Steroids: partial synthesis in medicinal chemistry
James R.
Hanson
*
Department of Chemistry, University of Sussex, Brighton, Sussex, UK BN1 9QJ
First published on 13th December 2005
Abstract
Covering: January to December, 2004
This article reviews the progress in the chemistry of the steroids that was published between January and December 2004. The reactions and partial synthesis of estrogens, androgens, pregnanes, cholic acid derivatives, cholestanes and vitamin D analogues are covered. There are 127 references.
1 Introduction
This review follows the pattern of its predecessors1 with sections on the major skeletal types of steroids. Reviews have appeared on the use of steroids in combinatorial chemistry,2 on the synthesis and biological activity of cephalostatin analogues,3 on directing effects in the hydroboration of steroidal alkenes4 and on the solid phase epoxidation of steroidal alkenes with potassium permanganate and metal salts.5 The methods that are employed for the identification of anabolic steroids that are abused in sport, have been reviewed.6 Special issues of the Journal of Steroid Biochemistry and Molecular Biology have been devoted to vitamin D7 and to the role of steroids in prostate cancer and its treatment.8,92 Estrogens
A number of novel syntheses of the estrane skeleton have been described10 including an enantioselective synthesis of estrone using a chiral oxazaborolidinium catalyst. A radical cascade cyclization has been used11 in a new total synthesis of estrone whilst the steroid backbone has been formed12 by a photo-induced domino-cyclization of silyl enol ethers.A gas chromatography–mass spectrometric assay of estradiol, catechol estradiols and methoxyestradiol in plasma has been developed13 whilst isotope dilution GC–MS methods have been used14 in the detection of estrogens and phytoestrogens in urine. The differentiation of steroid epimers by mass spectrometric methods has been examined.15 The complete 1H and 13C NMR assignments of some estradiols have been reported.16
A molecular dynamics simulation has been used17 to predict the binding affinities of estrogen analogues to the estrogen receptor. The need for selective targeting of estradiol dependent cancers has been discussed18 in the context of drug delivery systems for estrogenic hormone antagonists. 2-Methoxyestradiol has tumour growth inhibitory properties. In light of this, the anti-proliferative activity of 2-alkylsulfonyl estrogen derivatives has been examined.19 A series of 2-methoxyestradiol analogues, e.g.1, have been prepared20 in order to study their effects on cell proliferation and cytotoxicity in human cancer cell lines.
The oxidative coupling of estradiol through the aromatic ring to give 2 using hydrogen peroxide and peroxidase21 and the bromination of estradiol with N-bromosuccinimide22 have been described. The stability of the catechol estrogens in the presence of copper(I) salts has been examined.23 The anti-cancer agent, estramustane 3 has been synthesized24 bearing deuterium labels on rings A and D. An industrial synthesis of a 4-chloro-11β-arylestradiol 4 has been described.25
The way in which ICI 182780 5 binds to and regulates the estrogen receptor, has been examined.26 A further series of estradiol derivatives bearing sulfur containing substituents at the C-7a and C-11β positions have been reported.27 Some estradiol–taxol® conjugates have been prepared28 in which the estradiol is linked to the taxol® through the C-11 and C-16 positions.
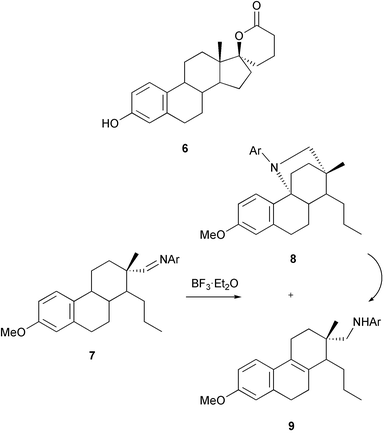
The modification of ring D of the estrogens has continued to be explored in the context of its effects on estrogen metabolism. A number of potential estrogenic inhibitors of the 17β-hydroxy-steroid dehydrogenase have been prepared including compounds bearing substituents at C-1629 and lactones at C-16,29e.g.6.30 The Mitsunobu inversion of sterically hindered 17β-hydroxy steroids has been examined.31 An unusual iminium ion induced overall 1,5-hydride shift 7
→
9 has been observed32 involving a D-seco isoquinuclidine 8.
3 Androstanes
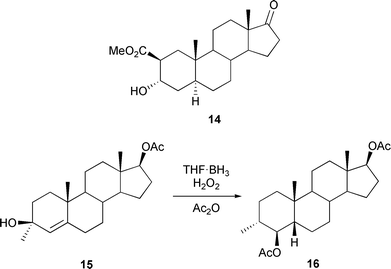
Studies on the androgen receptor as a potential target for the treatment of prostate cancer have been reported.33 The use of anti-androgens in the endocrine treatment of prostate cancer has been discussed.34 The loss of the androstane 19-methyl group in the aromatase sequence leading to the estrogens, has continued to be a topic of investigation with reports on the synthesis of 19-3H analogues of dehydroepiandrosterone.35 The determination of the stereochemistry of the reduction of the 19-carbonyl group of the 3-deoxyandrogens 10 and 11 by sodium borohydride has led36 to the preparation of some 19R- and 19S-labelled aromatase inhibitors. In both cases the 19S isomer predominated in the reduction. It is the 19-proR hydrogen which is lost on the aromatase oxidation to the aldehyde. The cleavage of the C(10)–C(19) bond of oxygenated androst-4-ene-3,6-diones (e.g.12 → 13) has been examined37 under various conditions.The selective carbonylation of 2α,3α-epoxyandrostan-17-one to give 2β-carbomethoxy-3α-hydroxyandrostan-17-one 14 in the presence of a cobalt octacarbonyl catalyst, has been reported.38 The hydroboration of 3-hydroxy-3-methylandrost-4-enes has been shown39 to give 4β-hydroxy-3α-methyl-5β(H)-steroids (15
→
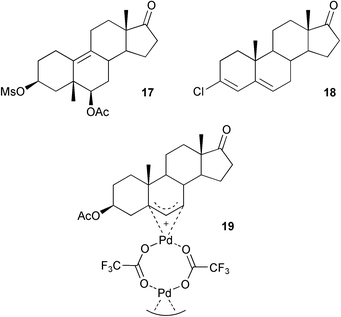
16) with the elimination of the 3-hydroxyl group irrespective of the stereochemistry at C-3. The β-face selective epoxidation of Δ5-steroids has continued to be of interest with reports40 on the use of oxygen in the presence of a silica supported cobalt catalyst. Whereas treatment of 5α,6α-epoxy-3β-methanesulfonoxyandrostan-17-one with hydrobromic acid in glacial acetic acid afforded a 4-methylestratriene by a dienol–benzene rearrangement, the reaction with sulfuric acid led to the product 17 of a Westphalen backbone rearrangement.41 3-Chloro-3,5-dienes 18 are easily prepared from the corresponding unsaturated ketones with oxalyl chloride. They have been shown42 to be oxidized with chromium trioxide to the corresponding Δ4-3,6-diones in good yield. The synthesis of some 3-hydroxy-3-methyl-6-oxoandrostanes has been reported.43 The crystal structure has been described44 of a trifluoroacetate bridged 5,6,7-π-allyl steroid palladium dimer 19 derived from the reaction of 3β-acetoxyandrost-5-en-17-one with palladium(II) trifluoroacetate.
11-Substituted androstanes have continued to attract interest. A cobalt mediated 2 + 2 + 2 cyclization of an allenediyne has been reported45 to give the 11-aryl steroid skeleton. The reactivity of 11-oxo steroids, e.g.20, towards organometallic reagents46 and the radical decomposition of oxalate esters47 have been examined in the context of preparing 11-substituted derivatives. Further work has been reported on the preparation of 13,14-seco-steroids by the Grob fragmentation of 14α-hydroxy-17β-tosyloxy steroids, 21
→
22,48 and by the lead tetra-acetate and iodine oxidation of 14α-hydroxy-17-keto steroids.49 The reaction of (13S)-13-iodo-16β-methoxy-3α,5α-cyclo-13,14-secoandrostan-14,17-dione with hydroxylamine has been examined.50
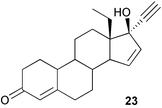
A simple synthesis of gestodene 23 from 18-methylestr-4-ene-3,17-dione has been reported.51 The detection of 17-alkyl substituted anabolic steroids by liquid chromatography and electrospray tandem mass spectrometry has been examined in relation to the abuse of these steroids.52 The microbiological hydroxylation of 17-methoxy steroids at C-6 and C-11 by the fungus, Cephalosporium aphidicola, has been reported.53
The synthesis of the furanosteroidal antibiotic, viridin 24 has been achieved.54 The biological activity of a library of viridin and wortmannin analogues as inhibitors of tumour cell growth has been examined.55
4 Pregnanes
The synthesis and the effect of 3α-amino-5α-pregnan-20-one on the GABA receptor has been studied56 in the context of the activity of pregnanes as neurosteroids. The synthesis and modification of 3β-steroidal glycosides of 3β,5α,6β-trihydroxypregnan-20-one has been reported.57 Some 5α-hydroxy-6-ketopregnanes, e.g.25, have been examined58 as analogues of brassinosteroid plant growth regulators. 17-Substituted pregnadienes have been prepared59 as potential inhibitors of testosterone 5α-reductase. An unusual Δ20-pregnene 26 which was obtained60 from an octacoral, has been shown to be an inhibitor of the mitochondrial respiratory chain.The hydrogenation of a C(20),C(22)-ketene dithioacetal has been used61 in the stereoselective synthesis of the unnatural C(20R) epimer of a C(22) aldehyde. The stereoselective reactions of the 20-dihydropyran 27 have been examined62 with the object of preparing compounds with modified steroidal side chains.
Steroidal tetrahydroxyoxazineones, e.g.28, have been examined63 as inhibitors of testosterone 5α-reductase. A number of heterocyclic steroids have been prepared from dehydropregnenolone acetate and D-secopregnenes including the tetrahydroquinoline analogue 29.64,65 The stereoselective addition of pyridyl lithium to a C-22 aldehyde has been reported.66 The effect of the modified steroid backbone of 5β-methyl-19-norpregn-9-enes on their biotransformation by Cephalosporium aphidicola, has been examined.67
An historical account has been given68 of the development of the Merck process for the synthesis of cortisone from bile acids. Some novel spironolactone analogues have been detected69 as impurities in commercial preparations of spironolactone. The flexibility of the progesterone receptor binding pocket has been explored70 using crystal structures of norethindrone and mometasone furoate 30 complexes. The synthesis and glucocorticoid activity of a series of 17-furoate esters, e.g.31,71 and of some C-21 nitroesters of prednisolone,72 have been explored. The products, including 32, from the photodegradation of the anti-inflammatory steroid loteprednol etabonate, have been identified.73
The synthesis of the biologically active cardenolides has been reviewed.74 Bufalin is a bioactive constituent of the Chinese drug Chan-Su, which shows potent anti-cancer activity. A number of cytotoxic bufadienolides have been obtained75 from bufalin by microbial hydroxylation with Mucor spinosus.
5 Bile acids
The partial synthesis of 3α,7α,14α-trihydroxy-5β-cholan-24-oic acid has been described.76 The C-14α hydroxyl group was introduced by oxidation with dimethyldioxirane. The farnesol X receptor is activated by endogenous bile acids and plays a variety of physiological roles related to the modulation of gene transcription. Some further bile acid analogues based on 33 and derived from chenodeoxycholic acid have been examined77 in this context. Bile acid conjugates, e.g.34 of mifepristone have been synthesized78 as liver selective glucocorticoid antagonists for the treatment of type 2 diabetes.The synthesis and X-ray crystal structures have been reported79–81 of a number of dinorcholane derivatives. The X-ray crystal structure of the monoethyl oxalate ester of 3α-hydroxy-5β-cholan-24-oic acid has been described.82 Some lithocholic acid derivatives have been synthesized.83 The hydrogen bonding network of 3-oxo-12α-hydroxy-5β-cholan-24-oic acid has been predicted84 from X-ray evidence.
The remote functionalization of 3-oxo-bile acids by oxidation with 2,6-dichloropyridine N-oxide catalysed by a ruthenium porphyrin has been shown85 to lead to lactonization at C-20 as in 35. Conjugates of gadolinium complexes with bile acids have been considered86 as hepatocyte directed contrast agents for magnetic resonance imaging.
6 Cholestanes
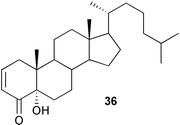
A series of gradient enhanced selective NMR experiments have been used87 to assign the 1H NMR signals of stigmasterol. The synthesis of cholest-1-en-3-one from the 2α-iodo-3-ketone by oxidation of the iodine with per-acid has been described.88 A series of spirostane analogues of the brassinosteroids have been prepared89 by the homogeneous potassium permanganate dihydroxylation of 2,3-enes. The oxidation of Δ2-, Δ2,4- and Δ4,6-cholestenes with ruthenium tetroxide to form diols and ketols, e.g.36 has been reported.90Methods for the attachment of glycosyl units at the C-3 of diosgenin have been examined.91,92 The synthesis of some spirostenols based on diosgenin which prevent β-amyloid induced neurotoxicity has been reported.93 Cholesterol surrogates containing a benzophenone moiety have been synthesized94 as potential photo-affinity labels for measuring cellular sterol efflux and HDL formation.
The design of chiral dimesogens containing cholesteryl groups for use in liquid crystals has been reviewed.95 The (2π + 2π) photo-cycloaddition of cholesteryl cinnamate to methyl phenanthrene-9-carboxylate has been examined96 in the context of stereoselectivity in the liquid crystalline phase. The dimethylaminobenzoate group linked to cholesterol has been used97 to examine the microenvironment of gel fibrils by fluorescence methods.
Iron picolinate complexes have been used in the stereoselective allylic 7α-hydroxylation of cholesteryl acetate with oxygen.98 The antibacterial and cytotoxicity of 7α- and 7β-spermidinyl cholesterol analogues, e.g.37, has been reported.99 A 7-hydroxy sterol 38 from a soft coral, has been shown100 to possess testosterone 5α-reductase inhibitory activity.
The cytotoxic sterols, 24-methylenecholesta-3β,5α,6β,19-tetraol and 24-methylenecholest-5-ene-3β,7α- and 3β,7β-diols have been synthesized101,102 from stigmasterol. The 22-spiroketal of diosgenin has been removed103 by a Clemmensen reduction to afford 3β,16β,26-trihydroxycholest-5-ene, 39. This methodology has also been used to produce deuteriated samples. The rearrangement of a 23-oxo-spirostane to a 22-oxo-23-spiroketal 40 has been investigated104 and the structure of the product established by X-ray crystallography.
The ecdysone relatives, viticosterone E 41105 and the 22R/S epimers of 2α,3α,20,22-tetrahydroxy-5α-cholestan-6-one106 have been synthesized and the binding of the latter to the ecdysterone receptor has been examined. A number of brassinosteroid relatives have been synthesized and their biological activity evaluated.107,108 (26,27-2H6)-Brassinosteroids have been prepared109 for metabolic studies. The preparation of 1α,25-diacetoxy-3β-hydroxycholesta-4,6-diene 42 from a 5,7-diene has been reported.110
Efficient methods for the separation of lanosterol and dihydrolanosterol from commercial mixtures and for the preparation of epimerically pure derivatives have been described.111,112
7 Vitamin D
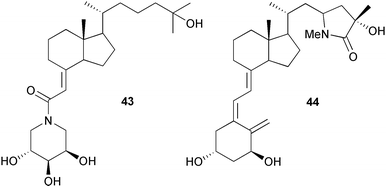
The proceedings of the 12th Vitamin D Workshop have been published.7 Some new derivatives of 1α,25-dihydroxy-19-norvitamin D3113 and 2-methylene analogues114 have been synthesized and their therapeutic potential has been discussed.115 Some 2-functionalized analogues of 1α,25-dihydroxyvitamin D3 have been shown to be116 potent inducers of cell differentiation and some further 2α-(ω-hydroxyalkoxy)-analogues have also been synthesized.117 C(3)-Carbamate derivatives of 1α,25-dihydroxyvitamin D3 showed118 a low affinity for the vitamin D receptor. The amide analogue 43 has been synthesized.119 The side chain lactam 44 has been reported120 to be a vitamin D antagonist.Calcitriol analogues with two different side chains at C(20) have been synthesized121 and their use as probes of the vitamin D receptor has been explored.122 The crystal structure of the vitamin D nuclear receptor liganded with the vitamin D side chain analogue calcipotriol 45, has been determined123 in order to provide an insight into the biological activity of these analogues.
24-Sulfone124 and sulfoxime analogues, e.g.46125 of 1α,25-dihydroxyvitamin D3 have been synthesized. The latter have low calcemic activity and are powerful inhibitors of the 24-hydroxylase involved in the catabolism of vitamin D. A number of other vitamin D derivatives have been synthesized.126,127
8 References
- J. R. Hanson, Nat. Prod. Rep., 2005, 22, 104 RSC.
- R. Maltais, M. R. Tremblay, L. C. Crobanu and D. Poirier, J. Comb. Chem., 2004, 6, 443 CrossRef CAS.
- T. Flessner, R. Jautelat, V. Scholz and E. Winterfeldt, Prog. Chem. Org. Nat. Prod., 2004, 87, 1 Search PubMed.
- J. R. Hanson, J. Chem. Res., 2004, 1 Search PubMed.
- J. A. R. Salvador and J. R. Hanson, J. Chem. Res., 2004, 513 Search PubMed.
- G. J. Trout and R. Kaslauskas, Chem. Soc. Rev., 2004, 33, 1 RSC.
- J. Steroid Biochem. Mol. Biol., 2004, 89, 1–633 Search PubMed.
- J. Steroid Biochem. Mol. Biol., 2004, 92, 219–325 Search PubMed.
- F. Labrie, L. Cusan, J. Gomez, V. Luu-The, B. Candas, A. Belanger and C. Labrie, J. Steroid Biochem. Mol. Biol., 2004, 92, 337.
- Q.-Y. Hu, P. D. Rege and E. J. Corey, J. Am. Chem. Soc., 2004, 126, 5984 CrossRef CAS.
- G. Pattenden, K. L. Reddy and A. Walter, Tetrahedron Lett., 2004, 45, 4027 CrossRef CAS.
- J. A. Bunte, S. Rinne and J. Mattay, Synthesis, 2004, 619 CAS.
- L. C. Zacharia, R. K. Dubey and E. K. Jackson, Steroids, 2004, 69, 255 CrossRef CAS.
- H. Adlercreutz, P. Kiuru, S. Rasku, K. Wahale and T. Fotsis, J. Steroid Biochem. Mol. Biol., 2004, 92, 399 CrossRef CAS.
- M. Mak, E. Francsics-Czinege and Z. Tuba, Steroids, 2004, 69, 831 CrossRef CAS.
- P. Cruffreda, S. Casati and A. Manzocchi, Magn. Reson. Chem., 2004, 42, 360 CrossRef.
- M. M. H. van Lipzig, A. M. ter Laak, A. Jongejan, N. P. E. Vermeulen, M. Wamelink, D. Geerke and J. H. N. Meerman, J. Med. Chem., 2004, 47, 1018 CrossRef CAS.
- T. Ameller, P. Legrand, V. Marsaud and J. M. Renoir, J. Steroid Biochem. Mol. Biol., 2004, 92, 1 CrossRef CAS.
- M. P. Leese, S. P. Newman, A. Purchit, M. J. Reed and B. V. L. Potter, Bioorg. Med. Chem. Lett., 2004, 14, 3135 CrossRef CAS.
- A. B. Edsall, A. K. Mohanakrishnan, D. Yang, P. F. Fanwick, E. Hamel, A. D. Hanson, G. E. Agoston and M. Cushman, J. Med. Chem., 2004, 47, 5126 CrossRef CAS.
- A. Pezzella, L. Lista, A. Napolitano and M. d'Ischia, J. Org. Chem., 2004, 69, 5652 CrossRef CAS.
- A. J. Lee, J. W. Sowell, W. E. Cotham and B. T. Zhu, Steroids, 2004, 69, 61 CrossRef CAS.
- P. A. Thibodeau, C. Pasquier and M. A. Gougerot-Pocidalo, Steroids, 2004, 69, 419 CrossRef CAS.
- D. Giribone and E. Fontana, J. Labelled Compd. Radiopharm., 2004, 47, 161 CrossRef CAS.
- D. Prat, F. Benedetti, C. F. Girard, L. N. Bouda, J. Larkin, C. Wehrey and J. Lenay, Org. Process Res. Dev., 2004, 8, 219 Search PubMed.
- M. W. Wang, R. J. Traystman, P. D. Hum and T. Liu, J. Steroid Biochem. Mol. Biol., 2004, 92, 51 CrossRef CAS.
- D. Spera, G. Cabrera, R. Fiaschi, K. E. Carlson, J. A. Katzenellenbogen and E. Napolitano, Bioorg. Med. Chem., 2004, 12, 4393 CrossRef CAS.
- C. Liu, J. S. Strobl, S. Bane, J. K. Schilling, M. McCracken, S. K. Chatterjee, R. Rahim-Bata and D. G. I. Kingston, J. Nat. Prod., 2004, 67, 152 CrossRef CAS.
- M. Berube and D. Poirier, Org. Lett., 2004, 6, 3127 CrossRef CAS.
- P. Bydal, S. Auger and D. Poirier, Steroids, 2004, 69, 325 CrossRef CAS.
- P. Tapolosanyi, J. Wolfling, E. Mernyak and G. Schneider, Monatsh. Chem., 2004, 135, 1129.
- J. Wolfling, E. Frank, G. Schneider and L. F. Tietze, Eur. J. Org. Chem., 2004, 90 CrossRef.
- A. F. Santos, H. Huang and D. J. Tindall, Steroids, 2004, 69, 79 CrossRef CAS.
- T. Tammela, J. Steroid Biochem. Mol. Biol., 2004, 92, 287 CrossRef CAS.
- I. Cerny, V. Pouzar, M. Budesinsky, M. Bicikova, M. Hill and R. Hampl, Steroids, 2004, 69, 161 CrossRef CAS.
- M. Numazawa, N. Sohtome and M. Nagaoka, Chem. Pharm. Bull., 2004, 52, 722 CrossRef CAS.
- M. Nagaoka and M. Numazawa, Chem. Pharm. Bull., 2004, 52, 983 CrossRef CAS.
- A. Balazs, C. Benedek, G. Szalontai and S. Toros, Steroids, 2004, 69, 271 CrossRef CAS.
- C. Uyanik, J. R. Hanson, L. Nisius and P. B. Hitchcock, J. Chem. Res., 2004, 471 Search PubMed.
- S. M. Silvestre, J. A. R. Salvador and J. H. Clark, J. Mol. Catal., 2004, 219, 143 Search PubMed.
- S. G. Knights and J. R. Hanson, J. Chem. Res., 2004, 830 Search PubMed.
- I. Kiran and J. R. Hanson, J. Chem. Res., 2004, 208 Search PubMed.
- H. Chodounska, V. Pouzar, M. Budesinsky, B. Slavikova and L. Kohout, Steroids, 2004, 69, 599 CrossRef.
- P. L. Ruddock, D. J. Williams and P. B. Reese, Steroids, 2004, 69, 193 CrossRef CAS.
- M. Petit, C. Aubert and M. Malacria, Org. Lett., 2004, 6, 3937 CrossRef CAS.
- V. Lecomte, E. Stephan, J. Vaissermann and G. Jaouen, Steroids, 2004, 69, 17 CrossRef CAS.
- V. Lecomte, E. Stephan, M. N. Rager and G. Jaouen, J. Org. Chem., 2004, 69, 3216 CrossRef CAS.
- V. A. Khripach, V. N. Zhabinskii, G. P. Fando, A. I. Kuchto, A. S. Lyakhov, A. A. Govorova, M. B. Groen, J. van der Louw and A. de Groot, Steroids, 2004, 69, 495 CrossRef CAS.
- V. A. Khripach, V. N. Zhabinskii, A. I. Kuchto, Y. Y. Zhiburtovitch, G. P. Fando, A. S. Lyakhov, A. A. Govorova, M. B. Groen, J. van der Louw and A. de Groot, Steroids, 2004, 69, 501 CrossRef CAS.
- V. A. Khripach, V. N. Zhabinskii, A. I. Kuchto, Y. Y. Zhiburtovitch, G. P. Fando, A. S. Lyakhov, A. A. Govorova, M. B. Groen, J. van der Louw and A. de Groot, Steroids, 2004, 69, 511 CrossRef CAS.
- P. Ferraboschi, P. Grisenti, A. Onofri and P. Prestileo, Synlett, 2004, 1838 CrossRef CAS.
- A. Leinonen, T. Kouranne, T. Kotiaho and R. Kostiainen, Steroids, 2004, 69, 101 CrossRef CAS.
- I. Kiran, J. R. Hanson and A. C. Hunter, J. Chem. Res., 2004, 362 Search PubMed.
- E. A. Anderson, E. J. Alexanian and E. J. Sorensen, Angew. Chem., Int. Ed., 2004, 43, 1998 CrossRef CAS.
- P. Wipf, D. J. Minion, R. J. Halter, M. I. Berggren, C. B. Ho, G. G. Chiang, L. Kirkpatrick, R. Abrahams and G. Powis, Org. Biomol. Chem., 2004, 2, 1911 RSC.
- L. Matyas, A. Kasal, Z. B. Riera and C. E. Sunol, Collect. Czech. Chem. Commun., 2004, 69, 1506 CrossRef CAS.
- S. M. Wang, W. Z. Ge, H. M. Liu, D. P. Zou and X. B. Yan, Steroids, 2004, 69, 599 CrossRef.
- D. G. Rivera, F. Coll and F. Leon, J. Chem. Res., 2004, 284 Search PubMed.
- M. Cabeza, E. Flores, I. Heuze, M. Sanchez, E. Bratoeff, E. Ramirez and V. A. Francoluge, Chem. Pharm. Bull., 2004, 52, 535 CrossRef CAS.
- M. L. Ciavatta, M. P. Lopez Gressa, E. Manzo, M. Gavagnin and S. Wadihulla, Tetrahedron Lett., 2004, 45, 7745 CrossRef CAS.
- B. P. Shingate, B. G. Hazra, V. S. Pore, R. G. Gonnade and M. M. Bhadbhade, Chem. Commun., 2004, 2194 RSC.
- A. Magyar, Z. Szendi, P. Forgo, M. Mak, H. Gorls and F. Sweet, Steroids, 2004, 69, 35 CrossRef CAS.
- J. Wolfling, L. Hackler, E. Merriyak, G. Schneider, I. Toth, M. Szecsi, J. Julesz, P. Schars and A. Csampai, Steroids, 2004, 69, 451 CrossRef CAS.
- G. Schneider, J. Wolfling, E. Mernyak and I. Toth, Magy. Kem. Foly., 2004, 109, 40.
- A. Magyar, J. Wolfling, M. Kubas, J. A. C. Seijo, M. Sevvana, R. Herbst-Immer, P. Forgo and G. Schneider, Steroids, 2004, 69, 301 CrossRef CAS.
- G. Visbal and A. Alvarez-Auler, Synth. Commun., 2004, 34, 533 CrossRef CAS.
- J. R. Hanson, P. B. Hitchcock and K. Yildirim, J. Chem. Res., 2004, 519 Search PubMed.
- S. H. Pines, Org. Process Res. Dev., 2004, 8, 708 Search PubMed.
- H. Chen, X. Y. Wang, Z. D. Yang and Y. C. Li, Steroids, 2004, 69, 647 CrossRef CAS.
- K. P. Madauss, S. J. Deng, R. J. H. Austin, M. H. Lambert, I. McLay, J. Pritchard, S. A. Short, E. L. Stewart, I. J. Uings and S. P. Williams, J. Med. Chem., 2004, 47, 338.
- D. A. Sandham, L. Barker, D. Beattie, D. Beer, L. Bidlake, D. Bentley, K. D. Butler, S. Craig, D. Farr, C. Ffoulkes-Jones, J. R. Fozard, S. Haberthuer, C. Hawes, D. Hynx, S. Jeffers, T. H. Keller, P. A. Kirkham, J. C. Maas, L. Mazzoni, A. Nicholls, G. R. Pilgrim, E. Schaebulin, G. M. Spooner, R. Stringer, P. Tranter, K. L. Turner, M. F. Tweed, C. Walker, S. J. Watson and B. M. Cuenoud, Bioorg. Med. Chem., 2004, 12, 5213 CrossRef CAS.
- P. G. Baraldi, R. Romagnoli, M. C. Nunez, M. Perretti, M. J. Paul-Clark, M. Ferrario, M. Govoni, F. Benedini and E. Ongini, J. Med. Chem., 2004, 47, 711 CrossRef CAS.
- Y. Shirasaki, K. Inada, J. Inoue and M. Nakamura, Steroids, 2004, 69, 23 CrossRef CAS.
- G. Schneider and J. Wolfling, Curr. Org. Chem., 2004, 8, 1381 CrossRef CAS.
- M. Ye, G. Qu, H. Guo and D. Guo, J. Steroid Biochem. Mol. Biol., 2004, 91, 87 CrossRef CAS.
- G. Kakiyama, T. Iida, T. Goto, N. Mano, J. Goto and T. Nambara, Chem. Pharm. Bull., 2004, 52, 371 CrossRef CAS.
- R. Pellicciari, G. Costantino, E. Camaioni, B. M. Sadeghpour, A. Entrena, T. M. Willson, S. Fiorucci, C. Clerici and A. Gioiello, J. Med. Chem., 2004, 47, 4559 CrossRef CAS.
- T. W. von Geldern, N. Tu, P. R. Kym, J. T. Link, H. S. Jae, C. Lai, T. Apelquist, P. Rhonnstaad, L. Hagberg, E. Koehler, M. Grynfarb, A. Goos-Nilsson, J. Sanberg, M. Osterland, T. Barkhem, M. Hoglund, J. Wang, S. Fung, D. Wilcox, P. Nguyen, C. Jakob, C. Hutchins, M. Fornegardh, B. Kauppi, L. Ohman and P. B. Jacobsen, J. Med. Chem., 2004, 47, 4213 CrossRef CAS.
- L. Nahar and A. B. Turner, J. Chem. Res., 2004, 329 Search PubMed.
- P. J. Cox, L. Nahar, S. D. Sarker and A. B. Turner, J. Chem. Res., 2004, 192 Search PubMed.
- P. J. Cox, L. Nahar and A. B. Turner, J. Chem. Res., 2004, 372 Search PubMed.
- P. J. Cox, L. Nahar and A. B. Turner, J. Chem. Res., 2004, 691 Search PubMed.
- L. Nahar and A. B. Turner, J. Chem. Res., 2004, 747 Search PubMed.
- A. Jover, F. Meijide, V. H. Soto, J. V. Tato, E. R. Nunez, H. T. Ton-Nu and A. F. Hofmann, Steroids, 2004, 69, 379 CrossRef CAS.
- S. Ogawa, T. Iida, T. Goto, N. Mano, J. Goto and T. Nambara, Org. Biomol. Chem., 2004, 2, 1013 RSC.
- P. L. Anelli, L. Lattuada, V. Lorusso, G. Lux, A. Morisetti, P. Morosini, M. Serleti and F. Uggeri, J. Med. Chem., 2004, 47, 3629 CrossRef CAS.
- P. Forgo and K. E. Kover, Steroids, 2004, 69, 43 CrossRef CAS.
- C. A. Horiuchi, S. J. Ji, M. Matsushita and W. Chai, Synthesis, 2004, 202 CrossRef CAS.
- D. G. Rivera, O. Pando, V. Leliebre-Lara, D. Coll, F. Leon and F. Coll, J. Chem. Res., 2004, 53 Search PubMed.
- D. Musumeci, G. N. Roviello and D. Sica, Steroids, 2004, 69, 173 CrossRef CAS.
- G. Gu, Y. Du and R. J. Linhardt, J. Org. Chem., 2004, 69, 5497 CrossRef CAS.
- S. Reicheneder and C. Unverzagi, Angew. Chem., Int. Ed., 2004, 43, 4353 CrossRef CAS.
- L. Lecanu, W. Yao, G. L. Teper, Z. X. Yao, J. Greeson and V. Papadopoulos, Steroids, 2004, 69, 1 CrossRef CAS.
- T. A. Spencer, P. Wang, D. Li, J. S. Russel, D. H. Blank, J. Huusjonen, P. E. Fielding and C. J. Fielding, J. Lipid Res., 2004, 45, 1510 CAS.
- V. A. Mallia and N. Tamaoki, Chem. Soc. Rev., 2004, 33, 76 RSC.
- H. Maeda, A. Horiuchi, N. Koshio and K. Mizuno, Chem. Lett., 2004, 33, 966 CrossRef CAS.
- Y. Iwashita, K. Sugiyasu, M. Ikeda, N. Fujita and S. Shinkai, Chem. Lett., 2004, 33, 1124 CrossRef CAS.
- I. Okamoto, W. Funaki, K. Nakaya, E. Kotani and T. Takeya, Chem. Pharm. Bull., 2004, 52, 756 CrossRef CAS.
- B. Choucair, M. Dherbomez, C. Roussakis and L. El-Kihel, Bioorg. Med. Chem. Lett., 2004, 14, 4213 CrossRef CAS.
- P. Radhika, M. Cabeza, E. Bratoeff and G. Garcia, Steroids, 2004, 69, 439 CrossRef CAS.
- W. Lu, L. Zeng and J. Su, Steroids, 2004, 69, 445 CrossRef CAS.
- W. Lu, C. Zhang, L. Zeng and J. Su, Steroids, 2004, 69, 803 CrossRef CAS.
- L. Alessandrini, P. Ciuffreda, E. Santaniello and G. Terraneo, Steroids, 2004, 69, 789 CrossRef CAS.
- M. K. Cyranski, J. Frelek, I. Jasirzebska and J. W. Morzycki, Steroids, 2004, 69, 395 CrossRef CAS.
- I. V. Galyautdinov, S. R. Nazmeeva, R. G. Savchenko, N. A. Ves'kina, D. V. Nedopekin and A. A. Fatykhov, Russ. J. Org. Chem., 2004, 40, 675 CrossRef CAS.
- B. Watanabe, Y. Nakagawa, T. Ogura and H. Miyagawa, Steroids, 2004, 69, 483 CrossRef CAS.
- S. Uesusuki, B. Watanabe, S. Yamamoto, J. Otsuki, Y. Nakagawa and H. Miyagawa, Biosci., Biotechnol., Biochem., 2004, 68, 1097 CrossRef CAS.
- F. M. Michelini, J. A. Ramirez, A. Berra, L. R. Galagovsky and L. E. Alche, Steroids, 2004, 69, 713 CAS.
- A. P. Antonchick, B. Schneider, V. N. Zhabinskii and V. A. Khripach, Steroids, 2004, 69, 617 CrossRef CAS.
- S. D. van Arnum, B. K. Carpenter, D. R. Parrish and A. MacIntrye, J. Org. Chem., 2004, 69, 8529 CrossRef CAS.
- L. K. Kaviaradze, M. Manley-Harris and B. K. Nicholson, Steroids, 2004, 69, 227 CrossRef.
- L. K. Kaviaradze, M. Manley-Harris and B. K. Nicholson, Steroids, 2004, 69, 697 CrossRef.
- A. Glebocka, R. R. Sicinski and H. F. DeLuca, J. Steroid Biochem. Mol. Biol., 2004, 89, 25 CrossRef.
- P. Grzywacz, L. A. Plum, W. Sicinska, R. R. Sicinski, J. M. Prahl and H. F. DeLuca, J. Steroid Biochem. Mol. Biol., 2004, 89, 19 CrossRef.
- H. F. DeLuca, J. Steroid Biochem. Mol. Biol., 2004, 89, 67 CrossRef.
- T. Fujishima, A. Kittaka, M. Kurihara, N. Saito, S. Honzawa, S. Kishimoto, T. Sugiura, K. Waku and H. Takayama, J. Steroid Biochem. Mol. Biol., 2004, 89, 89 CrossRef.
- N. Saito, Y. Suhara, M. Kurihara, T. Fujishima, S. Honzawa, H. Takayanagi, T. Kozono, M. Matsumoto, M. Ohmori, N. Miyata, H. Takayama and A. Kittaka, J. Org. Chem., 2004, 69, 7463 CrossRef CAS.
- V. Gotor-Fernandez, S. Fernandez, M. Ferrero, R. Bouillon and A. Verstuyf, Bioorg. Med. Chem., 2004, 12, 5443 CrossRef CAS.
- Y. Suhara, K. Ono, A. Yoshida, T. Fujishima, N. Saito, S. Honzawa, S. Kishimoto, T. Sugiura, K. Waku, H. Takeyama and A. Kittaka, Heterocycles, 2004, 62, 423 CrossRef CAS.
- Y. Kato, Y. Nakano, R. Sano, A. Tanatani, H. Kobayashi, R. Shimazawa, H. Koshino, Y. Hashimoto and K. Nagasawa, Bioorg. Med. Chem. Lett., 2004, 14, 2579 CAS.
- H. Maehr, M. R. Vskokovic, L. Adorini and G. S. Reddy, J. Med. Chem., 2004, 47, 6476 CrossRef CAS.
- H. Maehr, M. R. Vskokovic, L. Adorini and G. S. Reddy, J. Steroid Biochem. Mol. Biol., 2004, 89, 35 CrossRef.
- G. Tocchini-Valentini, N. Rochel, J. M. Wurtz and D. Moras, J. Med. Chem., 2004, 47, 1956 CrossRef CAS.
- G. H. Posner, K. R. Crawford, H. W. Yang, M. Kahraman, H. B. Jeon, H. Li, J. K. Lee, B. C. Suh, M. A. Hatcher and T. Labonte, J. Steroid Biochem. Mol. Biol., 2004, 89, 5 CrossRef.
- M. Kahraman, S. Sinishtaj, P. M. Dolan, T. W. Kensler, S. Peleg, V. Saha, S. S. Chuang, G. Bernstein, B. Korczak and G. H. Posner, J. Med. Chem., 2004, 47, 6854 CrossRef CAS.
- P. J. DeClercq, I. Murad, L. J. Gao, Y. J. Chen, D. V. Haver, M. van de Walle, A. Verstuyf, L. Verlinden, C. Verboven and R. Bouillon, J. Steroid Biochem. Mol. Biol., 2004, 89, 61 CrossRef.
- E. Moman, D. Nicolletti and A. Mourino, J. Org. Chem., 2004, 69, 4615 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |