(1→6)-4-O-Ac-β-D-Glcp | CH3 | H | H | 45 | |
| 16 | β-D-Quip-(1→6)-β-D-Glcp-(1→4)-β-D-Fucp | CH3 | H | H | 47 | |
| 17 | β-D-Glcp-(1→6)-[α-L-Rhap-(1→2)]-β-D-Glcp-(1→4)-β-D-Fucp | CH3 | H | H | 47 | |
| 18 | β-D-Glcp-(1→4)-[α-L-Rhap-(1→2)]-β-D-Fucp | CH3 | H | H | 49 | |
|
7,8 | β-D-Galp | CH3 | O-β-D-Glcp | OH | 36,37 |
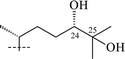
|
11 | H | CH3 | H | OH | 41 |
| 9 | α-L-Arap | CH3 | H | α-L-Rhap-(1→6)-O-β-D-Glcp | 39 | |
| 12 | H | CH2OH | H | OH | 42 | |
| 10 | α-L-Arap | CH2OH | H | α-L-Rhap-(1→6)-O-β-D-Glcp | 40 | |
| 13 | β-D-Xylp-(1→2)-β-D-Xylp | CH2OH | H | OH | 43 | |
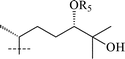
|
19 R5 = Ac | Ac | CH3 | β-D-Xylf-(1→6)-[α-L-Rhap-(1→2)]-β-D-Glcp | OH | 50 |
| 19a R5 = H | H | CH3 | H | OH | 50 | |
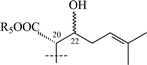
|
20 R5 = H | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | CH2OH | H | H | 51 |
| 21 R5 = β-D-Xylp-(1→6)-β-D-Glcp | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | CH2OH | H | H | 51 |
X-ray analysis of the artifact 1a established the conformation of the side chain which is stabilized by an intramolecular hydrogen bond between the hydroxyl group on C-16 and the O-atom of the tetrahydrofuran (THF) ring as shown on the Newman projection in Fig. 1a. Analysis of the 13C NMR chemical shifts of all the thalicogenin structures helped to establish the presence of the hydrogen bond. Comparing the experimental 13C chemical shifts with those calculated by the Beierbeck32 method confirmed the presence of an intramolecular hydrogen bond between the hydroxyl groups on C-16 and C-22, and helped to determine that the side chain conformation of the thalicogenin is as shown in Fig. 1b.
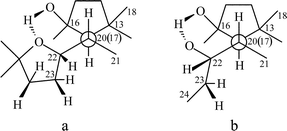 | ||
| Fig. 1 | ||
Two other saponins from T. minus, thalicoside C (3)33 and thalicoside A2 (4),34 both possessing thalicogenin, differed from 2 by the carbohydrate constituents: the first had a trisdesmoside with galactose and two glucose units; the second had arabinose and glucose in two separate carbohydrate chains.
Thalicoside E (5)35 was identified as a new saponin as a result of extensive spectroscopic analyses without degradation of the molecule. Thalicosides G1 (6),36 G2 (7)36 and H2 (8)37 are minor metabolites of T. minus and are isomers of each other. Thalicosides G2 and H2 were shown to be C-24 epimers, but which saponin of this couple is the 24R isomer and which one is the 24S remains unclear. The C-22 stereochemistry of glycosides 6–8 was recognized as S using Seo's process38 and made it possible to establish the stereochemistry of the hydroxyl groups in the chiral secondary alcohols using glycosylation effects observed in 13C NMR spectra.
As in the case of thalicoside A (1), cyclofoetosides A (9)39 and B (10)40 were isolated from T. foetidum at the beginning the 1980's. The isolation of the aglycone was important for their structural elucidation. Both saponins 9 and 10 have novel genins (11 and 12 respectively) and the same carbohydrate chains. Cyclofoetigenin A (11)41 was obtained by acid hydrolysis of 9 but in low yield, while cyclofoetoside B (10) yielded the artifact 10a during acid hydrolysis. The aglycon-cyclofoetigenin B (12)42 resulted from periodate oxidation of 10 followed by alkaline hydrolysis, whilst the α-diol group in the side chain was protected by esterification.42 The C-24 configuration of 12 was established as S.
Cyclofoetigenin B was also found to be a constituent of the new glycoside (13) isolated from the roots of T. smithii.43,44 From the roots of T. foeniculaceum a new trioside—thalifoenoside A (14) was isolated.45 Acid hydrolysis of 14 yielded glucose, rhamnose, quinovose and a new genin—3β,22(S),27-trihydroxycycloart-24-en (15). A comparison of 13C chemical shifts of 15 with those of 22S- and 22R-hydroxycholestanols46 established the C-22 configuration as S. Thalictosides A (16) and C (17) obtained from the aerial part of T. thunbergii47 have been reported to contain a new genin—talictogenin A (15). The S-configuration of C-22 in 16 and 17 was established by Mosher's procedure48 after acetylating the hydroxyl group on C-26. Talictogenin A (15) and the genin of 14 are likely to be the same compound since their NMR spectra are very similar. The Z-configuration of the double bond in both cases was determined by NOE data. A new trioside (18) from the aerial part of T. squarrosum49 also has the same genin 15.
A new glycoside (19)50 from the aerial part T. uchiyamai is the only example of a glycoside with an acetylated genin (on C-3 and C-24 atoms) and which is bound by an ester linkage to a carbohydrate chain. Alkaline hydrolysis of 19 produced artifact (19a). The structural assignment of 19 was carried out on the basis of very convincing spectroscopic evidence.
During investigation of the structure of a new genin, a constituent of both thalictosides V (20) and IX (21) from T. Herba,51 researchers selectively cleaved the ester linkage between the C-21 carboxylic acid group and the carbohydrate chain of 21 using anhydrous LiI and 2,6-lutidine in dry methanol. The product of this reaction turned out to be identical to 20. Glycoside 20 is 3-O-monodesmoside and 21 is 3, 21-di-O-bisdesmoside.
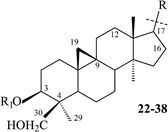
Cycloartanes with a THF ring on C-17 atom. Triterpenoids (28–31) as well as saponins (22–27, 32–38) of this group have been isolated from T. squarrosum, collected in the Baikal region of Russia, and from T. Herba, collected in Japan (Table 2).
| R | No. | R1 | Ref. |
|---|---|---|---|

|
28 | H (squarrogenin 1) | 53 |
| 22 | β-D-Glcp (squarroside A1) | 52 | |
| 23 | α-L-Rhap-(1→6)-β-D-Glcp (squarroside B1) | 52 | |
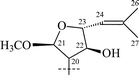
|
29 | H (squarrogenin 2) | 53 |
| 24 | β-D-Glcp (squarroside A2) | 52 | |
| 25 | α-L-Rhap-(1→6)-β-D-Glcp (squarroside B2) | 52 | |
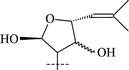
|
30 | H (squarrogenin B3) | 54 |
| 26 | α-L-Rhap-(1→6)-β-D-Glcp (squarroside B3) | 54 | |
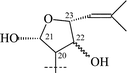
|
31 | H (squarrogenin B4) | 54 |
| 27 | α-L-Rhap-(1→6)-β-D-Glcp (squarroside B4) | 54 | |
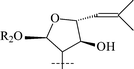
|
32 | α-L-Rhap-(1→6)-β-D-Glcp (squarroside C) | 55 |
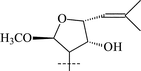
|
33 | α-L-Rhap-(1→2)-β-D-Glcp | 56 |
| 35 | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | 57 | |
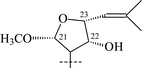
|
34 | α-L-Rhap-(1→2)-β-D-Glcp | 56 |
| 36 | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | 57 | |
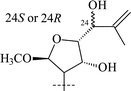
|
37 | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | 58 |
| 38 | α-L-Rhap-(1→2)-[α-L-Rhap-(1→6)]-β-D-Glcp | 58 |
From T. squarrosum several saponins52—squarrosides A1 (22), B1 (23), A2 (24), B2 (25), B3 (26), and B4 (27)—have been isolated, the genins of which have diastereomeric configuration at the C-21 atom. Squarrogenins 1 (28) and 2 (29),53 both possessing 21-OCH3, resulted from enzymatic hydrolysis of squarrosides A1, A2, B1, and B2 (22–25). The configuration of the C-21 atom was determined as R in 28 and S in 29 by NOE experiments. Enzymatic hydrolysis of squarrosides B3 (26) and B4 (27) afforded squarrogenins B3 (30) and B4 (31) having hydroxyl groups in positions 21S and 21R respectively.54 It should be noted that squarrosides B3 and B4 are chromatographically inseparable isomers. The genin of squarroside C (32)55 is squarrogenin B4 and so the 21-hydroxyl group has an α-relative stereochemistry and binds with the carbohydrate chain at the same time. A β-isomer of the trioside has not been found. The 21-O-CH3-glycosides (22–25) proved to be native, i.e. not modified during the isolation procedure. The absence of methanol during the isolation procedure provided evidence of the above conclusion. When ethanol was used all the expected saponins (22–27) as well as the 21-O-ethoxy derivatives were isolated, confirming the ease with which the alkylation of the hemiacetal hydroxyl group takes place. The possibility of alkylation of the hemiacetal hydroxyl group raises the question of whether compounds of this type are native or not.
Those saponins which have been isolated from T. Herba (Takatogusa) are diastereomeric with reference to C-21, (thalictosides I (33) and II (34)56 and thalictosides III (35) and IV (36)57), but unlike those isolated from T. squarrosum they have an α-configured 22-hydroxyl group. Interestingly, the same two monosaccharides comprise the disaccharide of both biosides from T. squarrosum and T. Herba, but the order of attachment is different: it is rhamnose(1→6)glucose in squarrosides B1 and B2 and rhamnose (1→2)glucose in thalictosides I and II. The difference between thalictosides XII (37) and XIII (38)58 from T. Herba consists of the orientation of the genin's C-24 hydroxyl group, the stereochemistry of which was inferred from spectroscopic data.58
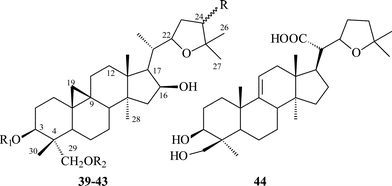
Cycloartanes with a THF ring on C-20 atom. This group is small in number consisting of the four glycosides 39–42 isolated from T. minus (Table 3). Thalicosides A1 (39) and A3 (40)34 are based on a new genin—thalicogenin A1 (43). Thalicosides H1 (41)59 and H3 (42)37 differ from thalicosides A1 and A3 by an additional hydroxyl group on C-24. Glycosides 39–42 have the 22S-configuration. Thalicoside H1 (41) has an S-configured C-24 atom while thalicoside H3 (42) has an R-configuration, as shown by 2D NMR techniques including NOE experiments.60
Squarrofuric acid (44)28,61 from T. squarrosum is believed to be an artifact produced from squarrogenins 1–4 (28–31) during acid hydrolysis of a methanolic extract of the plant, but the mechanism of transformation is not known.
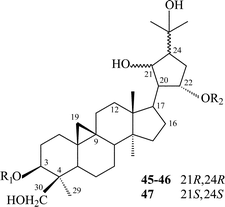
Cycloartanes with a cyclopentane ring on C-17 atom. In this group there are three saponins 45–47 isolated from T. thunbergii (Table 4). All the glycosides are bisdesmosides: their carbohydrate chains are bound with C-3 and C-22 hydroxyl groups. Thalictoside D (45) and thalictoside E (46) have the same genin,62 their differences lie in the structure of carbohydrate chain, glycoside 45 is a pentaoside while glycoside 46 is a hexaoside. The genin of thalictoside F (47)63 is a stereoisomer of the genin of 45 and 46 with opposite configuration of C-21 and C-24 atoms, 45 and 46 have R-configured C-21 and C-24-asymmetric centers, but 47 has these centers in an S-configuration. All structural features including the stereochemistry of the asymmetric centers have been resolved by extensive spectroscopic analyses using 2D NMR and chemical evidence.
| No. | R1–R5 | Name | Plant | Ref. |
|---|---|---|---|---|
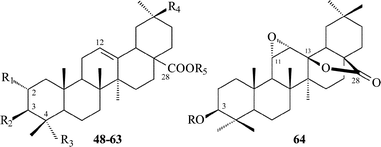
| 48 | R1 = H; R2 = OH; R3 = R4 = CH3; R5 = H | Oleanolic acid | T. minus | 65 |
| 61 | R1 = R2 = OAc; R3 = CH3; R4 = CH2OH; R5 = α-L-Rhap-(1→2)-β-D-Glcp | Aquilegifolin | T. aquilegifolium | 69 |
| 49 | R1 = R2 = OH; R3 = R4 = CH3; R5 = H | Maslinic acid | T. aquilegifolium | 64 |
| 62 | R1 = H; R2 = β-D-Glcp-(1→3)-α-L-Rhap-(1→4)-β-D-Xylp; R3 = CH2OH; R4 = CH3; R5 = β-D-Glcp | Thalictoside VI | T. Herba | 57 |
| 63 | R1 = H; R2 = α-L-Rhap-(1→3)-β-D-Glcp-(1→3)-α-L-Rhap-(1→4)-β-D-Xylp; R3 = CH2OH; R4 = CH3; R5 = β-D-Glcp | Thalictoside VIII | T. Herba | 57 |
| 64 | R = α-L-Rhap-(1→2)-β-D-Glcp-(1→4)-α-L-Ara | Thalicoside F | T. minus | 70 |
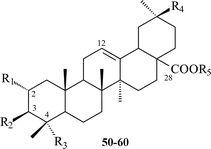
| No. | Glycosides of oleanolic acid (48) | Name | Plant | Ref. |
|---|---|---|---|---|
| 54 | R1 = H; R2 = α-L-Rhap-(1→2)-β-D-Glcp-(1→4)-α-L-Arap; R3 = R4 = CH3; R5 = β-D-Glcp | Thalicoside B | T. minus | 66 |
| 57 | R1 = H; R2 = α-L-Rhap-(1→2)-β-D-Glcp-(1→4)-α-L-Arap; R3 = R4 = CH3; R5 = β-D-Glcp-(1→6)-β-D-Glcp | Thalicoside D | T. minus | 67 |
| 58 | R1 = H; R2 = β-D-Xylp-(1→3)-α-L-Rhap-(1→2)-α-L-Arap; R3 = R4 = CH3; R5 = β-D-Glcp-(1→6)-β-D-Glcp | Foetoside C | T. foetidum | 68 |
| 59 | R1 = H; R2 = α-L-Rhap-(1→3)-β-D-Glcp-(1→3)-α-L-Rhap-(1→4)-β-D-Xylp; R3 = R4 = CH3; R5 = β-D-Glcp | Thalictoside VII | T. Herba | 57 |
| 55 | R1 = H; R2 = β-D-Glcp-(1→4)-α-L-Rhap-(1→2)-β-D-Xylp; R3 = R4 = CH3; R5 = β-D-Glcp | Squarroside II | T. squarrosum | 57 |
| 60 | R1 = H; R2 = β-D-Glcp-(1→4)-[α-L-Rhap-(1→2)]-β-D-Xylp; R3 = R4 = CH3; R5 = β-D-Glcp-(1→6)-β-D-Glcp | Squarroside III | T. squarrosum | 57 |
| 56 | R1 = H; R2 = β-D-Glcp-(1→3)-[β-D-Glcp-(1→3)-α-L-Rhap-(1→2)]-β-D-Xylp; R3 = R4 = CH3; R5 = β-D-Glcp | Squarroside IV | T. squarrosum | 57 |
| 50 | R1 = R5 = H; R2 = α-L-Arap; R3 = R4 = CH3 | T. minus | 65 | |
| 51 | R1 = R5 = H; R2 = β-D-Glcp-(1→4)-α-L-Arap; R3 = R4 = CH3 | T. minus | 65 | |
| 52 | R1 = R5 = H; R2 = α-L-Rhap-(1→2)-β-D-Glcp-(1→4)-α-L-Arap; R3 = R4 = CH3 | T. minus | 65 | |
| 53 | R1 = H; R2 = β-D-Glcp-(1→4)-α-L-Arap; R3 = R4 = CH3; R5 = β-D-Glcp | T. minus | 65 |
As mentioned above, the first triterpenoid glycosides were isolated from the plants of the Thalictrum genus, collected in Eastern Siberia. Triterpenoid compounds have not been discovered in the same species growing in other climatic areas (e.g. Middle Asia and Europe). This information points to a dependence of chemical composition of plants on ecological and on geographical growth conditions, and so we have suggested assigning Eastern Siberia's species to a Siberian chemorace.12 Further investigations by Japanese, Chinese and Korean research groups have shown a great diversity of triterpenoid saponins in plants of the Thalictrum genus collected exclusively in Asia. These observed differences in the chemical composition of the plants of the same Thalictrum species, which grow in different ecologo-georgraphic areas should be reflected in the morphology and anatomy of the plants, but that awaits a thorough study by botanists, morphologists, phylogenists, and ecologists, etc.
Having analyzed many cycloartane structures of the Thalictrum genus we would like to note that the same Thalictrum species produces both cycloartane and oleanane triterpenoids. All of the cycloartane compounds of the Thalictrum genus have the substituents at C-3 β-orientated; and in the A-ring substitution often occurs either at C-29 or C-30. This substitution site is rarely found and is not typical of cycloartanes in other species of plants. The CH2OH at position 29 is glycosylated in some cases (1–8). No glycosides of CH2OH at position 30 have been found. To our knowledge, besides the Thalictrum genus, only from Mangifera indica have two cycloartanes having 30-CH2OH been reported.71 One more position in the polycyclic fragment which can be oxygenated is C-16; those Thalictrum cycloartanes which have a 16-hydroxyl group, have it in the β-orientation. The majority of Thalictrum cycloartanes with an acyclic side chain contain a hydroxyl group at C-22, and where the C-22 configuration is known, it is found to be the S-configuration.
Cycloartane saponins from the Thalictrum genus occur as mono-, bis- and trisdesmosides; the only representative of trisdesmosides is thalicoside C (3). The highest possible number of carbohydrates (5–6) was found in bisdesmosides. The most typical site of glycosylation is on the C-3 of the genin, and in some glycosides the carbohydrate chain is branched. It is worth noting that in 11 and 12 in addition to C-3, the hydroxyl group of C-16, which is sterically hindered, is also glycosylated.
The carbohydrate part of the tetracyclic glycosides has the following sugars: D-glucose, D-galactose, L-rhamnose, D-quinovose, D-fucose, D-xylose and L-arabinose in pyranose form (except xylofuranoside 19 from T. uchiyamai25). An acetyl sugar was revealed in a single glycoside—14.
Pentacyclic saponins of Thalictrum have carbohydrate chains on C-3 and C-28. It is interesting to note that from 15 oleanane glycosides there are just two whose genins have nontrivial structures: diacyl genin in 61 (T. aquilegifolium)69 and a derivative of oleanolic acid (64) which possesses a 11α,12α-epoxy ring and 28,13β-γ-lactone ring (T. minus).70 The carbohydrate part of the pentacyclic glycosides has the following sugars: D-glucose, L-rhamnose, D-xylose and L-arabinose in pyranose form only.
3.2 Biological activity of the saponins of the Thalictrum genus
Natural triterpenoid glycosides show a wide spectrum of biological activity, including fungicidal, antibacterial, antiphlogistic, antineoplastic, antiallergic and immunostimulating activity.72 Saponins have a significant effect on the metabolism as well as on the cardiovascular system of mammals.73–75 There is also abundant evidence on the impact of triterpenoid glycosides on the fertility of animals.76–77It has been suggested that the basis for such diverse medical–biological actions of triterpenoid glycosides is their ability both to influence biological processes taking place in the cell, and to modify structural–functional features of biological membranes and thus to act as bioregulators.78,79
During their study of the biological activity of the triterpenoid saponins of plants of the Thalictrum genus, Japanese scientists, who have published extensively on the isolation of triterpenoid glycosides from the genus, indicate that the Thalictrum species have been used in folk medicine in Japan for treating stomach diseases. Among glycosides isolated by them there is a series of cycloartane compounds (with a THF ring on C-17) that demonstrate a high LTT activity (lymphocyto transformation test).5 Thalicoside A2 (4) showed inhibitory activity against Candida albicans (78.7%) and against Staphylococcus aureus (45.7%), both at a concentration of 1 mg mL−1.34
The sum of the triterpenoid glycosides from T. foetidum (cyclofoetosides A (9), B (10) and foetoside C (58)) in addition to foetoside C (58) alone were separately studied using mammals having an experimental endogenous hypercholesterinemia. The results of introducing the sum of the foetosides and foetoside C (7 days with a dose of 50 mg kg−1) showed that both preparations influenced both lipidic and cholesteric metabolisms. The sum of compounds 9, 10 and 58 decreased the level of cholesterol in blood serum by 25.5 mg%, while compound 58 reduced the level of cholesterol by 60.0 mg% relative to the control data.80–81
Some individual glycosides, isolated from T. minus and T. foetidum were investigated for antineoplastic activity on mammals.82 The most interesting results were obtained for foetoside C (58), thalicoside A (1) and cyclofoetoside B (10). Oleanane pentaoside 58 in a dose of 30 mg kg−1 inhibited the growth of transplantable tumor strains as follows: sarcoma: 45–91%, breast cancer RMK: 1–85%, Pliss malignant lymphoma (lymphosarcoma) and Walker carcino(sarco)ma: 84–86%. Cycloartane bioside 1 gently inhibited the growth of Pliss malignant lymphoma (lymphosarcoma), sarcoma and RMK-1 (73–77%). Sarcoma 45 was sufficiently sensitive to compound 10 that 87% of the tumor growth was inhibited.
Data obtained on the efficiency of compound 58 in experiments with rats using the resistant strains of transplantable tumor are of particular interest. The growth of sarcoma 45, which is resistant to sarcolysine applied in cancer clinics, slowed down under the effect of pentaoside 58 by 90%. The other two substances demonstrated a moderate (1) or weak (10) activity.82
Mats and co-authors76 studied in detail the contraceptive activity of triterpenoid glycosides T. minus, T. foetidum and T. squarrosum. Their results indicated that at the subcutaneous post coitum, introduction of the triterpenoid glycosides of T. minus demonstrated the greatest activity, while the glycosides of T. foetidum and T. squarrosum showed a smaller contraceptive effect.
Weakly toxic (LD50 1800 mg kg−1) cycloartane glycoside 1 of T. minus has shown a high (80–100%) post coitum activity in small doses (0.001–0.1 mg kg−1). Analysis of the mechanism of contraceptive action of thalicoside A indicates that the effect is due to the influence of this compound on the formation of endometrium and on the rate of ovule transport.76
Korkhov and co-authors77 have investigated the influence of compound 1 on ovulation and on the level of gonadotropins in blood serum of laboratory animals. Their results indicate that introducing glycoside 1 orally for 5 days at a dose of 1 mg kg−1 to the rabbits stimulates ovulation. The same scientists revealed that compound 1 changes the level of gonadotropin yield: at 5 days enteral introduction in a dose of 1 mg kg−1 to rats it reduced the content of luteinizing [interstitial cell-stimulating] hormone and increased the content of follicle-stimulating hormone during active stages of the proestrus–estrus cycle. The detected effect of disrhythmia in incretion of gonadotropins caused by thalicoside A, most likely can be used medicinally for different forms of polycystic ovary accompanied with unbalance of hormones.
These studies of the biological activity of triterpenoid saponins from the Thalictrum genus indicate possible directions of their potential usage, viz. as non-hormonal substances having significant antitumoral activity that have an influence on the reproductive system of mammals, even in small doses.
3.3 Flavonoids and their glycosides
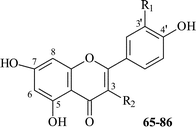
Flavonoids are widely distributed in species of Thalictrum, being mostly present as glycosides of flavones and flavonols (Table 7).| No. | R1, R2, carbohydrate chain (if present) | Plant | Ref. |
|---|---|---|---|
| a The site of attachment of the second glucose is unclear. | |||
| 65 | 7-O-β-D-Gal, R1 = R2 = H (talictin) | T. thunbergii | 83 |
| 66 | 7-O-diGlca, R1 = R2 = H | T. rugosum | 84 |
| 67 | 7, 4′-bis-O-β-D-Allop, R1 = R2 = H | T. thunbergii; | 85 |
| T. minus; | 86 | ||
| T. squarrosum | 86 | ||
| 68 | 7-O-(6″-mono-O-acetyl-β-D-Allop), 4′-O-β-D-Allop, R1 = R2 = H | T. thunbergii; | 85 |
| T. minus; | 86 | ||
| T. squarrosum | 86 | ||
| 69 | 7-O-(4″,6″-di-O-acetyl-β-D-Allop), 4′-O-β-D-Allop, R1 = R2 = H | T. thunbergii | 85 |
| 70 | 7-O-β-D-Glcp, R1 = OH, R2 = H (cinaroside) | T. rugosum | 84 |
| 71 | 7-O-(6″-O-α-L-Rhap)-β-D-Glcp, R1 = R2 = H, 4′-OCH3 (linarin) | T. aquilegifolium; | 88 |
| T. simplex; | 98 | ||
| T. przewalskii; | 91 | ||
| T. smithii Boivin | 99 | ||
| 72 | 7-O-[6″-O-(4‴-O-acetyl-α-L-Rhap)]-β-D-Glcp, R1 = R2 = H, 4′-OCH3 | T. aquilegifolium; | 87 |
| T. przewalskii | 91 | ||
| 73 | 7-O-[6‴-O-(4⁗-O-acetyl-α-L-Rhap)-3″-O-β-D-Glcp]-6″-O-acetyl-β-D-Glcp, R1 = R2 = H, 4′-OCH3 | T. przewalskii | 91 |
| 85 | R1 = H, R2 = OH (kaempferol) | T. atriplex | 95 |
| 86 | R1 = H, R2 = OCH3 (isokaempferide) | T. minus (roots) | 87 |
| 79 | R1 = H, R2 = O-(6″-O-α-L-Rhap)-β-D-Glcp | T. rugosum | 90 |
| 78 | R1 = H, R2 = O-β-D-Glcp (astragalin) | T. przewalskii | 91 |
| 80 | R1 = H, R2 = O-[6″-O-(3‴-O-acetyl-α-L-Ara)-β-D-Glcp] | T. atriplex | 92 |
| 81 | R1 = OH, R2 = O-β-D-Glcp | T. atriplex | 93 |
| 82 | R1 = OH, R2 = O-(6″-O-α-L-Rhap)-β-D-Glcp | T. foetidum; | 94 |
| T. minus; | 90 | ||
| T. rugosum | 90 | ||
| 83 | R1 = OH, R2 = O-β-D-Glcp, 7-O-CH3 | T. foetidum | 94 |
| 74 | 6-C-β-D-Glcp, R1 = R2 = H (saponaritin) | T. dasycarpum; T. minus; T. minus race B; T. rugosum | 90 |
| 75 | 6-C-β-D-Glcp, R1 = OH, R2 = H (isoorientin) | T. dasycarpum; T. minus; T. minus race B; T. rugosum | 90 |
| 76 | 8-C-β-D-Glcp, R1 = R2 = H (vitexin) | T. dasycarpum | 90 |
| 77 | 8-C-β-D-Glcp, R1 = OH, R2 = H (orientin) | T. dasycarpum; T. minus; T. minus race B; T. rugosum | 90 |
| 84 | 6-C-β-D-Glcp, 5-O-α-L-Rhap, R1 = R2 = H (isovitexin 5-rhamnoside) | T. rugosum | 84 |
Flavone O-glycosides are mostly those of apigenin, luteolin and acacetin 7-O-glycosides (65–73). There are two monoglycosides of apigenin (65, 66)83,84 and three apigenin diglycosides, being bisdesmosides (67–69),85–87 where the second carbohydrate chain is bound to the 4′-phenolic hydroxyl group. In glycosides 68 and 69, allopyranose, which is bound to the 7-OH group, is acetylated. Acetyl-sugars are characteristic of acacetin glycosides (72, 73), one of them being a disaccharide (72)88 and another—a trisaccharide (73).89 It is interesting to note that acetyl-derivatives of flavonoid glycosides have only been isolated from Thalictrum species growing in Asia. The only reported glycoside of luteolin is 7-O-glucoside (70)84 from T. rugosum.
Flavone C-glycosides are widespread in nature and those present in Thalictrum are 6-C-glucosides of apigenin (saponaretin) (74),90 luteolin (isoorientin) (75),90 8-C-glucosides of apigenin (vitexin) (76),90 and luteolin (orientin) (77).90 Apigenin 6-C-glucoside, 5-O-rhamnoside (84)84 has been isolated from T. rugosum. Flavone C-glycosides have only been isolated from Thalictrum species growing in Europe.
Flavonol glycosides found in Thalictrum are exclusively those of the 3-O-glycosides. There are three kaempferol glycosides known, one of them: monoglycoside (78),91 the others are diglycosides (79, 80).89,9280 contains acetyl-arabinose and has been isolated from T. atriplex collected in China. The quercetin glycosides include glucoside (81)93 and rutinoside (82).90,94 The only glycoside of rhamnetin found in T. foetidum is a glucoside (83).94 It should be noted that of the 22 flavonoids represented in Table 7, nine are new glycosides; these include 65,8367–69 from T. thunbergii,85T. minus, T. squarrosum,86,877288 from T. aquilegifolium, 7389 from T. przewalskii, 8092 from T. atriplex, 8294 from T. foetidum, and 8484 from T. rugosum.
Kaempferol (85)95 itself was found in T. atriplex, its methyl ether (86)86 was reported to be found in the roots of T. minus. Kaempferol and quercetin, together with two unidentified flavonoids, have been identified by analytical paper chromatography in the leaves and flowers of T. delavay Franch, T. flavum L., T. foetidum, and T. medium Poir.96 By the same method, hydrolyzates of extracts of T. angustifolium Jacq., T. coronellum D.C., T. fendleri Engelm, T. minus var. adiantifolium, T. minus L., T. petaloideum, T. purpurascens D.C., T. sparsiflorum Rurch, T. squarrosum Steph., and T. thuberosum L. have been screened, and apigenin, luteolin, kaempferol and quercetin have been identified in the plants.84,97 Quercetin has been found in T. aquilegifolium L., and kaempferol in T. calabricum Spreng, while both of them have been found in T. rugosum.84 These articles represent the first reports devoted to the investigation of Thalictrum flavonoids.
3.4 Cyanogenic glycosides
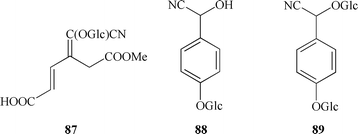
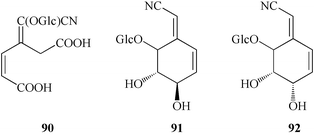
In the Thalictrum genus, cyanogenesis has been discovered by van Itallie et al.,100 who reported that the leaves of T. aquilegifolium liberated HCN (about 0.05% of fresh weight) on distillation after being ground and allowed to stand at 30 °C for a period. In addition to HCN, acetone was also detected in the distillate, so acetone cyanhydrin was postulated to have been the cyanogenic principle. Later Sharples et al.101–103 established the structures of the cyanogenic glycosides from T. aquilegifolium. The major cyanogenic constituent was found to be the monomethyl ether of isotriglochinin (87), which accounted for 90% of the cyanogenic content of the plant. The structures of the minor glycosides were shown to be p-glucosyloxymandelonitrile (88) and p-glucosyloxymandelonitrile-β-D-glucoside (89), each of them accounting for 5% of the cyanogenic content.Tryglochinin (90) was discovered in T. polygamum and T. aquilegifolium,104,105 and the authors of the first mentioned work made the assumption that tryglochinin is an indigenous glycoside of T. aquilegifolium and that its esterification (and probably isomerisation) to monomethyl ether of isotriglochinin took place during extraction and purification performed by Sharples etc.
A cyclic unsaturated cyanogenic glycoside 91 (lithospermoside) has been isolated from T. rugosum Ait., T. revolutum106 and T. orientale Boiss.,107 and its 4-epimer 92 (dasycarponin) has been found in T. dasycarpum Fisch. and Lall.106 The absolute stereochemistry of the asymmetric centers of the molecules was proved convincingly using circular dichroism, and NMR spectral data.
In addition to the above mentioned Thalictrum species, several other species have also been found to be cyanophoric: T. foetidum L. (weakly cyanophoric), T. flexuosum Berng. and T. minus L. (very weakly cyanophoric), T. dipterocarpum Franch.104 (releases trace amounts of HCN), and T. polycarpum,108 however the composition of their cyanogenic components has not been established.
The biosynthesis and subsequent accumulation of cyanogenic glycosides probably represents a defence for the plant from attack by herbivores. Within the Thalictrum genus species; the ability to synthesize cyanogens is expressed differently and appears to correlate with ecologo-geographic conditions of growth and numbers of herbivores.109
3.5 Hydrocarbons and high molecular weight alcohols
Hydrocarbons and high molecular weight alcohols are common metabolites in higher plants. In the Thalictrum genus these constituents have only been discovered in two species: T. aquilegifolium110 and T. simplex.98 The identification and relative quantities of the hydrocarbons were determined by gas liquid chromatography-mass spectroscopy (GC-MS), (the alcohols were preliminary acetylated). In the dried whole plant (including the roots) of T. aquilegifolium 13 n-alkanes and 9 alcohols were found. These included normal saturated hydrocarbons C23–C35, in which n-C29H60 and n-C31H64 were the major components. All identified alcohols had normal saturated structures (C28–C36) and the majority consisted of n-C32H65OAc along with n-C34H69OAc.From T. simplex all the above-mentioned compounds have been characterized including two other hydrocarbons (C21 and C22). It was reported that the C23–C28 fraction was dominant in this instance.
3.6 Phenolic compounds
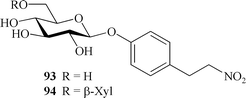
Some Thalictrum species represent a source of unusual nitrogen-containing compounds, namely, T. aquilegifolium110–111 and T. orientale.107 Besides the cyanogenic glycosides described above, other types of phenolic glycosides containing a nitro group have been isolated from these species. The first representative was thalictoside (93).110–111 Its structure was established by NMR spectroscopy and synthetic methods. T. aquilegifolium is poisonous for domestic animals. Research into the poisonous compounds of this plant enabled the authors to isolate thalictoside, but a toxicity test, performed by injection of 93 into the abdominal cavities of mice did not show the expected level of toxicity.A compound (94) isolated from the underground part of T. orientale contained a disaccharide fragment that was bound with the same phenylethyl moiety as (93).107 The complete assignment of the structure was based on 2D NMR and HR-MALDI mass spectral data.
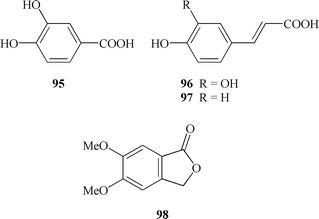
Three phenolic acids have been isolated from the aerial part of T. atriplex.95 These are protocatechuic acid (95), caffeic (96) and p-coumaric acids (97).
From the roots of T. dioicum, used medicinally in Mexico as a diuretic, m-meconina (5,6-dimetoxiftalid) (98) has been isolated.112
3.7 Fatty acids
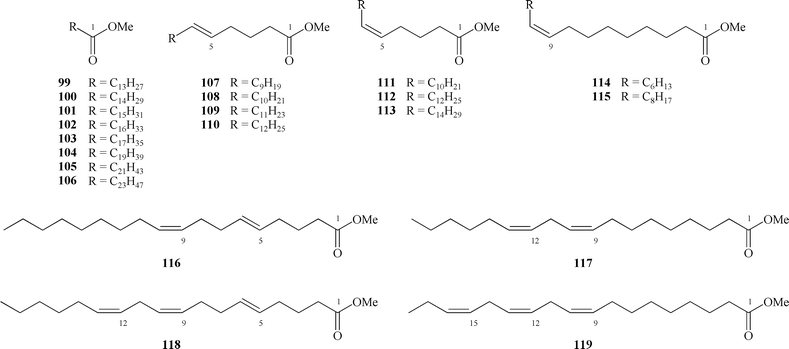
The fatty acids of the Thalictrum genus have been studied rather extensively: in 17 plant species studied (including various populations of the same species) 21 saturated and unsaturated acids have been identified. The Thalictrum genus is the only known natural source of a unique class of fatty acids having an isolated trans-double bound at position-5.The first report of fatty acids from Thalictrum (T. polycarpum) was by Bagby, et al.113 in which 5-trans-octadecenoic acid (110), and 5-trans,9-cis,12-cis-octadecetrienoic acids (118) were identified, and two other C18 acids which were not characterized (C18-dienoic acid and an unknown C18-acid). Bhatty et al.114 have established that the seed oil of T. venulosum contains acids 110, 118, and the previously unknown 5-trans-hexadecenoic (108) and 5-trans,9-cis-octadecadienoic (116) acids. Traces of 5-trans-pentadecenoic acid (107) and 5-trans-heptadecenoic acid (109) were also detected. Moreover, Bhatty et al. found fatty acids having only a cis-double bond (111–115, 117, 119) as well as other saturated acids (99–106). Thus, T.venulosum oil has been studied with more detail than any other species of Thalictrum.
The composition of seed oils from other studied species of Thalictrum closely resembles that reported from T. polycarpum and T.venulosum.
The presence of four unsaturated 5-trans series fatty acids—108, 110, 116, and 118—has been reported by Rankoff et al. in different species of the Thalictrum genus (Bulgarian populations: T. aquilegifolium, T. adiantifolium, T. glaucum, T. minus Balchik, T. minus Losen, T. minus Sofia),115 by Wu (T. revolutum D.C.),116 by Ucciani (T. flavum and T. morisinii),117 and by Freiman (Uzbekistan population: T. minus, T. flavum, T. foetidum, T. simplex and T. sultanobadens).118–119 In leaves of T. simplex (Eastern Siberia population) Trofimova et al.98 have isolated saturated fatty acids (99–104), in which acids C16 and C18 were dominant. Thus, from 21 fatty acids detected in the Thalictrum genus, 8 acids (99–106) are saturated (5–10%), 9 acids (107–115) are monoenes (12–49%), 2 acids (116, 117) are dienes (22–27%) and 2 acids (118, 119) are trienes (30–56%). The fatty acid composition of seed oils of all investigated species were shown to consist of about 50–60% fatty acids having double bonds in a trans-position. The major component of the oil is 5-trans,9-cis,12-cis-octadecatrienoic acid. The number, the position and the configuration of double bonds in the unsaturated acids was correctly established using chemical (studying the products of oxidation and reduction) and spectroscopic methods (IR-, UV-, MS- and GLC of methyl esters). All acids were identified as the methyl esters by gas chromatography.
3.8 Phytosterols and their glycosides
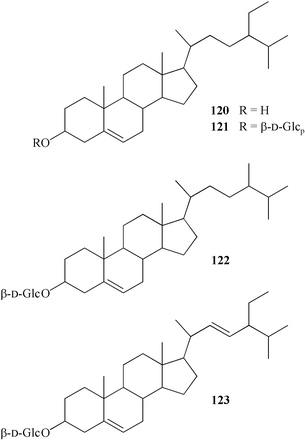
β-Sitosterol (120) has been isolated from the roots of T. dioicum,112 and the aerial parts of T. aquilegifolium,110T. atriplex95 and T. foeniculaceum.45 β-Sitosterol 3-O-β-D-glucopyranoside (121) was also obtained from the aerial part of T. aquilegifolium,110T. minus L., T. squarrosum St. ex Willd, and T. foetidum.120 From T. simplex a fraction of phytosterol glucosides was isolated.98 After acid hydrolysis of this fraction β-sitosterol [M+ 414], campesterol [M+ 400] (122), and stigmasterol [M+ 412] (123) were identified by GS-MS spectral analysis. Glucose was the only carbohydrate in the aqueous part of the hydrolysate.4 References
- Flora Sibiri, ed. L. I. Malyshev and G. I. Peshkova, Nauka, Novosibirsk, 1993, vol. 6, pp. 198–206 Search PubMed.
- Rastitel'nii resursi USSR, ed. A. A. Fedorov, Nauka, Leningrad, 1984, 90 pp Search PubMed.
- A. I. Shreter, Lekarstvennaya flora sovetskogo Dal'nego Vostoka, Medisina, Moskva, 1975, 384 pp Search PubMed.
- V. V. Telyat'ev, Poleznie rasteniya Tsentral'noi Sibiri, Vostochno-Sibirskoe izdatelstvo, Irkutsk, 1985, 328 pp Search PubMed.
- T. Nohara, S. Yahara and J. Kinjo, in: Advances in experimental medicine and biology, vol. 404, Saponins used in traditional and modern medicine, ed. G. R. Waller and K. Yamasaki, Plenum Press, New York, London, 1996, p. 263 Search PubMed.
- S.-B. Chen, S.-L. Chen and P.-G. Xiao, J. Asian Nat. Prod. Res., 2003, 5, 263–271 Search PubMed.
- P. L. Shiff, in: Alkaloids: Chemical and Biological Perspectives, Pergamon, New York, 1987, p. 271 Search PubMed; P. L. Shiff, Chem. Abstr., 1988, 107, 228242u Search PubMed.
- P. L. Shiff, in: Alkaloids: Chemical and Biological Perspectives, vol. 11, Pergamon, New York, 1996, pp. 1-171 Search PubMed.
- S. Ju. Junusov, Alkaloidi, FAN, Tashkent, 1981 Search PubMed.
- R. Djurkovic, O. Gasic and Y. Popovic, Hem. Pregl., 1991, 32, 86–92 Search PubMed; R. Djurkovic, O. Gasic and Y. Popovic, Chem. Abstr., 1993, 118, 22445f.
- B. Kuzmanov and H. Dutschewska, J. Nat. Prod., 1982, 45, 766–771 CrossRef CAS.
- A. A. Semenov, V. I. Lutsky, A. S. Gromova, T. V. Ganenko, E. A. Khamidullina and N. N. Trofimova, in: Advances in experimental medicine and biology, Vol. 405, Saponins used in food and agriculture, ed. G. R. Waller and K. Yamasaki, Plenum Press, New York and London, 1996, p. 193 Search PubMed.
- A. S. Gromova, V. I. Lutsky, E. V. Kosteleva, N. N. Dedkova and A. A. Semenov, Izv. Sib. Otd. Akad. Nauk SSSR, 1981, 129–132 Search PubMed.
- A. A. Semenov, V. I. Lutsky, A. S. Gromova, E. V. Slavyanov and V. N. Biushkin, in: Abstracts of the First International Conference on Chemistry and Biotechnology of Biologically Active Natural Products, vol. 3-1. Varna, Bulgaria, 1981, pp. 135-139 Search PubMed.
- T. V. Ganenko, A. S. Gromova, V. I. Lutsky and A. A. Semenov, Khim. Prir. Soedin., 1982, 262 CAS.
- A. S. Gromova, V. I. Lutsky, A. A. Semenov, E. V. Slavyanov, V. N. Biushkin and G. I. Malinovsky, Khim. Prir. Soedin., 1982, 718–723 CAS.
- A. S. Gromova, V. I. Lutsky, A. A. Semenov, V. A. Denisenko and V. V. Isakov, in: Abstracts of the Second International Conference on Chemistry and Biotechnology of Biologically Active Natural Products, Budapest, Hungary, 1983, p. 160 Search PubMed.
- A. Cronquist, An Integrated system of Classification of flowering plants, Columbia University Press, New York, 1981, 1249 pp Search PubMed.
- A. L. Takhtadxhan, Sistema I filogenia tsvetkovih rastenii, Nauka, Moskow–Leningrad, 1966, vol. 67. p. 611 Search PubMed.
- Zhizn rastenii, ed. A. L. Takhtadzhan, Prosvechenie, Moskva, 1980, vol. 5(1), pp. 210–216 Search PubMed.
- S. N. Ziman, Bot. Zh., 1980, 65, 1120–1130 Search PubMed.
- W. C. Gregory, Trans. Am. Philos. Soc., 1941, 31, 443–521 Search PubMed.
- V. V. Bochanseva, Bot. Zh., 1973, 57, 1109–1110 Search PubMed.
- M. Tamura, Sci. Rep. Osaka Univ., 1968, 17, 41–56 Search PubMed.
- G. I. Ponomarchuk and K. N. Ulanova, Prirodnaya flora Dal'nego Vostoka, DVGY, Vladivostok, 1977, pp. 126–131 Search PubMed.
- I. A. Gubanov, I. L. Krylova and V. L. Tikhonova, Dikorastoochie poleznii rastenia USSR, Misl', Moskva, 1976, p. 360 Search PubMed.
- T. V. Ganenko, M. I. Isaev, A. S. Gromova, N. D. Abdullaev, V. I. Lutskii, M. F. Larin, A. A. Semenov, M. B. Gorovits and N. K. Abubakirov, Khim. Prir. Soedin., 1986, 312–320 CAS.
- A. S. Gromova, V. I. Lutskii, A. A. Semenov, M. F. Larin and R. B. Valeev, Khim. Prir. Soedin., 1987, 376–384 CAS.
- A. S. Gromova, V. I. Lutskii, A. A. Semenov, V. A. Denisenko and V. V. Isakov, Khim. Prir. Soedin., 1984, 213–219 CAS.
- A. S. Gromova, V. I. Lutskii, A. A. Semenov, V. A. Denisenko and V. V. Isakov, Khim. Prir. Soedin., 1984, 207–213 CAS.
- F. W. Wehrli and T. Nishida. in Progress in the Chemistry of Organic Natural Products, vol. 36, Springer-Verlag, Wien, New York, 1979, pp. 1–229 Search PubMed.
- H. Beierbeck, J. K. Saunders and J. W. ApSimon, Can. J. Chem., 1977, 55, 2813–2828 CAS.
- A. S. Gromova, V. I. Lutskii, S. V. Zinchenko, N. N. Trofimova, A. A. Semenov and N. A. Nahova, Khim. Prir. Soedin., 1993, 103–109.
- A. S. Gromova, V. I. Lutsky, D. Li, S. G. Wood, N. L. Owen, A. A. Semenov and D. M. Grant, J. Nat. Prod., 2000, 63, 911–914 CrossRef CAS.
- A. S. Gromova, V. I. Lutskii, S. V. Zinchenko, T. V. Ganenko and A. A. Semenov, Khim. Prir. Soedin., 1993, 567–571 CAS.
- N. N. Trofimova, A. S. Gromova, V. I. Lutskii, A. A. Semenov, S. A. Avilov, A. I. Kalinovskii, D. Li and N. L. Owen, Russ. Chem. Bull., 1998, 47, 1395–1398 CrossRef CAS.
- N. N. Trofimova, A. S. Gromova, V. I. Lutsky and A. A. Semenov, in: Medicinal raw material and phytopreparations for medicine and agriculture (Abstracts of International Conference) Karaganda, 1999, p. 91 Search PubMed.
- S. Seo, J. Tomita, K. Tori and J. Yashimura, J. Am. Chem. Soc., 1978, 100, 3331–3339 CrossRef CAS.
- T. V. Ganenko, M. I. Isaev, V. I. Lutsky, A. A. Semenov, N. D. Abdullaev, M. B. Gorovits and N. K. Abubakirov, Khim. Prir. Soedin., 1986, 66–71 CAS.
- T. V. Ganenko, M. I. Isaev, A. S. Gromova, N. D. Abdullaev, A. A. Semenov, M. B. Gorovits and N. K. Abubakirov, Khim. Prir. Soedin., 1986, 341–345 CAS.
- T. V. Ganenko, M. I. Isaev, M. B. Gorovits, N. D. Abdullaev, V. I. Lutsky, A. A. Semenov and N. K. Abubakirov, Khim. Prir. Soedin., 1985, 370–375 CAS.
- T. V. Ganenko, M. I. Isaev, A. S. Gromova, N. D. Abdullaev, V. I. Lutsky, M. F. Larin, A. A. Semenov, M. B. Gorovits and N. K. Abubakirov, Khim. Prir. Soedin., 1986, 312–320 CAS.
- S. C. Yu, Q. L. Wu, L. W. Wang and P. G. Xiao, Chin. Chem. Lett., 1999, 10, 485–486 CAS.
- S. C. Yu, Q. L. Wu, L. W. Wang and P. G. Xiao, Zhongcaoyao, 1999, 30, 321–323 Search PubMed.
- Y. Yijun and W. Zhixing, J. China Pharm. Univ., 1991, 22, 270–274 Search PubMed.
- Y. Letourneux, Q. Khuong-Huu and M. Gut, J. Org. Chem., 1975, 40, 1674–1675 CrossRef CAS.
- H. Yoshimitsu, K. Hayashi, K. Shingu, J. Kinjo, S. Yahara, K. Nakano, K. Murakami, T. Tomimatsu and T. Nohara, Chem. Pharm. Bull., 1992, 40, 2465–2468 CAS.
- J. A. Dale and H. S. Moscher, J. Am. Chem. Soc., 1973, 95, 512–519 CrossRef CAS.
- H. Yoshimitsu, M. Nishida, Z.-Z. Qian, Z.-H. Lei and T. Nohara, Chem. Pharm. Bull., 2000, 48, 828–831 CAS.
- Y.-H. Choi, N. G. Kim and I. R. Lee, Arch. Pharmacal. Res., 1996, 19, 429–431 Search PubMed.
- H. Yoshimitsu, K. Hayashi, M. Kumabe and T. Nohara, Phytochemistry, 1995, 38, 939–942 CrossRef CAS.
- E. A. Khamidullina, A. S. Gromova, V. I. Lutskii, A. L. Vereschagin, A. A. Semenov and M. F. Larin, Khim. Prir. Soedin., 1989, 516–523 CAS.
- V. I. Lutskii, E. A. Khamidullina, A. S. Gromova and A. A. Semenov, Khim. Prir. Soedin., 1989, 510–516 CAS.
- E. A. Khamidullina, A. S. Gromova, V. I. Lutskii, S. V. Zinchenko and A. A. Semenov, Russ. Chem. Bull., 1996, 45, 1476–1480 CrossRef.
- E. A. Khamidullina, A. S. Gromova, V. I. Lutsky, D. Li and N. L. Owen, J. Nat. Prod., 1999, 62, 1586–1588 CrossRef CAS.
- H. Yoshimitsu, K. Hayashi, M. Kumabe and T. Nohara, Chem. Pharm. Bull., 1993, 41, 786–788 CAS.
- H. Yoshimitsu, K. Hayashi, M. Kumabe and T. Nohara, Chem. Pharm. Bull., 1994, 42, 101–104 CAS.
- H. Yoshimitsu, K. Hayashi, M. Kumabe and T. Nohara, Nat. Med. (N. Y.), 1997, 51, 131–133 Search PubMed.
- N. N. Trofimova, A. S. Gromova, V. I. Lutsky, A. A. Semenov, S. A. Avilov, D. Li and N. L. Owen, Russ. Chem. Bull., 1999, 48, 596–599 CrossRef CAS.
- N. N. Trofimova, Issledovanie minornikh triterpenoidnikh glikozidov Thalictrum minus L., PhD thesis, Irkutsk State University, 1999 Search PubMed.
- Ju. V. Gatilov, I. Ju. Bagryanskaya, V. I. Lutskii, A. S. Gromova and A. A. Semenov, Khim. Prir. Soedin., 1987, 533–537.
- H. Yoshimitsu, M. Nishida, S. Yahara and T. Nohara, Tetrahedron Lett., 1998, 39, 6919–6920 CrossRef CAS.
- H. Yoshimitsu, M. Nishida and T. Nohara, Tetrahedron, 2001, 57, 10247–10252 CrossRef.
- H. Ina and H. Iida, Chem. Pharm. Bull., 1986, 34, 726–729 CAS.
- A. S. Gromova, V. I. Lutsky, A. L. Vereschagin and A. A. Semenov, Khim. Prir. Soedin., 1987, 107–111 CAS.
- A. S. Gromova, V. I. Lutsky, A. A. Semenov, R. B. Valeev, G. A. Kalabin and Yu. N. El'kin, Khim. Prir. Soedin., 1985, 670–676 CAS.
- A. S. Gromova, A. A. Semenov, V. I. Lutsky, S. V. Zinchenko, N. N. Trofimova and Ya. V. Rashkes, Khim. Prir. Soedin., 1994, 398–403 CAS.
- T. V. Ganenko, M. I. Isaev, T. T. Gorovits, A. S. Gromova, V. I. Lutsky, A. A. Semenov and N. K. Abubakirov, Khim. Prir. Soedin., 1984, 458–463 CAS.
- H. Ina, Y. Ohta and H. Iida, Phytochemistry, 1985, 24, 2655–2657 CrossRef CAS.
- A. S. Gromova, V. I. Lutsky, A. A. Semenov, D. Li and N. L. Owen, Phytochemistry, 1998, 47, 437–440 CrossRef CAS.
- M. A. Khan, S. S. Nizami, M. N. I. Khan, S. W. Azeem and Z. Ahmed, J. Nat. Prod., 1994, 57, 988–991 CrossRef CAS.
- M. F. Balandrin, in: Advances in experimental medicine and biology, vol. 404, Saponins used in traditional and modern medicine, ed. G. R. Waller and K. Yamasaki, Plenum Press, New York, London, 1996, pp.1–14 Search PubMed.
- S. B. Mahato and A. K. Nandy, Phytochemistry, 1991, 30, 1357–1390 CrossRef CAS.
- S. B. Mahato, S. K. Sarkar and G. Poddar, Phytochemistry, 1988, 27, 3037–3067 CrossRef.
- M. M. Anisimov and V. Ya. Chirva, Usp. Sovrem. Biol., 1980, 90, 351–364 Search PubMed.
- M. N. Mats, V. V. Korkhov, V. I. Lutsky, A. S. Gromova, T. V. Ganenko and A. A. Semenov, Rastit. Resur., 1988, 570–574 Search PubMed.
- V. V. Korkhov, V. V. Boikova and E. A. Lesik, Eksp. Klin. Farmakol., 1995, 58, 43–44 Search PubMed.
- M. M. Anisimov, L. I. Strigina, P. G. Gorovoi, D. L. Aminin and I. G. Agafonova, Rastit. Resur., 2000, 1, 107–129 Search PubMed.
- B. A. Salahutdinov, D. N. Dalimov, T. F. Aripov, I. I. Tukfatulina, R. X. Ziyatdinova, A. Zh. Dzhuraev, F. G. Kamaev, L. Ju. Izotova, B. T. Ibragimov, I. Mavridis and P. Giastas, Khim. Prir. Soedin., 2002, 209–215.
- T. V. Ganenko, Triterpenoid glycosides and their genins of Thalictrum foetidum L.; PhD thesis, Irkutsk Institute of organic chemistry, 1986 Search PubMed.
- T. V. Ganenko, A. S. Gromova, V. I. Lutsky and A. A. Semenov, in: Abstracts of Third International Conference on Chemistry and Biotechnology of Biologically Active Natural Products, Sofia, Bulgaria, 1985, p. 330 Search PubMed.
- K. D. Rakhimov, S. M. Vermenichev, V. I. Lutsky, A. S. Gromova, T. V. Ganenko and A. A. Semenov, Khim.-Farm. Zh., 1987, 1434–1436 CAS.
- H. Sekiguti, Yakugaku Zasshi, 1960, 80, 759–760.
- L. Kuczynski and T. Chyczewski, Diss. Pharm. Pharmacol., 1971, 23, 519–527 Search PubMed.
- E. Shimizu, T. Tomimatsu and T. Nohara, Chem. Pharm. Bull., 1984, 32, 5023–5026 CAS.
- E. A. Khamidullina, A. S. Gromova, V. I. Lutskii, A. A. Semenov, D. Li and N. L. Owen, Russ. Chem. Bull., 1999, 48, 390–392 CrossRef CAS.
- E. A. Khamidullina, N. N. Trofimova, A. S. Gromova and V. I. Lutskii, Khim. Interesakh Ustoich. Razvit., 1999, 7, 195–197 Search PubMed.
- H. Ina and H. Iida, Phytochemistry, 1981, 20, 1176–1177 CrossRef CAS.
- C. S. Yu, L. Q. Wu, W. L. Wang and G. P. Xiao, Chin. Chem. Lett., 1999, 10, 295–296.
- H. Wagner and M. A. Iyengar, Phytochemistry, 1971, 10, 2553–2554 CrossRef CAS.
- Y. Shi-Chun, W. Qing-Li, W. Li-Wei, Y. Jun-Shan and X. Pei-Gen, J. Asian Nat. Prod. Res., 1999, 1, 301–306 Search PubMed.
- G. Guangyao, C. Sibao, Y. Junshan and X. Peigen, Fitoterapia, 2000, 71, 627–629 CrossRef CAS.
- G. Guangyao, C. Sibao, W. Liwei, Y. Junshan and X. Peigen, Zhongcaoyao, 2000, 31, 324–326 Search PubMed.
- Z. S. Nuralieva, W. I. Litwinienko and P. K. Alimbaiewa, Khim. Prir. Soedin., 1969, 369–371 CAS.
- G. Guangyao, C. Sibao, W. Liwei, L. Maochuan, Y. Shichun and X. Peigen, Zhongguo Zhongyao Zazhi, 1999, 24, 191 Search PubMed.
- K. Egger, Z. Naturforsch., B, 1959, 14, 401–403.
- Z. Kowalevski, I. Frencel and I. Schumacher, Acta Pol. Pharm., 1966, 23, 305–310.
- N. N. Trofimova, A. S. Gromova, V. I. Lutsky, A. L. Vereshchagn and A. A. Semenov, Khim. Prir. Soedin., 1992, 723–724 CAS.
- Y. Shichun, W. Qingli, W. Liwei, Y. Junshan and X. Peigen, Zhongcaoyao, 1999, 30, 321–323 Search PubMed.
- L. van Itallie, Pharm. Weekbl., 1910, 47, 442–445 Search PubMed.
- D. Sharples and J. R. Stoker, Phytochemistry, 1969, 8, 597–601 CrossRef CAS.
- D. Sharples, M. S. Spring and J. R. Stoker, Phytochemistry, 1972, 11, 2999–3002 CrossRef CAS.
- D. Sharples, M. S. Spring and J. R. Stoker, Phytochemistry, 1972, 11, 3069–3071 CrossRef CAS.
- J. W. Jaroszewski and M. G. Ettlinger, Phytochemistry, 1981, 20, 819–821 CrossRef CAS.
- L. T. S. Fat, Proc. K. Ned. Akad. Wet., Ser. C, Biol. Med. Sci., 1979, 82, 197–209 Search PubMed.
- J. Wu, E. H. Fairchild, J. L. Beal and T. Tomimatsu, J. Nat. Prod., 1979, 42, 500–511 CrossRef CAS.
- F. Z. Erdemgil, K. I. C. Baser, P. Akbay, O. Sticher and I. Calis, Z. Naturforsch., C: Biosci., 2003, 58, 632–636 CAS.
- Y. P. Abrol, Indian J. Biochem., 1969, 6, 227–229 Search PubMed.
- A. A. Semenov, Ocherki khimii prirodnich soedinenii, Nauka, Novosibirsk, 2000, 664 pp Search PubMed.
- H. Ina and H. Iida, Chem. Pharm. Bull., 1986, 34, 726–729 CAS.
- H. Iida, T. Kikuchi, K. Kobayashi and H. Ina, Tetrahedron Lett., 21, 759–760 Search PubMed.
- X. A. Dominguez, R. O. Franco, G. Cano and S. G. Socorro, Rev. Latinoam. Quim., 1981, 12, 61–62 Search PubMed.
- M. O. Bagby, J. Smith, J. K. L. Mikolajczak and I. A. Wolff, Biochemistry, 1961, 1, 632–639.
- M. K. Bhatty and B. M. Craig, Can. J. Biochem., 1966, 44, 311–318 CAS.
- D. Rankoff, A. Popov, P. Panov and M. Daleva, J. Am. Chem. Soc., 1972, 48, 700–701.
- J. Wu, A. Ghosh and J. L. Beal, J. Nat. Prod., 1980, 43, 360–364 CrossRef CAS.
- E. Ucciani, A. Debal, M. Gruber and R. Z. Wolff, Z. Naturforsch., C: Biosci., 1996, 51, 151–154 CAS.
- P. E. Freiman and A. L. Markman, Khim. Prir. Soedin., 1970, 167–169.
- P. E. Freiman and A. L. Markman, Khim. Prir. Soedin., 1968, 246–247.
- A. S. Gromova, V. I. Lutskii, E. A. Tucallo, T. V. Ganenco and V. Y. Vitkovsky, Izv. Sib. Otd. Acad. Nauk SSSR, Ser. Khim. Nauk, 1986, 83–86 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |