Marine natural products
John W. Blunt*a, Brent R. Coppb, Murray H. G. Munroa, Peter T. Northcotec and Michèle R. Prinsepd
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bDepartment of Chemistry, University of Auckland, Auckland, New Zealand
cSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 9th January 2006
Abstract
Covering: 2004. Previous review: Nat. Prod. Rep., 2005, 22, 15
This review covers the literature published in 2004 for marine natural products, with 693 citations (491 for the period January to December 2004) referring to compounds isolated from marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates and echinoderms. The emphasis is on new compounds (716 for 2004), together with their relevant biological activities, source organisms and country of origin. Biosynthetic studies (8), and syntheses (80), including those that lead to the revision of structures or stereochemistries, have been included.
 John W. Blunt | John Blunt obtained his BSc (Hons) and PhD degrees from the University of Canterbury, followed by postdoctoral appointments in Biochemistry at the University of Wisconsin-Madison, and with Sir Ewart Jones at Oxford University. He took up a lectureship at the University of Canterbury in 1970, where he is now a Professor. His research interests are with natural products, and the application of NMR techniques to structural problems. |
 Brent R. Copp | Brent Copp received his BSc (Hons) and PhD degrees from the University of Canterbury, where he studied the isolation, structure elucidation and structure–activity relationships of biologically active marine natural products under the guidance of Professors Blunt and Munro. He undertook postdoctoral research with Jon Clardy at Cornell and Chris Ireland at the University of Utah. 1992–1993 was spent working in industry as an isolation chemist with Xenova Plc, before returning to New Zealand to take a lectureship at the University of Auckland, where he is currently an Associate Professor. |
 Murray H. G. Munro | Murray Munro, University of Canterbury, Christchurch New Zealand, has worked on natural products, mainly of New Zealand origin, right through his career. Following a sabbatical with Ken Rinehart at the University of Illinois in 1973 interest in marine natural products developed with a particular focus on bioactive compounds. In recent years his research interests have widened to include terrestrial and marine fungi and actinomycetes as well as marine invertebrates. |
 Peter T. Northcote | Peter Northcote received his BSc and PhD degrees from the University of British Columbia, Canada where he was a member of R. J. Andersen's marine natural products research group. He carried out postdoctoral research with Professors Blunt and Munro at the University of Canterbury before taking a position as a senior research scientist at Lederle Laboratories, American Cyanamid Co. He joined the faculty of the Victoria University of Wellington in 1994 where he is currently an Associate Professor in organic chemistry. |
 Michèle R. Prinsep | Michèle Prinsep received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied the isolation and structural elucidation of biologically active secondary metabolites from sponges and bryozoans under the supervision of Professors Blunt and Munro. She undertook postdoctoral research on cyanobacteria with Richard Moore at the University of Hawaii before returning to New Zealand to take up a lectureship at the University of Waikato, where she is currently a Senior Lecturer. |
1 Introduction
Previous reviews in this series, published in 2003 and 2004, noted the passing of D. John Faulkner and Paul J. Scheuer, and acknowledged their contributions to the field of marine natural products. Sadly, we now have to note the death of Professor Kenneth L. Rinehart in June 2005. Professor Rinehart had an academic career at the University of Illinois that spanned nearly five decades (1954–2001). Rinehart, like Faulkner and Scheuer, was a pioneer in the study of natural products with significant contributions in the application of mass spectrometry to the solving of natural product structures and the emphasis placed on the search for bioactive compounds sourced from marine organisms. Professor Rinehart pioneered the use of bioassays in the field during collecting expeditions, thus identifying species that should be recollected in larger amounts while still at the site of collection. Lasting contributions from Professor Rinehart will be from his work on the ecteinascidins and didemnins, two of the still relatively few marine natural products in advanced stages of clinical trials (Yondelis™ and Aplidine™).This review is of the literature for 2004 and describes 716 new compounds from 259 articles, numbers that have increased by ∼10% from those of each of the past few years. As in previous reviews, we show structures only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described, are referenced, but separate structures are generally not shown.
2 Reviews
In 2004 a larger than customary number of reviews were published covering many different aspects of marine natural products, with two reviews1,2 giving overall coverage of the 2003 literature. Several reviews describe work derived from regional collections, including Southern Africa,3 the Japanese coasts with emphasis on drug leads from marine invertebrates,4 ecosystem comparisons in New Caledonia,5 cytotoxic natural products from Taiwan,6 bioactive compounds from Brazil,7 diterpenes from Dictyotacean brown algae from the tropical Atlantic American region,8 and the efforts of Faulkner, Scheuer, Paul and the NCI in Palau.9Reviews with an ecological and/or antifouling focus include an account of biogenic compounds from marine algae with potential as antifouling agents,10 a general discussion of biofouling and antifouling,11 a review of the recent literature on marine chemical ecology,12 an account of chemical defence strategies of marine organisms,13 and some insights into the antimicrobial defences of selected marine invertebrates.14 There have been three articles that explore the nature of immune systems in various phyla, including marine invertebrates.15–17 A somewhat speculative article compares selected marine and non-marine toxins, raising issues such as convergence and the role of symbiotic organisms.18
Synthetic efforts on marine natural products continue to expand, with reviews covering a variety of topics including trans-fused polycyclic ethers,19 the convergent syntheses of polycyclic ethers,20 the use of Suzuki–Miyaura cross-couplings for polycyclic ethers,21 and the total syntheses of oxazole-containing natural products.22
Several reviews focus on studies on compounds from specific types of organisms, such as cytotoxic metabolites from marine algae,23 anticoagulants from marine algae,24 metabolites of marine-derived fungi,25 antimicrobials and antifungals from marine microorganisms,26 toxins from microalgae,27 enzyme inhibitors from marine microbes,28 glycosides from sea cucumbers,29 medicinal and pharmaceutical products from macroalgae,30 bioactive polypeptides from Anemonia sulcata,31 metabolites from several species of sponges of the genus Plakortis32 and specifically from Plakortis simplex,33 peptide and peptide-like pheromones and kairomones from crustaceans,34 toxins from Northern Adriatic mussels,35 metabolites from the gorgonian corals of the genus Junceella,36 and metabolites from symbiotic bacteria.37
A broad range of bioactivities of marine natural products is reviewed in several articles, including marine pharmacology in 2000,38 the merging of the potential of microbial genetics with biological and chemical diversity,39 drugs and cosmetics from the sea,40 unconventional natural sources for future drug discovery,41 new structures and bioactivities for small-molecule natural products,42 and marine natural products and related compounds in clinical and advanced preclinical trials.43
Treatments for specific disease types is the subject of several reviews including the search for new candidates for cancer chemotherapy from natural products,44 antitumour and cytotoxic compounds reported in 2001–2002,45 mechanism-targeted discovery of antitumour marine natural products,46 antiviral marine natural products,47 antifungal compounds from marine organisms,48 and natural antimycobacterial metabolites.49
Specific compound classes are reviewed in antimicrobial peptides from marine invertebrates,50 bioactive peptides from marine sources,51 mycosporine-like amino acids,52 simple indole alkaloids and those with a nonrearranged monoterpenoid unit,53 bioactive alkaloids,54 bisindole alkaloids from sponges,55 quinolizidine alkaloids from marine sources,56 purines from marine organisms,57 siderophores from marine microorganisms,58 secosteroids of marine origin (1972–2004),59 biologically active natural glycosides,60 drimane sesquiterpenoids (1990–2002),61 diterpenoids (2002),62 diterpenoids (2003),63 organohalogen compounds,64 marine isocyanides and related compounds,65 and allenic compounds.66
Research on specific compounds is reviewed in articles on discodermolide,67 curacin A,68 agelasphin KRN7000,69 ecteinascidin 743, aplidine and kahalalide F,70,71 amphidinolides (1986–2003),72 shark-repelling saponins mosesins and pavoninins,73 lamellarins,74 and the bisindole alkaloids dragmacidins and hamacanthins.75
A brief review on “Drugs from the Sea – Who are the producers?” explores the hypothesis that microorganisms, either by way of ingestion or as symbionts, are the true source of bioactive compounds from marine invertebrates.76 Two reviews on the use of mass spectrometry in peptidomics77 and analysis of marine toxins78 should be of general interest. The Marinlit database79 continues to be updated and has again been used as the basis for the preparation of this present review.
3 Marine microorganisms and phytoplankton
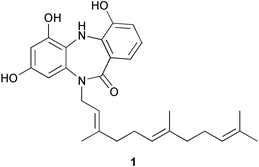
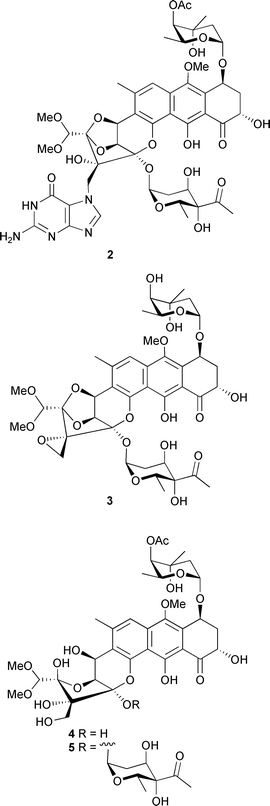
Increasingly, the field of marine microbes is becoming a focal point for research and rising numbers of novel compounds continue to be identified. An actinomycete, Micromonospora sp. obtained from Didemnum proliferum (Shishijima Is., Japan), produced the dibenzodiazepine alkaloid, diazepinomicin 1, which exhibited modest antimicrobial activity against selected Gram-positive bacteria.80Gutingimycin 2 is a highly polar trioxacarcin derivative from a Streptomyces species isolated from sediment (Laguna de Terminos, Gulf of Mexico).81 The absolute configuration was determined by X-ray analysis.82 The same Streptomyces species81 also yielded trioxacarcins D–F 3–5, in addition to the known trioxacarcins A–C.83–85 The structures and absolute configurations of the new trioxacarcins followed from the X-ray analysis of gutingimycin 2 and the known stereochemistry of L-trioxacarcins A and B.85 The trioxacarcins and gutingimycin 2 exhibited strong antibacterial activity against a range of test organisms. The antitumour activity of trioxacarcin D 3 was similar to that previously reported for trioxacarcins A–C,83–85 while trioxacarcin A and trioxacarcin D 3 were also potently antiplasmodial.86
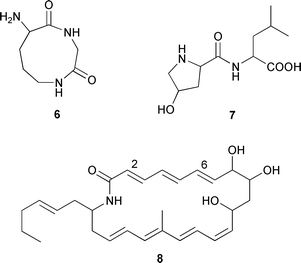
S. acrimycini, isolated from sediment (São Sebastião channel, Brazil), provided two dipeptide derivatives, 8-amino-[1,4]diazonane-2,5-dione 6 and leucyl-4-hydroxyproline 7.87 A cultured Nereus™ strain of S. aureoverticillatus isolated from marine sediment (source not given) produced a macrocyclic lactam, aureoverticillactam 8, possessing moderate activity against human tumour cell lines.
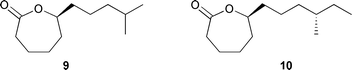
Some stereochemistry (2E,6E) could be determined, but other olefins could not be assigned due to spectral overlap.88 Two caprolactones 9 and 10 were produced from a Streptomyces species (mangrove sediment, Papua New Guinea). The proposed structures were based on GCMS experiments and proven by synthesis, while the absolute configurations of 9 and 10 were established using chiral GC to compare the natural and synthetic stereoisomers.
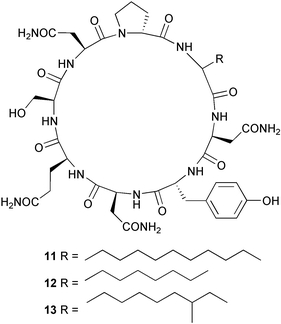
Compounds 9 and 10 displayed moderate phytotoxicity and concentration-dependent inhibition of human cancer cell lines, with concomitant low general cytotoxicity.89 A Bacillus species obtained from mud near the Arctic pole yielded three cyclopeptides, mixirins A–C 11–13. The absolute stereochemistries of the amino acid residues were determined (Marfey's), but not those of the fatty acid residues. The mixirins A–C inhibited the growth of human colon tumour cells (HCT-116).90
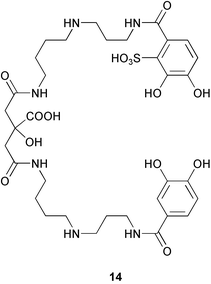
Petrobactin sulfonate 14, along with petrobactin,91 was isolated from the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus,91,92 and is the first marine siderophore containing a sulfonated 3,4-dihydroxy aromatic ring. The structure of petrobactin sulfonate was elucidated from spectral data, resulting in a revision of the NMR assignments for petrobactin.93
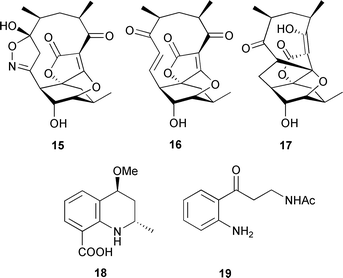
An actinomycete, Verrucosispora sp. (deep sediment, Sea of Japan), yielded the abyssomicins B–D 15–17, whose structures and relative stereochemistries were supported by X-ray analyses. The absolute stereochemistry of abyssomicin D 17 was determined (Mosher and Helmchen methods) and, through structural analogy, assumed to be the same for abyssomicins B 15 and C 16.94 Abyssomicin C 16 was identified as an inhibitor of the pathway between chorismic and para-aminobenzoic acids and was strongly active against Gram-positive bacteria.95 Helquinoline 18, and the N-acetylkynuramine 19, were produced by a North Sea bacterium Janibacter limosus. Helquinoline displayed moderate activity against B. subtilis, Streptococcus viridochromogenes and Staphylococcus aureus.96
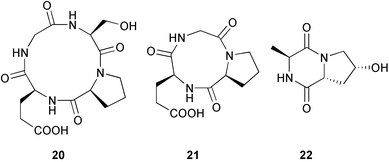
Two cyclic peptides 20 and 21 were isolated from a bacterial Ruegeria species associated with cell cultures of a sponge Suberites domuncula (Gulf of Naples, Italy).97 Two further peptides from the culture were known synthetic compounds,98,99 but isolated for the first time from nature. A known peptide100 and a new diastereoisomer 22 were also obtained, in addition to a peptide first reported from rabbit skin tissue.101
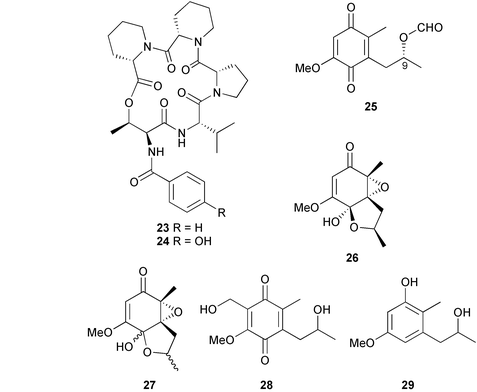
Cyclopeptides 20 and 21 exhibited moderate activity against B. subtilis.102 Two cyclodepsipeptides, petrosifungins A 23 and B 24, have been isolated from Penicillium brevicompactum derived from a sponge Petrosia ficiformis (Elba, Italy). The absolute configurations of the amino acids were determined (Marfey's).103 A mixed culture of two P. corylophilum strains, (deep water sediment, Fiji/Matuka), provided the known fungal pigments anserinones A and B104 along with three other pentaketides, (+)-formylanserinone B 25, (−)-epoxyserinone A 26 and (+)-epoxyserinone A 27. Two further minor constituents of the extract, hydroxymethylanserinone B 28 and deoxyanserinone B 29, were isolated but not completely purified.
The relative stereochemistries of 25 and 26 were determined (NOE experiments and molecular mechanics calculations), but the stereochemistry of (+)-formylanserinone B 25105 was subsequently revised from (9S) to (9R) based on recognition of discrepancies in published data.104,106 Similarly, the structure of (−)-epoxyserinone A 26 has been clarified as the structure was incorrectly drawn in the original report.105 The revised stereochemistry for 25 requires the absolute stereochemistry of 26 to be (2R,3S,4S,9R).107 Anserinone B and (+)-formylanserinone B 25 were the most active against a range of tumour cell lines.105P. citrinum, isolated from a red alga Actinotrichia fragilis (Okinawa, Japan), was the source of citrinadin A 30 that displayed modest cytotoxicity against L1210 and KB cell lines.108 A Penicillium species, from a sponge Axinella verrucosa (Elba, Italy), produced the known compound communesin B109 and the new congeners communesins C 31 and D 32. Communesins B–D exhibited moderate antiproliferative activity against several human leukemia cell lines and were active against brine shrimp.110 A mussel Mytilus edulis (Toyama Bay, Japan Sea) was the source of an Aspergillus species that yielded a polyketide, aspermytin A 33, with the absolute configuration being deduced from the CD spectrum.
Aspermytin A 33 induced neurite outgrowth in rat pheochromocytoma cells.111 Golmaenone 34, a diketopiperazine alkaloid, was obtained from an Aspergillus species isolated from a red alga Lomentaria catenata (Ulsan City, Korea). The absolute stereochemistry was established (Marfey's) as were significant radical scavenging and UV-A protecting properties.112A. pseudodeflectus, isolated from an alga Sargassum fusiform (Miura Peninsula, Japan), was the source of pseudodeflectusin 35, an isochroman cytotoxic against human cancer cell lines.113 A diketopiperazine dimer 36 was obtained from a marine-derived A. niger supplied by the Australian Institute of Marine Sciences, and the absolute stereochemistry determined (chiral HPLC).114
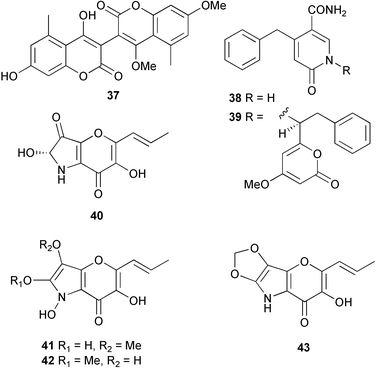
A. niger, isolated from a sponge Axinella damicornis (near Elba, Italy), yielded seven metabolites; the bicoumanigrin 37, the structurally unusual 4-benzyl-1H-pyridin-6-one derivatives aspernigrins A 38 and B 39 and the pyranonigrins A–D 40–43, which contain the unprecedented pyrano[3,2-b]pyrrole skeleton.
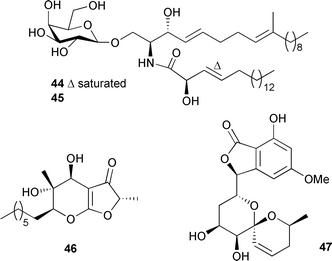
The absolute configurations of aspernigrin B 39 and pyranonigrin A 40 were established by quantum chemical calculations of the CD spectra. Also isolated was the known fungal pigment cycloleucomelone,115,116 which was moderately cytotoxic against human cancer cell lines in vitro, while aspernigrin B 39 was established as strongly neuroprotective.117 Cultivated mycelium of A. flavipes, isolated from a sea anemone Anthopleura xanthogrammica (Qingdao Bay, eastern China Sea), produced two cerebroside analogues, flavicerebrosides A 44 and B 45, with moderate activities against the KB cell line.118Aigialus parvus, isolated from mangrove wood (Thailand), provided the ketene acetal aigialone 46 and the spiroacetal aigialospirol 47.
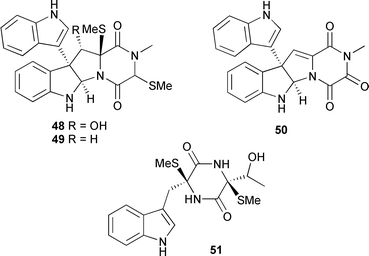
X-ray analysis of 46 established the relative stereochemistry only.119 The moderately cytotoxic oxopiperazine metabolites, gliocladins A–C 48–50, and glioperazine 51 were isolated from a strain of Gliocladium species separated from a sea hare Aplysia kurodai (Kata coast, Japan).
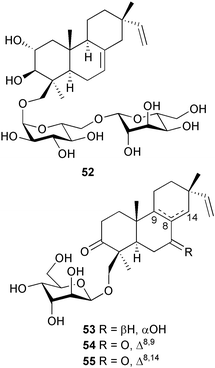
Relative stereochemistries at all centres, except for C-3 in 48 and 49 and for C-8 in 51, were determined (NOESY).120 Four cytotoxic diterpene glycosides, virescenosides R–U 52–55, were isolated from Acremonium striatisporum originating from a holothurian Eupentacta fraudatrix (Kitovoe Rebro Bay, Sea of Japan).121
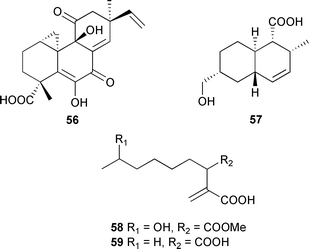
The stereochemistry of C-13 in 55 was determined by comparison against known compounds.122Apiospora montagnei, isolated from the inner tissue of an alga Polysiphonia violacea (North Sea near Helgoland), was the source of myrocin A 56, apiosporic acid 57, methyl 9-hydroxyhexylitaconate 58 and the (−)-enantiomer 59 of the known (+)-hexylitaconic acid.123–125
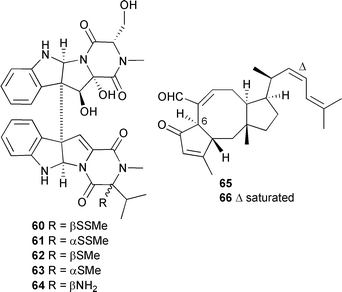
Five leptosins O–P 60–64 were obtained from a Leptosphaeria species, originally separated from Sargassum tortile (Tanabe Bay, Japan),126 but structural and absolute stereochemistry determinations were performed on the derived acetates. Leptosins O 60 and P 61 exhibited significant cytotoxicity against P388 cells while leptosins O 60 and S 64 were moderately cytotoxic to 39 human tumour cell lines.127Emericella variecolor, (marine sediment, Gokasyo Gulf, Japan), provided two new sesterterpenes, 6-epi-ophiobolin G 65 and 6-epi-ophiobolin N 66 along with six previously reported ophiobolins. All isolated compounds were cytotoxic against a neuroblastoma cell line.128
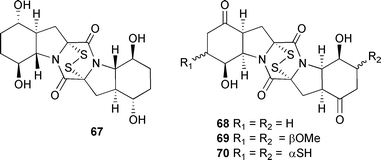
The modestly cytotoxic cyclic dipeptides rostratins A–D 67–70 were isolated from Exserohilum rostratum, a fungal strain associated with a marine cyanobacterial mat (Lanai, Hawaii). Absolute configurations of the rostratins were determined (Mosher's).129
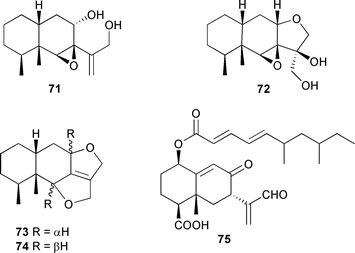
Peribysins A–D 71–74, from a strain of Periconia byssoides originating from Aplysia kurodai,130 are eremophilane sesquiterpenoids of which 73 and 74 represent a new class of furanofuran, and are potent inhibitors of the adhesion of HL-60 cells to HUVEC, with 74 being the most active.131 An eremophilane sesquiterpene, 7H239-A 75, was obtained from a xylariaceous fungus isolated from a Nypa (mangrove) palm frond (Kuala Selangor, Malaysia) and was cytotoxic towards a variety of cancer cell lines, with some selectivity for the CCRFCEM leukemia line.132
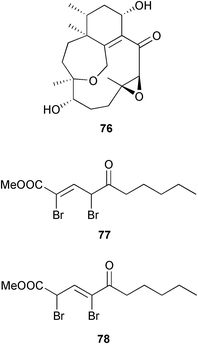
The diterpene phomactin H 76, with a novel skeleton including an oxepane moiety, was obtained from an unidentified surface fungus isolated from a brown alga Ishige okamurae (Tateishi, Japan). The structure and relative stereochemistry were determined by X-ray analysis.133 Two halogenated alkenoates, 77 and 78 were isolated from an unidentified surface fungus of a red alga Gracillaria verrucosa (Jinha, Korea).134
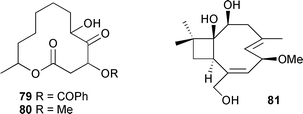
A total synthesis, using a Cr(II)–Ni(II) macrocyclisation, has been reported135 for racemic phomactin G, from a marine fungal Phoma species.136 A Cladosporium species from a brown alga Actinotrichia fragilis (Okinawa, Japan), produced two cytotoxic macrolides, sporiolides A 79 and B 80. Sporiolide A 79 was also moderately antifungal against a range of organisms, while both sporiolides were active against Micrococcus luteus.137 Fuscoatrol A 81, a caryophyllene sesquiterpene, was isolated from Humicola fuscoatra associated with an unidentified colonial ascidian (Shikotan Is., Kuril Isles) with the structure being confirmed by X-ray analysis.
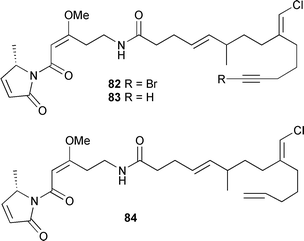
Fuscoatrol A 81 displayed moderate antimicrobial activity and was cytotoxic against sea urchin eggs.138Lyngbya majuscula (Hector's Bay, Jamaica) was the source of three lipopeptides, jamaicamides A–C 82–84.
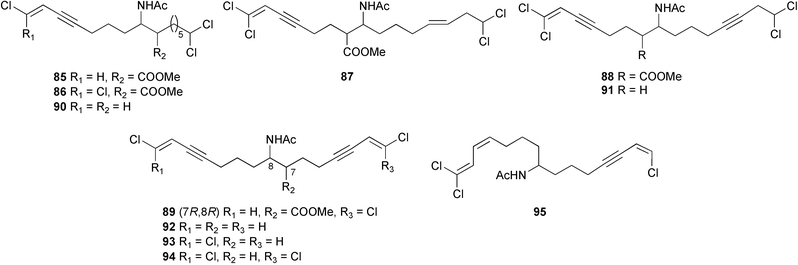
Structural elucidation included the use of the Accordion 1,1-ADEQUATE experiment.139 This experiment detects 1H–13C–13C spin systems for a wide range of 13C–13C coupling values and permits unequivocal distinction between 2- and 3-bond HMBC correlations. Only the C-23 stereochemistry of jamaicamide A 82 could be determined (Marfey's). Biosynthetic experiments suggested that the jamaicamides result from alternating PKS and NRPS assembly units, confirmed through cloning and identification of the biosynthetic gene cluster. The jamaicamides displayed cytotoxic, sodium channel blocking and ichthyotoxic activities.140 Eleven chlorinated lipids, taveuniamides A–K 85–95, were isolated from two mixed samples of L. majuscula and Schizothrix sp. (Taveuni Is., Fiji).
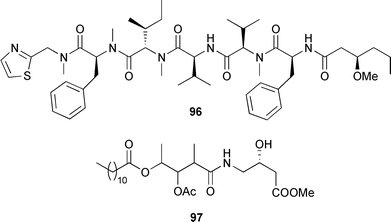
Taveuniamides F 90, G 91 and K 95 exhibited relatively potent brine shrimp toxicity.141 From a total synthesis of kalkitoxin and its congeners, the absolute stereochemistry of this potent neurotoxin isolated from L. majuscula142 could be assigned as (3R,7R,8S,10S,2′R).143 Kalkitoxin, from a second total synthesis, exhibited potent activity against HCT-116.144 A Symploca species (Fingers Reef, Guam) was the source of two cytotoxic compounds, micromide 96 and guamamide 97.
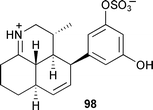
Chiral HPLC and chemical derivatisation followed by comparison with synthetic standards established the absolute stereochemistry of 96.145 Isolation of 8-hydroxy-3,5-dimethyl-isochroman-1-one146 from a mangrove fungus (coastal South China Sea) is the first report of this compound from a marine source.147 A symbiotic dinoflagellate Symbiodinium species, from an acoel flatworm Amphiscolops species (source not given), produced the amphoteric iminium compound symbioimine 98. The absolute stereochemistry was determined by X-ray analysis.
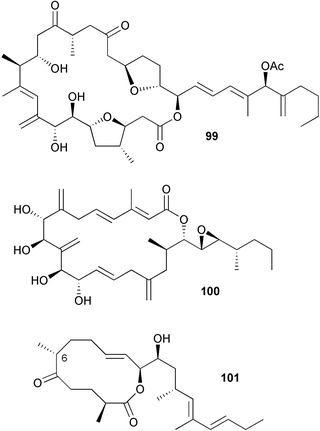
Symbioimine 98 inhibited osteoclastogenesis of the murine monocytic cell line RAW264 and therefore has potential for use as an antiresorptive for prevention and treatment of osteoporosis in postmenopausal women.148 The cytotoxic 25-membered macrolide amphidinolide C2 99 was obtained from a dinoflagellate Amphidinium species isolated from an acoel flatworm Amphiscolops species (Sunabe, Okinawa).149 Structural elucidation of (+)-amphidinolide A 100, also from an Amphidinium species,150,151 has been completed through a combination of NMR analysis and total synthesis.152 Amphidinolide H153 attenuated actin depolymerisation induced by diluting F-actin and enhanced the binding of phalloidin to F-actin and is therefore a useful tool for analyzing actin-mediated cell function.154 A total synthesis of amphidinolide W 101, a 12-membered macrolide isolated from an Amphidinium species,155 has been completed with revision of the C-6 stereochemistry.156
A total synthesis of amphidinolide X157 has also been reported.158 A dinoflagellate Amphidinium species, originally isolated from the surface wash of seaweeds (Lingshui Bay, Hainan Province, China), was the source of the potent cytotoxin lingshuiol 102.159 The same culture yielded two further lingshuiols, A 103 and B 104.160
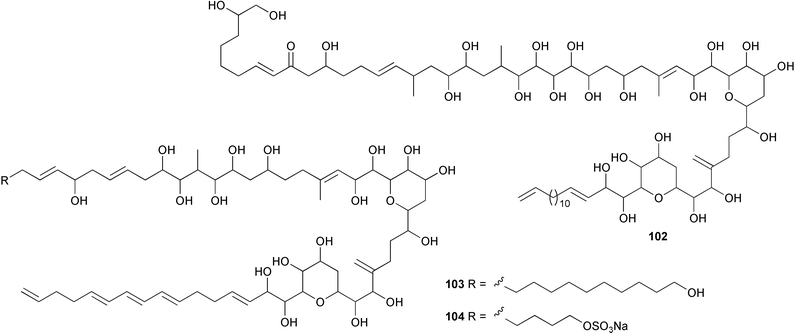
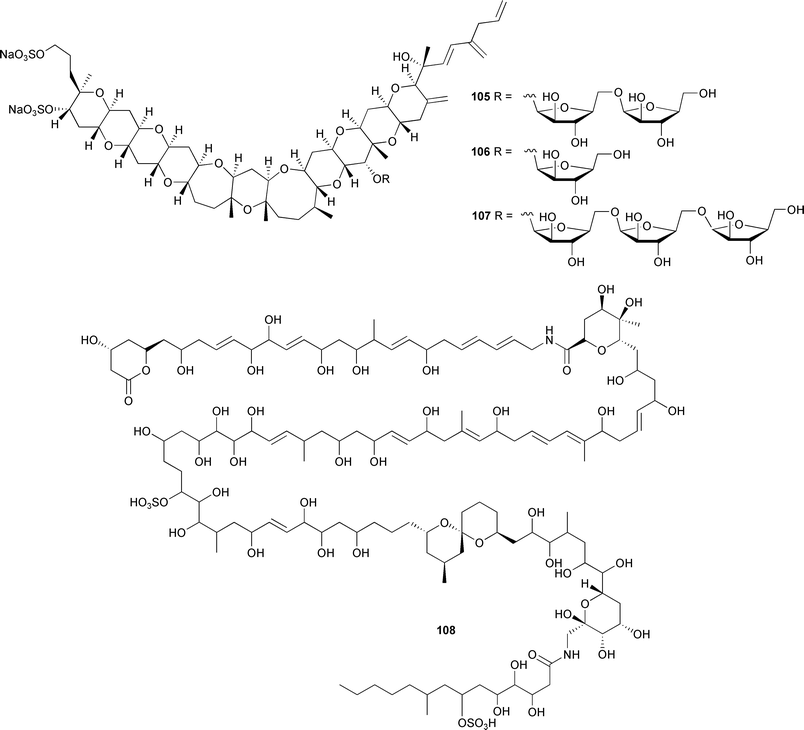
Two strains of a dinoflagellate Protoceratium cf. reticulatum (Sanriku, Iwate, Japan) were the source of four potent antitumour compounds, protoceratins 1–4. Protoceratin 1 was identical to the shellfish toxin 2-homoyessotoxin,161 while protoceratins 2–4 105–107 were determined to be the di-, mono- and tri-arabinosides of 2-homoyessotoxin respectively.162 A Symbiodinium species, isolated in the free-living state from a Hawaiian tide pool, gave the polyhydroxy metabolite zooxanthellamide B 108, a δ-lactone analogue of zooxanthellamide A163 which had previously been obtained from the same dinoflagellate.164
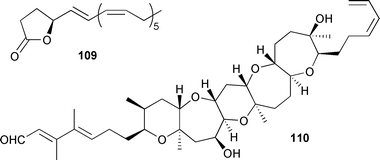
Several Symbiodinium strains obtained from different researchers around Japan produced zooxanthellactone 109. The original strain used for the production was isolated from the foraminifer, Amphisorus hemprichii. The absolute stereochemistry of zooxanthellactone 109, which was modestly cytotoxic against two human squamous cell carcinomas, was also established.165 The polyether brevenal 110, obtained from laboratory cultures of Karenia brevis as well as collections off the west coast of Florida during a red tide, competes with brevetoxin for a site associated with the voltage-sensitive sodium channel thus protecting fish from the neurotoxic effects of brevetoxin exposure.
Brevenal may have utility as a model compound for the development of therapeutics to prevent, or reverse intoxication in red tide exposures.166 A study in rats using short-term inhalation exposure to brevetoxin 3167 suggested that the immune system may be a target of toxicity following brevetoxin inhalation.168 The rapid effects of brevetoxin-2169 and saxitoxin170 on embryonic murine frontal cortex neuronal networks on microelectrode arrays have been reported.171 NMR studies on two synthetic diastereomeric models corresponding to the CDE/FG ring of prymnesins,172,173 suggested that the earlier stereochemical assignment of the E/F ring juncture in the prymnesins needs to be revised.174 A total synthesis of oscillarin 111, an antithrombotic peptide from Oscillatoria agardhii,175 has been achieved, confirming the absolute stereochemistry proposed and correcting the structure of a subunit to the Δ3-pyrroline-containing unit shown. Bioassays confirmed that 111 is a potent thrombin inhibitor while the originally assigned structure is inactive. An X-ray analysis of a ternary complex between oscillarin and α-thrombin has been reported.176 Synthetic (1S,2S) grenadamide had equal but opposite optical rotation from natural grenadamide from Lyngbya majuscula,177 which is therefore the (1R,2R)-enantiomer 112.178
Both enantiomers of xestodecalactone A179 have been synthesised, and CD and chiral HPLC comparisons have confirmed the (R)-configuration for the natural product.180 Using inexpensive starting materials, concise total syntheses of the maculalactones A,181 B182 and C,182 from a cyanobacterium Kyrtuthrix maculans, have been achieved. Results from optical rotation measurement, chiral HPLC and NMR spectroscopy in the presence of a chiral shift reagent established that natural maculalactone A is present in K. maculans as a partially racemic mixture.183 Total synthesis of the Bacillus laterosporus polyketide antibiotics basiliskamide A and B184 confirmed the assignment of absolute configuration as (7S,8S,9R,10S).185 The first total synthesis of (+)-phomopsidin, a microtubule assembly inhibitor and isolated from a Phomopsis species,186 has been achieved via a diastereoselective transannular Diels–Alder reaction.187 The total synthesis of halovir A,188 one of a series of linear, lipophilic peptides produced by a Scytalidium species, has been completed as part of a structure–activity relationship study of the series.189 A one-pot multistep domino reaction from a starting enone of established absolute configuration was used to complete a total synthesis of the mangrove fungus metabolite (+)-xyloketal D.190,191 The dinoflagellate metabolite yessotoxin192 has been shown to induce a cyclosporin A-sensitive permeability transition in rat liver mitochondria and Morris Hepatoma 1C1 cells in the presence of permissive levels of calcium, and also induces membrane depolarisation.193 The potent ichthyotoxin antillatoxin from Lyngbya majuscula,194 along with three synthetic stereoisomers, were assessed for ichthyotoxicity and cytotoxicity in five different assay systems. In all assays the natural antillatoxin was the most potent.195 Euplotin C,196 a sesquiterpene from a marine ciliate protist Euplotes crassus, displayed moderate activity against the protozoa Leishmania major and L. infantum and mild activity against Candida albicans.197 An assay for inhibitors of lymphocyte function-associated molecule 1/intercellular cell adhesion molecule-1 (LFA-1/ICAM-1) mediated cell–cell adhesion was used to identify new pharmacologically active compounds from marine cyanobacteria and algae.198 Six of the sixty marine natural products tested significantly inhibited LFA-1/ICAM-1 mediated cell adhesion after primary and secondary testing. These compounds were cymathere aldehyde,199 microcolins B200 and D,198 avrainvilleol,201 cymopol202 and hormothamnione diacetate.203–205 The stereochemistry of a cyclisation product, and results of incorporation experiments with [1-13C]- and [1,2-13C2]-acetates, were used to propose the course of geranylgeranyl diphosphate cyclisation in the biosynthesis of the phomactins206–208,136 from a marine fungal Phoma species.209 Incubation of a diatom Rhizosolenia setigera with [1-13C]-acetate indicated that the polyunsaturated monocyclic sesterterpene and triterpene metabolites are produced predominantly via the mevalonate pathway.210
4 Green algae
Very few new secondary metabolites from green algae have been reported. Capisterones A 113 and B 114 are triterpene sulfate esters isolated from Panicillus capitatus (Cat Cay, Bahamas). Both compounds exhibited potent antifungal activity against the marine algal pathogen Lindra thallasiae.2115 Brown algae
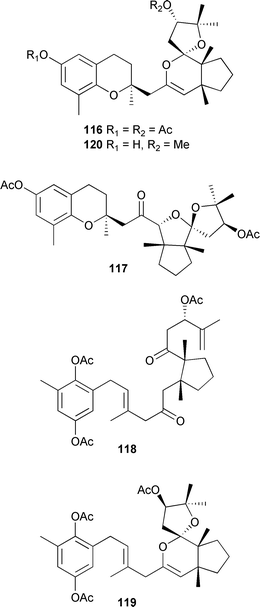
As noted in previous reviews, terpenes and polyphenolic compounds are the predominant metabolite classes found in brown algae. A sample of Eisenia bicyclis, a very common Pacific-coast Japanese species, was purchased from a Japanese company and yielded a new phloroglucinol derivative 115, and two known phloroglucinols, eckol212 and dieckol.213,214All three compounds inhibited glycation and α-amylase, so may have an effect on complications of diabetes.215 Phloroglucinol216 and the derivatives eckstolonol,217 eckol,212 phlorofucofuroeckol A218 and dieckol,213 isolated from Ecklonia stolonifera are tyrosinase inhibitors.219 Five meroditerpenes, amentol chromane diacetate 116, cystoseirone diacetate 117, preamentol triacetate 118, 14-epi-amentol triacetate 119 and 14-methoxyamentol chromane 120 were obtained from a Cystoseira species (Canary Islands).220
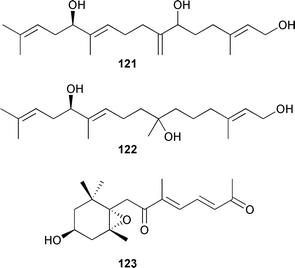
Two cytotoxic trihydroxylated diterpenes based on 12-hydroxygeranylgeraniol, 121 and 122, were isolated from Bifurcaria bifurcata (Atlantic coast of Morocco). The absolute configuration at C-12 in both compounds was established (Mosher's), but the stereochemistries of C-6 in 121 and C-7 in 122 could not be unambiguously determined.221Scytosiphon lomentaria, cultured in deep seawater, produced the bisnorditerpene 123.222
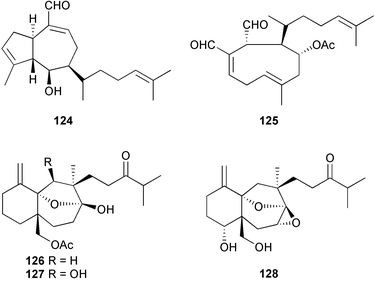
The secondary metabolite compositions of Dictyota dichotoma (Aegean Sea) and D. linearis (Chios Is., Greece), were examined and new diterpenes isolated; isopachydictyolal 124 from D. dichotoma and 4α-acetyldictyodial 125 from D. linearis.223D. dichotoma (Karachi coast in the Arabian Sea) yielded three C-16 oxidised seco-dolastanes, dichotenols A–C 126–128.224
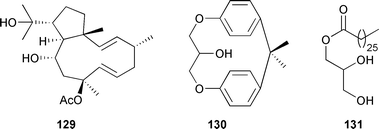
A new dolabelladiene derivative 129 and the previously isolated 10,18-diacetoxy-8-hydroxy-2,6-dolabelladiene225 were characterised from D. pfaffii (Northeast Brazil).226 Both compounds showed strong anti-HSV-1 activity in vitro but little inhibition of HIV-1 reverse transcriptase. 10,18-Diacetoxy-8-hydroxy-2,6-dolabelladiene225 was the antifeedant component of D. pfaffii against the sea urchin Lytechinus variegatus and generalist fishes.227 The diterpenes (6R)-6-hydroxydichotoma-3,14-diene-1,17-dial, and the 6-acetate derivative, from D. menstrualis,228 originally isolated from D. dichotoma,229 exhibited antiretroviral activity in vitro.228 Two glycerol derivatives, 130 and 131, were obtained from Sargassum parvivesiculosum (Hainan Province, China).230
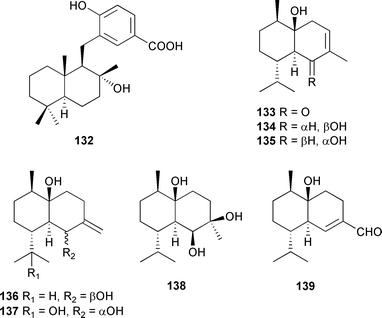
The sesquiterpene-substituted benzoic acid, dictyvaric acid 132, was isolated from Dictyopteris divaricata (Shandong coast, China),231 while seven cadinane sesquiterpenes, 133–139, were obtained from D. divaricata (Qingdao coast, China).
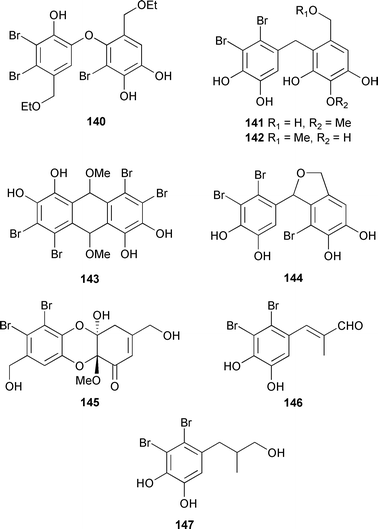
Structures and absolute configurations of 133–139 were established from spectroscopic data and X-ray analysis of 133 and 136.232 Six dibenzyl bromophenols 140–145,233 with different dimerisation patterns, and two propyl bromophenol derivatives, 146 and 147,234 were isolated from Leathesia nana (Shandong Province, China).
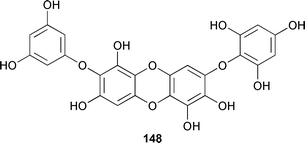
X-Ray analysis determined that 145 was a racemate. Compound 142 showed moderately selective cytotoxicity, and 140 may have been an artifact of isolation.233 Surprisingly, compound 146 was reported in another paper by the same authors.235 The moderately cytotoxic diphlorethohydroxycarmalol 148, from Ishige okamurae (Boso Peninsula, Japan),236 was characterised by conversion to the known nonaacetate.237
An enantioselective synthesis of the proposed structures for the bioactive (E)- and (Z)-usneoidones, from Cystoseira usneoides,238 has been completed by a convergent strategy.239 However, the spectroscopic data of the synthetic products differed significantly from the reported values. The phlorotannin derivatives 8,8′-bieckol240 and 8,4‴-dieckol,241 from Ecklonia cava (Korean coasts), are inhibitors of HIV-1 reverse transcriptase (RT) and protease. Both compounds inhibited the RT more potently than the protease and the inhibitory activity of 8,8′-bieckol against HIV-1 RT was comparable to that of nevirapine, a reference compound.242 Fucosterol243,244 was isolated from Pelvetia siliquosa and in vivo testing demonstrated that it is the main antidiabetic principle from P. siliquosa.245
6 Red algae
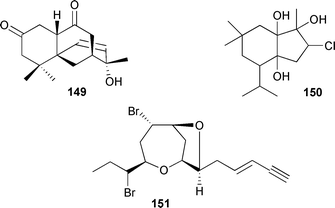
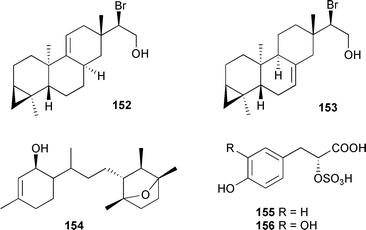
As for the brown algae, terpenes and polyphenolic compounds continue to be the predominant metabolite classes reported from red algae. Laurencia obtusa (Isla Grande, Panama), contained the oxygenated sesquiterpene chamigrenelactone 149.246 The sesquiterpene 150 and a halogenated C15 acetogenin 151, a stereoisomer of neoisoprelaurefucin,247 were isolated from L. obtusa (Tekirova, Turkey).248Two halogenated diterpenes, 15-bromoparguer-9(11)-ene-16-ol 152 and 15-bromoparguer-7-ene-16-ol 153, were obtained from L. nipponica (Troitsa Bay, Sea of Japan).249 Bioassay-guided fractionation of an extract from L. intricata (Discovery Bay, Jamaica), gave the diterpene laurenditerpenol 154 and the absolute configuration at C-1 was established (Mosher's). 154 was a potent inhibitor of hypoxia-activated hypoxia-inducible factor-1 and of the angiogenic factor hypoxia-induced VEGF in T47D cells.250 Two phenylpropanoic acid derivatives, tichocarpols A 155 and B 156, were isolated from Tichocarpus crinitus (Katsurakoi coast of Japan).
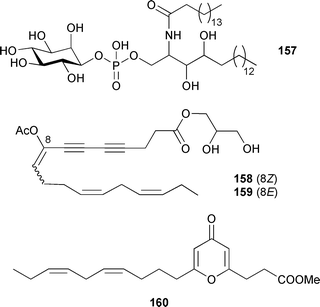
These two compounds, along with floridoside,251 also isolated from the alga, exhibited antifeedant activity against the sea urchin Strongylocentrotus intermedius.252 Inositol phosphoceramide 157 was isolated from Gracilaria verrucosa (Sea of Japan) and identified by GC and GCMS analysis.253Peyssonnelia caulifera (Yanuca Is., Fiji) was the source of two enediyne ω3 monoacyl glycerides, peyssonenynes A 158 and B 159, and a pyrone-containing ω3 fatty acid methyl ester, peyssopyrone 160, but the instability of the peyssonenynes prevented complete stereochemical assignment.
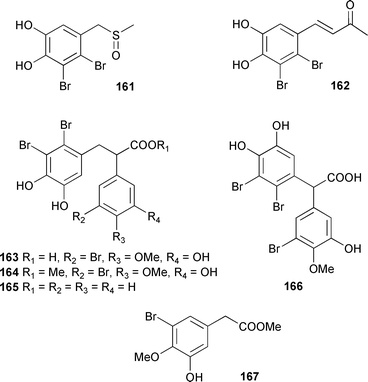
Both peyssonenynes displayed activity at a similar level in a DNA methyltransferase enzyme inhibition assay.254Rhodomela confervoides (Qingdao coast, China) was the source of seven new bromophenol derivatives 161–167, together with two known bromophenol compounds. X-Ray analysis of 161 and 162 supported the structural assignments.
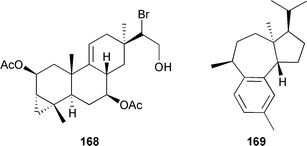
The known synthetic compounds 3-bromo-5-hydroxy-4-methoxyphenylacetic acid255 and 3-bromo-5-hydroxy-4-methoxybenzoic acid,256 were obtained as natural products for the first time.257 Seven brominated diterpenes of the parguerene and isoparguerene series isolated from Jania rubens (Red Sea coast of Egypt) included the novel deoxyparguerol-7-acetate 168. All the isolated diterpenes had anthelmintic activity and exhibited antitumour activity against Ehrlich ascites carcinoma in vitro with isoparguerol derivatives being slightly more effective than parguerol derivatives.258 The first total synthesis of (−)-presphaerene 169259 from Sphaerococcus coronopifolius was achieved from (R)-glyceraldehyde260 and established the relative and absolute stereochemistries of 169 as well as the absolute stereochemistries of the co-occurring sphaeroanes (+)-presphaerol,261,262 isosphaerodiene 1263 and isosphaerodiene 2.263
The first enantioselective total synthesis of (+)-brasilenyne264,265 was reported, starting from (S)-malic acid.266 Also reported in 2004 was the first total synthesis of the sesquiterpenoid (+)-palisadin B from Laurencia cf. palisada,267 utilising a new method for the formation of trans-anti cyclogeranyl-oxepene systems.268 Furoplocamioid C, prefuroplocamioid, pirene and a tetrachlorinated cyclohexane from Plocamium cartilagineum269 exhibited selective cytotoxicity against human tumour cell lines with pirene showing a specific and irreversible effect on SW480 cells.270 Halogenated metabolites from several species of Laurencia were tested for antibacterial activity against 22 strains of human pathogenic bacteria, including seven strains of antibiotic-resistant bacteria. Laurinterol,271 isolaurinterol,272allo-laurinterol,273 cupalaurenol274 and 2,3,5,6-tetrabromoindole275 displayed a wide spectrum of antibacterial activity against Gram-positive bacteria including methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae and vancomycin-resistant Enterococcus faecalis and E. faecium. Laurinterol and allo-laurinterol were particularly effective.276 Studies on vanadium bromoperoxidase isolated from marine red algae have established it as a catalyst in the biosynthesis of brominated cyclic sesquiterpene structures in the algae.277
7 Sponges
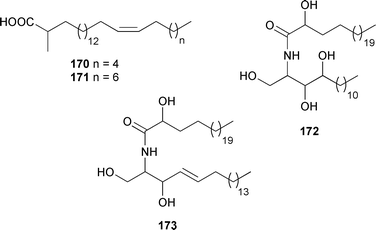
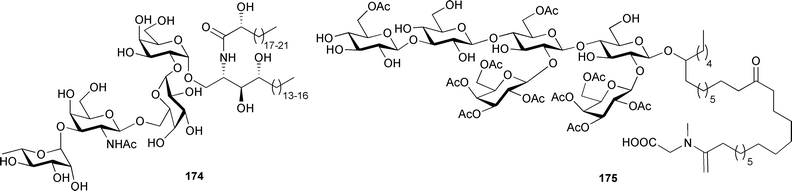
Unusual fatty acid derived metabolites are often isolated from sponges, and some motifs seem to be characteristic of the phylum; 2-methoxylated fatty acids are one such group of metabolites. A series of these compounds were synthesised, found to be active against mycobacteria, and proposed as a possible chemical defence mechanism in sponges.278 Two long 2-methyl substituted fatty acids 170 and 171 were isolated as methyl esters from Halichondria panicea (Sea of Japan, Russia).279 A Chinese Spongia species yielded the ceramides spongiamines A 172 and B 173.280Clarhamnoside 174, a glycolipid containing an unusual L-rhamnose unit, was isolated from the Bahamian Agelas clathrodes.281 The relative stereochemistry of the sugar units was determined from proton coupling constants determined with the use of a 13C-coupled HMQC experiment. The absolute stereochemistry of the alkyl chain oxymethine of caminoside A, originally isolated from Caminus sphaeroconia,282 has been determined as (10R) by the preparation of a TPP diester of the aglycone.283 An inhibitor of bacterial proteases, pachymoside A 175, was isolated from Pachymatisma johnstonia (Isle of Man).284
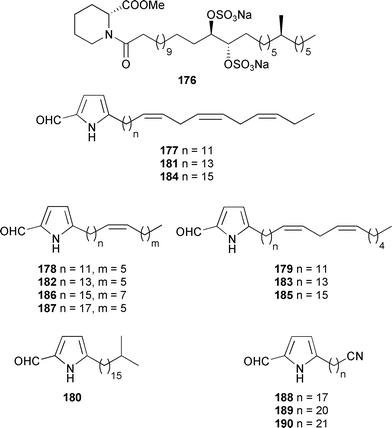
An α-glycosidase inhibitor, penasulfate A 176, was obtained from a Japanese Penares species using recycling HPLC.285Mycale cecilia (Gulf of California, Mexico) yielded fourteen cytotoxic long chain pyrroles, mycalazals 3–13 177–187, and three nitrile-containing congeners, the mycalenitriles 1–3 188–190.286
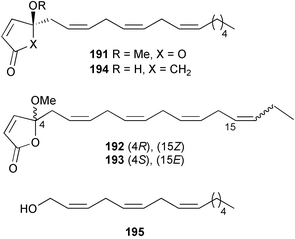
A Korean Homaxinella species yielded three weakly cytotoxic butenolides, homaxinolides A–C 191–193, along with the moderately cytotoxic cyclopentenone, homaxinone A 194. Also isolated from the same collection was an unsaturated alcohol 195 previously known only as a synthetic intermediate.287
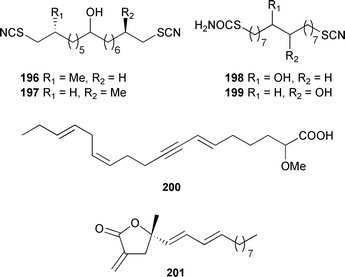
A pair of isomeric bisthiocyanates, thiocyanatins D1196 and D2197, and the related thiocyanate-thiocarbamates, thiocyanatins E1198 and E2199, were obtained as inseparable pairs from a South Australian Oceanapia species; all compounds were potent nematocides. Several related compounds were also tentatively identified by mass spectrometry.288 An acetylenic acid 200 has been obtained from a Chinese Cinachyrella australiensis.289 A Plakortis species (Canary Islands) contained plakolide A 201, a γ-lactone that inhibits inducible nitric oxide synthase activity.290
Two isomeric cytotoxic trans epoxides, plakorstatins 1 202 and 2 203, were isolated from Plakortis nigra (Sulawesi, Indonesia).291 A Dominican Plakortis anglospiculatus provided two highly branched tricylic acids, spiculoic acids A 204 and B 205 which are thought to be the result of a Diels Alderase-catalyzed intramolecular [4 + 2] cycloaddition.292
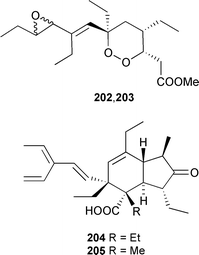
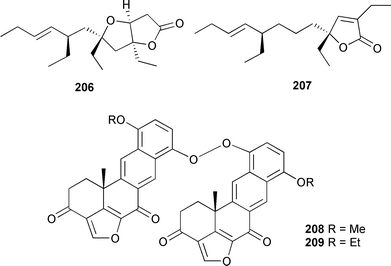
A series of glycosphingolipids were partially characterised from four Agelas species using tandem HPLC-ESIMS and HPLC-LSIMS.293 Dideoxypetrosynol A, a polyacetylene originally obtained from a Petrosia species,294 induced apoptosis in human melanoma cells.295 Manzamenone A296 was a potent inhibitor of β DNA polymerase and only weakly active against the α form of the enzyme.297 A lysophosphatidylcholine, originally isolated from Spirastrella abata,298 has been shown to increase intracellular Ca2+ concentration in human leukemia cells.299 Mycalazal 2 and mycalazol 11, pyrrole-containing lipids isolated from Mycale micranthoxea,300 have been synthesised using a Stille coupling.301 A synthesis of (2R,5Z,9Z)-2-methoxyhexacosa-5,9-dienoic acid, from Topsentia roquensis,302 has been accomplished using a novel HgO mediated O-alkylation.303 A racemic synthesis of plakortone E 206304 has established the relative stereochemistry,305 and an asymmetric synthesis of plakortone G 207306 defined both the relative and absolute stereochemistries.307 Two dimeric peroxides, dihalenaquinolides A 208 and B 209, were isolated from a Taiwanese Petrosia elastica.308
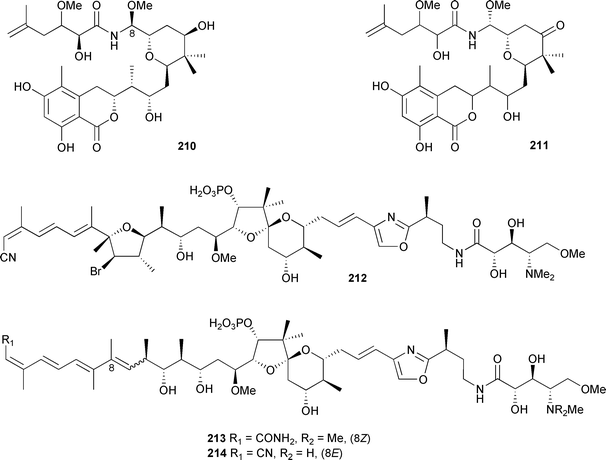
A very potent cytotoxin, psymberin 210, related to the pederin family of metabolites, was obtained from a series of Papua New Guinean collections of Psammocinia species.309 In an independent study, a compound named irciniastatin A, having the same NMR characteristics as 210 but claiming different stereochemistry at C-8, and the keto analogue irciniastatin B 211 were isolated from Ircinia ramosa (Borneo).310 Four calyculin analogues, calyculin J 212, calyculinamide F 213, des-N-methylcalyculin A 214 and the known calyculinamide A,311 were obtained from a Japanese Discodermia calyx.
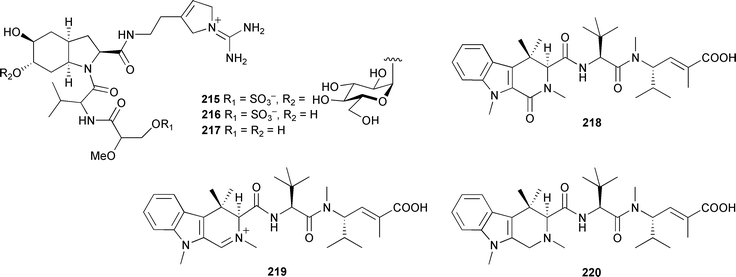
All four compounds were potent inhibitors of protein phosphatase 2A.312 A prokaryotic gene cluster, proposed to be responsible for the biosynthesis of onnamide A, has been isolated from the metagenome of Theonella swinhoei. The specific PKS cluster is distinct from many others by the lack of an acyltransferase domain, a characteristic that it shares with the pederin gene cluster. The onnA cluster was found only in the chemotypes of T. swinhoei that produce onnamide A and the theopederins.313 In a series of five consecutive papers the large-scale synthesis of discodermolide is described, culminating in the production of 60 g of enantio-pure material.314 Discodermolide, a microtubule stabilizing agent, was originally isolated from a deepwater Discodermia disoluta.315 The level of commitment involved in this large-scale synthesis of a highly complex marine natural product is a measure of the importance of the field of marine natural products to medical research and the pharmaceutical industry. Sponges continue to be a rich source of novel and biologically active peptides. The dysinosins B–D 215–217, from a Queensland Lamellodysidea chlorea, were inhibitors of serine protease factor VIIa and thrombin.316 The microtubule-destabilizing peptide, milnamide C 218, was obtained from an Auletta species (Papua New Guinea).317 Milnamide D 219, isolated from a Cymbastela species and reported in 2003,318 was inadvertently omitted from the previous review.1 An asymmetric synthesis of milnamide A 220319 and the subsequent autoxidation to milnamide D established the relative and absolute stereochemistry of these two potent antimitotic compounds.320
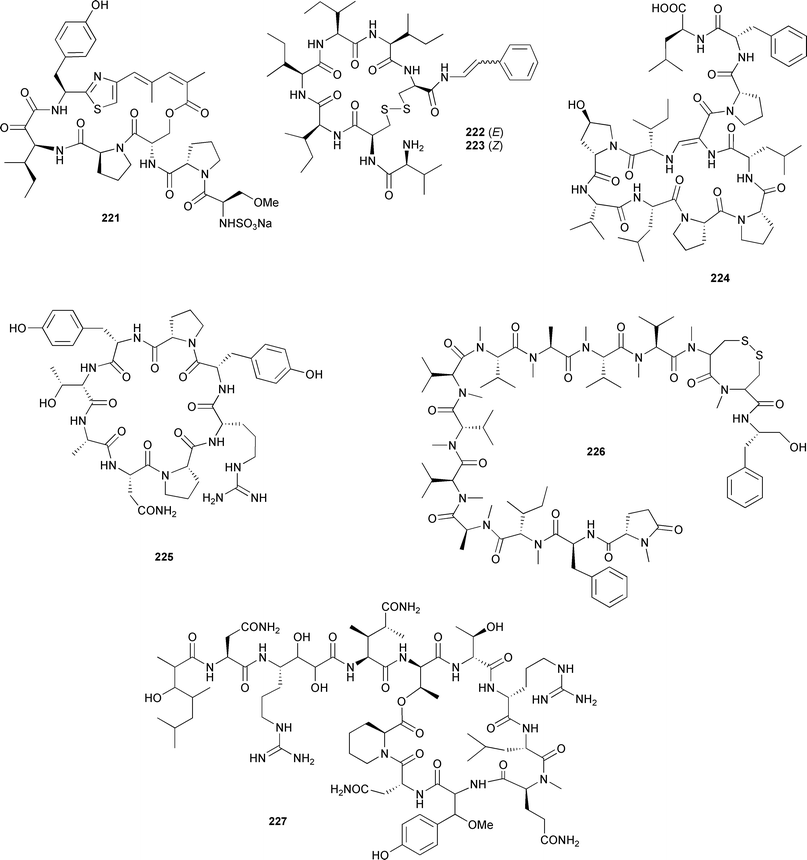
Scleritoderma nodosum (Philippines) contained the cytotoxic cyclic peptide scleritodermin A 221.321 Another Philippine sponge, Clathria (Thalysias) abietina, yielded two isomeric disulfide-bridged heptapetides, microcionamides A 222 and B 223, with cytotoxic and antitubercular activities.322 An unusually cyclised peptide, callynormine 224, isolated from Callyspongia abnormis (Kenya), represents a new class of heterodectic cyclic peptide, the endiamino peptides, which contain an α-amido-β-aminoacrylamide functionality.323Axinella carteri (Caroline Islands) yielded the octapeptide cyclonellin 225.324 Kendarimide A 226, a linear peptide obtained from a Haliclona species (Sulawesi, Indonesia), reversed P-glycoprotein-mediated multi-drug resistance in mammalian cells.325 A large depsipeptide, neamphamide A 227, with potent cytoprotective activity against HIV-1 was isolated from a Papua New Guinean Neamphius huxleyi.326
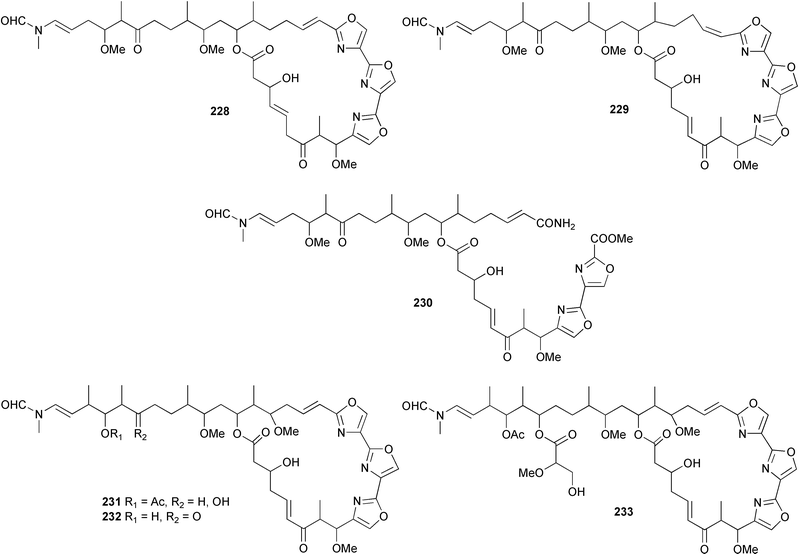
Phakellistatin 13, recently isolated from Phakellia fusca,327 has been synthesised, confirming the natural L configuration of the tryptophan residue.328 Phakellistatin 13 was also converted by photo-oxidation into phakellistatin 3 and isophakellistatin 3, both originally obtained from P. carteri.329 Miraziridine A, isolated from Theonella aff. mirabilis,330 has been synthesised and is an inhibitor of three classes of proteases.331 Callipeltin A, originally isolated from a New Caledonian Callypelta species,332 acts as an ionophore, increasing the contraction of vascular tissues in the presence of Na+ ions.333 The complex structures and often spectacular biological activity of sponge-derived macrolides and their related congeners provide attractive targets and considerable challenges to natural product and synthetic chemists alike. Examples of these include the two oxazole-containing macrolides neohalichondramide 228, (19Z)-halichondramide 229 and a related ring opened compound secohalichondramide 230 obtained from Chondrosia corticata (Guam),334 and the three cytotoxic mycalolides, 30-hydroxymycalolide A 231, 32-hydroxymycalolide A 232, and 38-hydroxymycalolide B 233 isolated from a Japanese Mycale magellanica.312
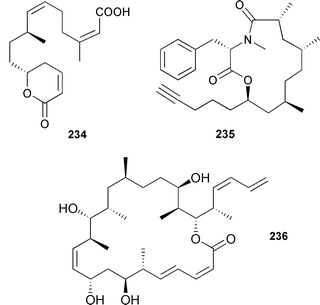
Latrunculeic acid 234, from Negombata (Latrunculia) magnifica335 (Gulf of Eilat, Red Sea), is clearly related to the latrunculin macrolides, isolated previously from the same sponge.336 Spongidepsin 235, originally isolated from a Spongia species (Vanuatu),337 has been synthesised independently by two groups defining both relative and absolute configurations.338,339 The relative stereochemistry of dictyostatin 236, originally obtained from a Spongia species,340 has been determined (Murata's method), revising the original structure.341
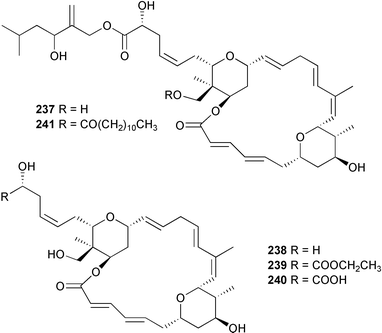
Two independent groups have confirmed this relative stereochemistry and absolute configuration by synthesis.342,343 Five antiproliferative lasonolide congeners, lasonolides C–G 237–241, were isolated from a Forcepia species (west coast of Florida).344
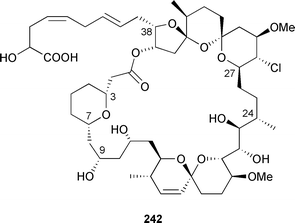
A larger recollection of the producing organism, Spirastrella coccinea, yielded sufficient spirastrellolide A 242 for further spectroscopic examination and derivative preparation.
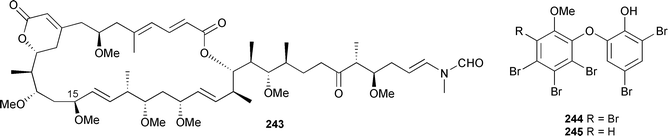
Originally reported with a molecular formula of C53H86O19,345 an extensive re-analysis led to the determination of a new molecular formula, exchanging H3O2 for Cl, and structural revision (as shown). The relative stereochemistries of segments C-3–C-7, C-9–C-24, and C-27–C-38 were established from ROESY data obtained at 800 MHz.346 The synthesis of a series of diastereomers of a degradation fragment of reidispongiolide A 243, originally isolated from Reidispongia coerulea,347 has led to a reassignment of the relative stereochemistry of the C-7–C15 fragment.348 The potent cytotoxin 13-deoxytedanolide, from Mycale adhaerans,349 has been shown to bind to the 60S large ribosomal subunit.350 Two brominated biphenyl ethers 244 and 245 were isolated from Phyllospongia dendyi (Palau), along with a series of related known compounds.
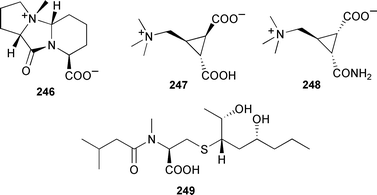
Some of the known dihydroxy congeners showed inhibitory activity towards the assembly of microtubules.351 A tricyclic dipeptide betaine, dysibetaine PP 246, and two cyclopropane betaines, dysibetaines CPa 247 and CPb 248, were isolated from an aqueous extract of Dysidea herbacea (Yap, Micronesia).352 A Japanese Spongia species produced the moderately antifungal spongiacysteine 249.353
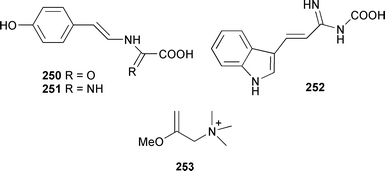
Three aromatic amino acid-derived alkaloids, hamigeroxalamic acid 250, hamigeramine 251, and hamigeramide 252 were isolated from a Mediterranean Hamigera hamigera.354 Esmodil 253, known previously as a synthetic drug, has been found as a metabolite of a South Australian Raspailia species.355
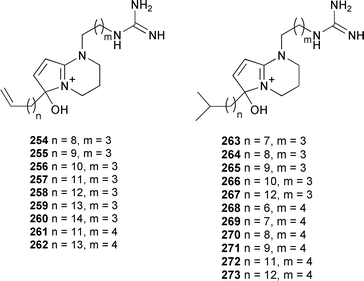
A series of twenty new phloeodictine alkaloids 254–273, together with several known congeners, were obtained from Oceanapia fistulosa (New Caledonia).
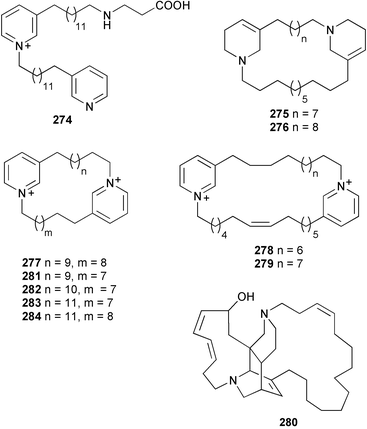
The compounds were characterised by LCMS from a complex mixture that showed antiplasmodial activity against chloroquine-resistant Plasmodium falciparum.356 Alkylpyridine-derived alkaloids are a characteristic of sponges that continue to provide an assortment of unusual metabolites. Viscosaline 274 was obtained from Haliclona viscosa (Russian Arctic Ocean).357 The same sponge, but from Svalbard in the Norwegian Arctic, yielded haliclamines C 275 and D 276.358 Three inhibitors of histone deacetylase, cyclostellettamine G 277 and dehydrocyclostellettamines D 278 and E 279, were isolated from a Japanese Xestospongia species.359 A Brazilian Pachychalina species yielded the cytotoxic ingenamine G 280, the previously described cyclostellettamine G, and four new congeners, cyclostellettamines H, I, K and L 281–284.360
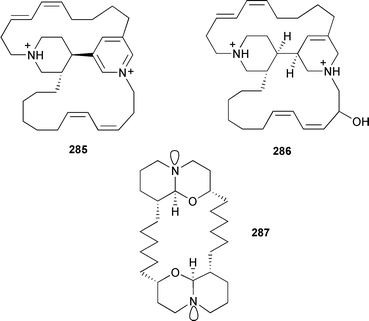
An Amphimedon species (Southern Japan) contained tetrahydrohalicyclamine 285 and 22-hydroxyhalicyclamine 286, both of which inhibit the growth of mammalian cells.361 A new aruguspongine/xestospongine analogue, araguspongine M 287, has been described from Neopetrosia exigua (formerly Xestospongia exigua) from Palau.362
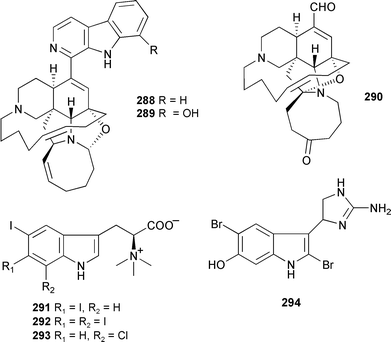
Hachijodine B, originally isolated from a Xestospongia species,363 has been synthesised, along with the proposed structure of ikimine B originally isolated from an unidentified sponge,364 but the spectra of the synthetic ikimine B did not match that of the natural product suggesting a possible incorrect assignment of the original structure.365 Three manzamine alkaloids 288–290 have been isolated from an Indonesian Acanthostrongylophora species. The activities of several known manzamine alkaloids as potent antiinflammatory, antifungal, and anti-HIV-1 agents were also reported.366 Indole-based alkaloids are well represented among sponge metabolites. Three further iodinated indole alkaloids, plakohypaphorines D–F 291–293 are reported from Plakortis simplex (Bahamas).
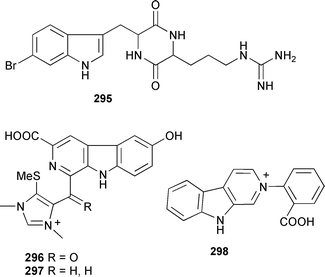
Plakohypaphorines B–D have significant antihistamine activity.367 The weakly cytotoxic guanine-containing 6-hydroxydiscodermindole 294 was obtained from Discodermia polydiscus (Bahamas).368 An antifouling alkaloid, 8,9-dihydrobarettin 295, has been isolated from Geodia barretti.369 The recently reported revised structure for barettin,370 from G. baretti,371 has been synthesised confirming the revised structure.372 Using LCMS, barettin has been detected at biologically-significant concentrations in water surrounding live G. barretti specimens.369 An enantiospecific synthesis of the (+)-dragmacidin F has established the absolute configuration of the natural product as (4′S,6″S,6‴S).373 Two thiomethyl-containing β-carbolines, dragmacidonamines A 296 and B 297, were isolated from a Dragmacidon species (Adaman Islands, India).374 A Thorectandra species (Palau) produced a β-carboline alkaloid 298 with moderate cytotoxicity.375
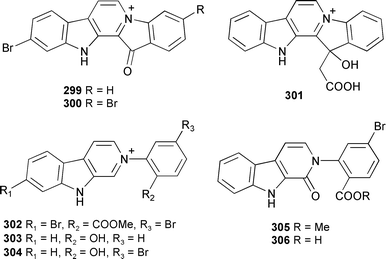
A series of fused bisindole-derived compounds 299–306 in the fascaplysilin series were isolated from Fascaplysinopsis reticulata (Indonesia).376
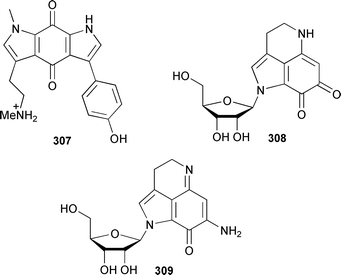
Pyrroloquinones and pyrroloiminoquinones are associated with sponges, particularly members of the Order Poecilosclerida. Zyzzyanone A 307, which is moderately cytotoxic and inhibits division of fertilised sea urchin eggs, was isolated from an Australian Zyzzya fuliginosa.377 Two β-D-ribopyrroloquinoline alkaloids 308 and 309 were obtained from a South African Strongylodesma aliwaliensis.378
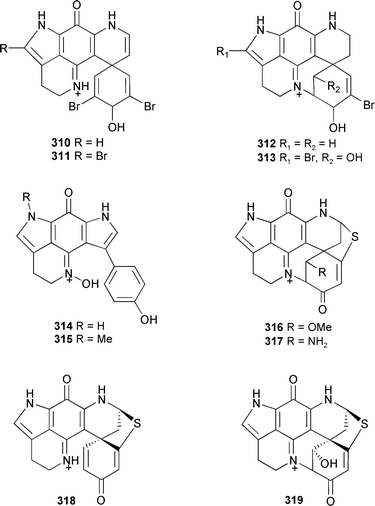
Four new discorhabdins, 3-dihydro-7,8-dehydrodiscorhabdin C 310, 14-bromo-3-dihydro-7,8-dehydrodiscorhabdin C 311, discorhabdin V 312, and 14-bromo-1-hydroxydiscorhabdin V 313 were isolated from the South African Tsitsikamma pedunculata. In the same article, the tsitsikammanamines A 314 and B 315 are reported from T. favus along with 1-methoxydiscorhabdin D 316 and 1-aminodiscorhabdin D 317 from Latrunculia bellae.379 Also isolated from L. bellae was discorhabdin G*, identical in structure with discorhabdin I 318 which was reported, along with discorhabdin L 319, from Latrunculia brevis (Argentina).380
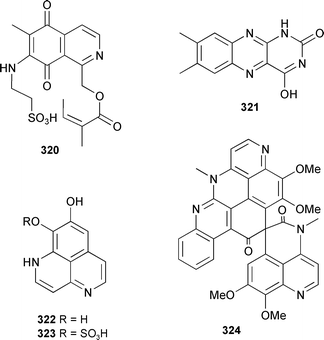
Fortunately, some of the confusion over the earlier naming of the discorhabdin series has been resolved in a 2005 review on pyrroloiminoquinones.381 Cribrostatin 7 320, a cytotoxic quinone alkaloid was obtained from a Petrosia species (Philippines).382 A Chinese Cinachyrella australiensis contained isolumichrome 321.289 Bisdemethylaaptamine 322 and the 9-O-sulfate analogue 323 were isolated from an Indonesian Aaptos species.383 The racemic spiro-polycyclic aromatic alkaloid lihouidine 324, obtained from a new species of Suberea (Lihou reef, Coral Sea), showed cytotoxic activity.384
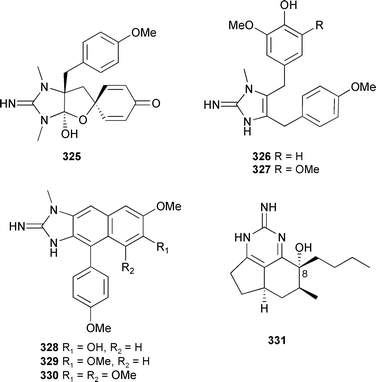
Syntheses of 7-methoxy-1,6-dimethylisoquinoline385 from a Xestospongia species,386 6-hydroxy-7-methoxyisoquinolinemethanol387 from a Haliclona species,388 and cribrostatin 6389 from a Cribrochalina species,390 have been completed. Renieramycin A, from a Reniera species,391 had antileishmanial activity.392 A Leucetta species (Papua New Guinea) provided spiroleucettadine 325 which showed antibacterial activity against Enterococcus durans.393Leucetta chagosensis (Sulawesi, Indonesia) contained naamines F 326 and G 327 as well as kealiinines A–C 328–330. Naamine G was strongly antifungal.394Monanchora unguifera (Jamaica) provided 1,8a;8b,3a-didehydro-8α-hydroxyptilocaulin 331, together with the known 8β isomer, which co-crystallised in perfect order with an approximate inversion centre.395
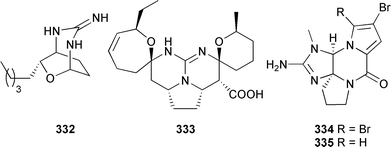
Monanchorin 332, a new bicyclic metabolite and the pentacyclic crambescidin acid 333, previously reported as the ethyl ester,396 have both been isolated from Monanchora ungiculata (Maldive Islands).397 Two phakellin alkaloids, 334 and 335, active as human lipoxygenase inhibitors, were found in an Agelas species (Papua New Guinea).398
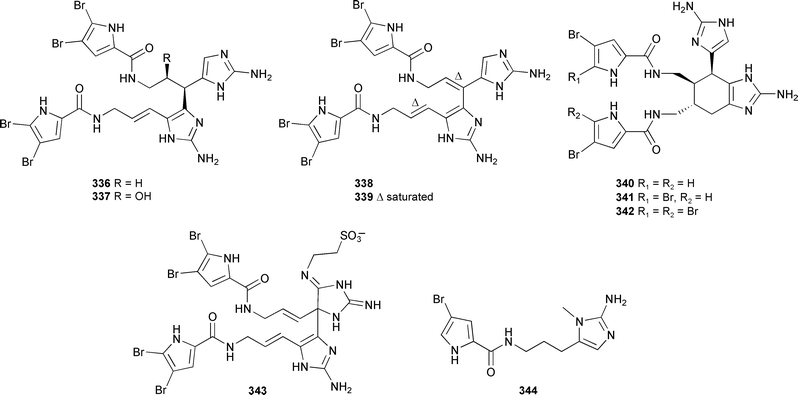
An Okinawan Agelas species provided the nagelamides A–H 336–343 and 9,10-dihydrokeramadine 344. The nagelamides were antibacterial, while nagelamide G inhibited protein phosphatase 2A activity.399
A systematic analysis of CD spectra and Mosher acid derivatives suggested that the configuration of longamide A, from Agelas longissima,400 be revised to (4R).401 (±)-Sceptrin,402,403 from Agelas sceptrum,404 and (±)-dibromosceptrin403 from Agelas conifera,405 have both been synthesised. The conversion of ageliferin into sceptrin using microwave irradiation also revealed a possible alternative biosynthetic pathway linking the pyrrole-imidazole alkaloids.406 The synthesis of dehydrobatzelladine C, originally isolated from two Monanchora species396 has been reported.407 The first enantiospecific synthesis of agelastatin A, isolated from Agelas dendromorpha,408 has been achieved.409 The HIV-active alkaloid batzelladine A, from a Batzella species,410 has been synthesised in an enantio-pure form.411 Crambescidin 800, originally isolated from Crambe crambe,412 caused myelogenous leukemia cells to differentiate into erythrocytes.413 The schulzeines A–C 345–347 were isolated from Penares schulzei (Hachijo-kojima Is., Japan).414
The configurations of these unusual sulfonated long chain aromatic alkaloids, potent inhibitors of yeast α-glucosidase, were determined from a combination of chemical degradation and modified Mosher's method. A series of bromotyrosine alkaloids, the purpurealidins A–H 348–355, were reported from a Psammaplysilla purpurea (India).415
Purpurealidin B 349 was antibacterial. Two potent antagonists of the sarcoplasmic reticulum calcium channel RyR1-FKBP12, the sulfonates 356 and 357, were isolated from Ianthella basta (Guam), along with 34-O-sulfatobastadin-9 358.416 A sulfur-containing nucleoside, hamiguanosinol 359, was obtained from a Mediterranean Hamigera hamigera.354
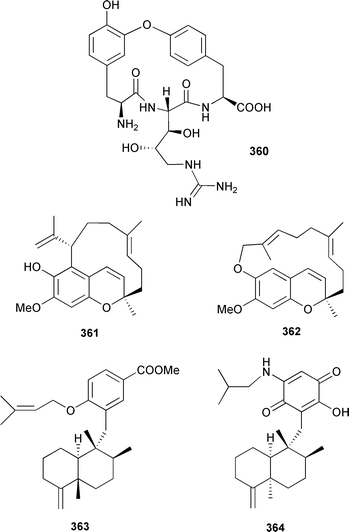
A bromotyrosine oxime that inhibits mycothiol-S-conjugate amidase, isolated from an Oceanapia species,417 has been synthesised.418 The same molecule was also synthesised independently through a trifluoromethyloxazole intermediate.419 A total synthesis of the eurypamides, first isolated from a Palauan Microciona eurypa,420 resulted in revised stereochemistry for eurypamide A 360 and confirmed the structures of eurypamides B–D.421 Two macrocyclic merosesquiterpenoids, likonides A 361 and B 362, were obtained from a Kenyan Hyatella species.422 17-O-Isoprenyldictyoceratin-C 363 was isolated from a Philippine Spongia species.423 An amino-merosesquiterpenoid, 5-epi-smenospongorine 364, isolated from Dactylospongia elegans (Flores, Indonesia), caused the differentiation of K562 leukemia cells into erythrocytes.424
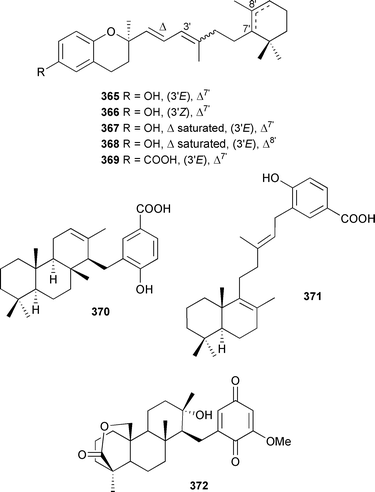
A Papua New Guinean Psammocinia species contained chromarols A–E 365–369, inactivators of human 15-lipoxygenase through a redox mechanism similar to that reported for a range of known meroterpenoids.425 Makassaric acid 370 and subersic acid 371, isolated from an Acanthodendrilla species (Sulawesi, Indonesia), were inhibitors of MAPKAP kinase 2.426Petrosia (Strongylophora) corticata (Papua New Guinea) contained the anti-invasion active meroditerpenoid strongylophorine-26 372.427
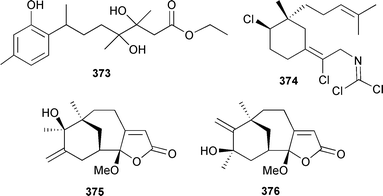
A series of merosesquiterpenoids from a variety of sponge sources were assessed for hemolytic and cytotoxic properties.428 Similarly, a further range of sponge-derived merosesquiterpenoids were active as antioxidants.429 Smenospongine, originally isolated from a Smenospongia species,430 caused K562 leukemia cells to differentiate into erythrocytes.431 A series of polyprenylated hydroquinones and benzoic acids of sponge origin inhibited phosphatase CDC25A.432 Puupehenone, originally isolated from a Hawaiian sponge,433 inhibited the differentiation of endothelial cells into tubular structures, an important step in angioneogenesis.434 Chloropuupehenone433 has been synthesised.435 An aromatic sesquiterpenoid 373 was isolated from a Didiscus species (Thailand).436Stylotella aurantium (Great Barrier Reef) yielded a carbonimidic dichloride stylotellane D 374.437 The weakly antibacterial hyrtiosenolides A 375 and B 376 were isolated from a Red Sea Hyrtios species.438
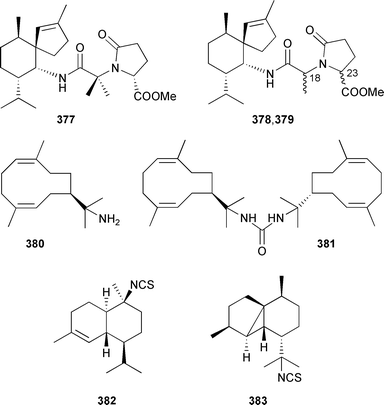
The boneratamides A–C were isolated as their methyl esters 377–379 from an Indonesian Axynyssa aplysinoides.439 The relative stereochemistry of boneratamide A 377 was established by X-ray analysis, while boneratamides B 378 and C 379 differ in their relative configurations at either one or both of C-18 and C-23. The antimicrobial germacrane sesquiterpenoid 380, and the bis-urea analogue 381, were obtained from an Axinyssa species (Thailand).440 Two sesquiterpene isothiocyanates 382 and 383 were obtained from an Okinawan Stylissa species.441
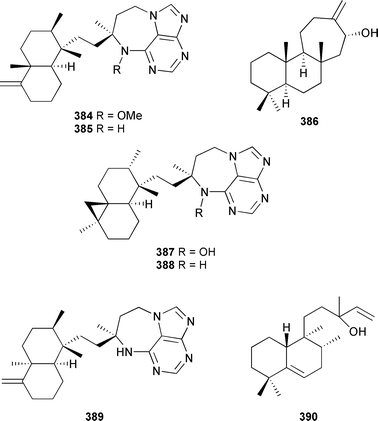
The biosynthesis of the carbonimidic dichloride sesquiterpenoids, the stylotellanes A and B, was examined in the producing organism Stylotella aurantium (Great Barrier Reef). Feeding experiments with 14C-labeled metabolites established that CN, SCN, and farnesyl isothiocyanate were intermediates in the biosynthetic pathway to these dichloroimines.442 A total synthesis of hamigeran E,443 from a New Zealand Hamigera tarangaensis, has been reported.444 (6R,7S)-7-amino-7,8-dihydro-α-bisabolene, from a Halichondria species,445 has been synthesised.446 Helianane, isolated from Haliclona fascigera,447 was synthesised employing a vacuum thermolytic ring expansion step.448 A Kenyan Raspailia species gave two purine alkaloid-derivatised diterpenes asmarines G 384 and H 385 and the diterpenoid barekol 386, together with several related known compounds.449 A Raspailia species (Madagascar) produced further asmarines, I–K 387–389, and the diterpenoid nosyberkol 390.450
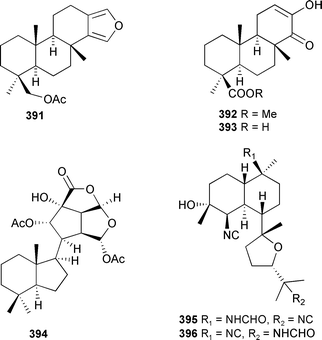
A furanoditerpenoid 391, and two related norditerpenoids 392 and 393, that inhibit the lyase activity of DNA polymerase β, were obtained from an unidentified Dictyoceratid sponge from Papua New Guinea.451 A bright yellow encrusting Aplysillid sponge, tentatively identified as Aplysilla sulfurea from S. E. Queensland, contained the cytotoxic and nematocidal compound chromodorolide C 394, along with the known A and B congeners.452Acanthella cavernosa (Philippines) provided 10-formamido-kalihinol F 395 and 15-formamido-kalihinol F 396, both of which inhibited bacterial folate biosynthesis.453
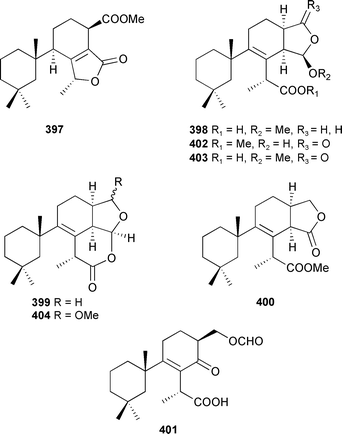
A norgracilane 397 and three new aplysulfurane-type spongian diterpenoids 398–400 were isolated from Dendrilla membranosa (South Shetlands, Antarctic),454 and by forming complexes between (R) and (S) 2,2,2-trifluoro-1-(9-anthryl)ethanol and the γ-methylbutenolide of 397 the absolute stereochemistries were established (as drawn). Aplysulfurane-type spongian diterpenoids, with antiinflammatory properties, were also reported from a New Zealand Chelonaplysilla violacea.455 Two compounds from this series, named as pourewic acid A 398 and cadlinolide C 399, were found in both collections along with the formate-containing pourewanone 401, methyl pourewate B 402, 15-methoxypourewic acid B 403, and an inseparable mixture of the diastereoisomeric cadlinolides D 404, with relative stereochemistries established as drawn.
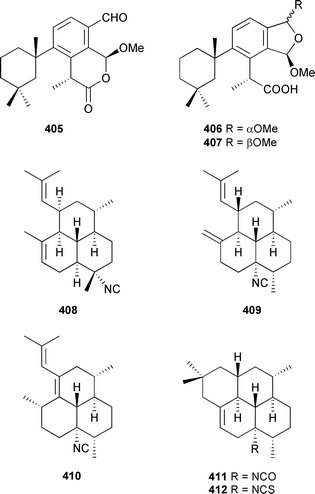
The optical rotation of the New Zealand-derived pourewic acid A, which was small, was comparable to but opposite in sign to that reported for the Antarctic sample, but the measurements were made in different solvents (CH2Cl2vs. CHCl3). Further resolution of the absolute stereochemistries will require more rigorous chiroptical evaluation of the samples. In contrast, an Anvers Is., Antarctic, collection of Dendrilla membranosa yielded the aromatic antimicrobial spongian diterpenoids, membranolides B–D 405–407.456 The three diterpenoid isocyanides 408–410, isocyanate 411, and isothiocyanate 412 were isolated from the same Okinawan Stylissa species that had yielded the previously described isothiocyanate sesquiterpenes 382 and 383.441
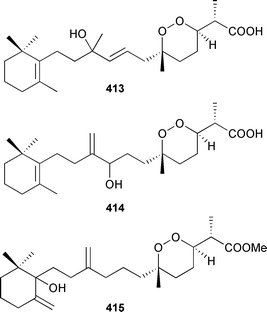
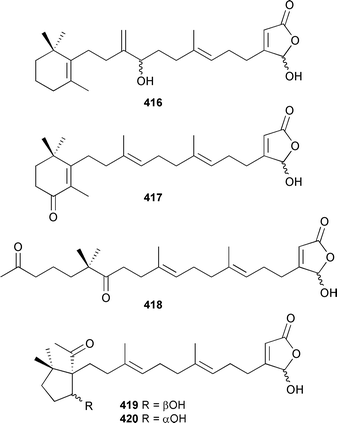
All metabolites displayed weak cytotoxicity. The biosynthetic origins of the isocyanide and isothiocyanate carbon of a variety of sesqui- and diterpenes isolated from Amphimedon terpenensis and an Axinyssa species (Great Barrier Reef), have been investigated. For all compounds specific incorporation of 14C-thiocyanate was demonstrated.457 A racemic synthesis of kalihinol C, first isolated from an Acanthella species,458 has been reported.459 Three cytotoxic norsesterterpenoids, tasnemoxides A–C 413–415, have been isolated from a Red Sea Diacarnus erythraenus.460 An Acanthodendrilla species (Indonesia) provided the cytotoxic acantholides A–E 416–420.
Acantholide B 417 was antimicrobial.461 A Korean Psammocinia species contained three cytotoxic sesterterpenoids, psammocinins A1421, A2422 and B 423.462 (6Z)-Neomanoalide-24,25-diacetate 424 was obtained from a Palauan Luffariella species.463 A Brachiaster species (Thailand) yielded the same manoalide congener 424, the (E) isomer 425 and the potently antitubercular 12-deacetoxyscalarin-19-acetate 426.464
Five moderately cytotoxic sesterterpenoids, three scalaranes 427–429 and two linear furanoterpenoids 430 and 431, were obtained from a Korean Smenospongia species.465
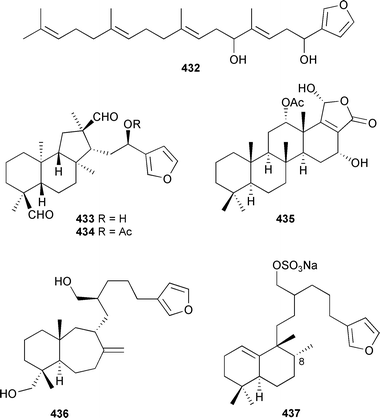
Hyrtios erectus (Hainan Is., China) contained the linear furanoterpenoid 5,10-dihydroxyfurospinulosine-1 432, two formylhyrtiosal congeners 433 and 434, and 12α-O-acetylhyrtiolide 435.466Stoeba extensa (Japan) gave the cytotoxic furanosesterterpenoid shinsonefuran 436 along with halisulfate 7 437.467
The C-8 stereochemistry of halisulfate 7, originally isolated from a Coscinoderma species,468 has been revised to that shown on the basis of careful NOE analysis.467 A scalarane-type sesterterpenoid 438, and a related oxycyclic congener 439, were obtained from a Madagascan Phyllospongia madagascarensis.469 Two different, undescribed Madagascan species of Lendenfeldia contained a linear furanoterpenoid 440 with potent HIV-1 reverse transcriptase inhibitory activity, along with four scalarane-type sesterterpenoids 441–444.470
Suberites caminatus (King George Is., Antarctica) contained oxaspirosuberitenone 445 and 19-epi-suberitenone 446.471 In an independent study, a Suberites species, also collected from King George Is., Antarctica, yielded suberitenones C 447 and D 448, and suberiphenol 449.472
Untenospongin B from an Okinawan Hippospongia species,473 has antimicrobial activity.474 A structural comparison of the PLA2 inhibitory activity of the petrosaspongiolides, isolated from Petroaspongia nigra,475 was reported.476 Using bioassay-guided fractionation, heteronemin477 was reisolated as a farnesyl transferase inhibitor from Hyrtios reticulata.478 Cacospongionolide E from Fasciospongia cavernosa479 has been synthesised.480 A synthesis of (−)-(18S)-variabilin from Ircinia variabilis481 has been achieved.482 Four cytotoxic steroids 450–453 were obtained from Axinellacf.bidderi (Yemen, Indian Ocean).483Phorbas amaranthus (Florida) contained four moderately cytotoxic nor-ring A steroids, phorbasterones A–D 454–457 of which 456 and 457 were isolated as C-24 epimeric mixtures.484
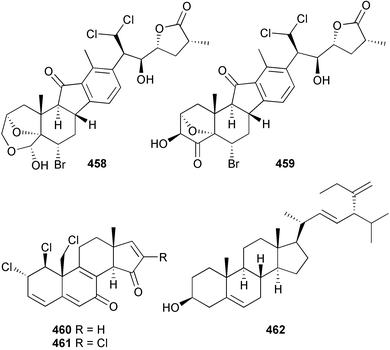
Nakiterpiosin 458 and nakiterpiosinone 459, both potent cytotoxins, were obtained from Terpios hoshinota (Okinawa).485 An Italian Cliona nigricans produced the potently cytotoxic polychlorinated steroids clionastatins A 460 and B 461.486 The C31 steroid, ophirasterol 462, was isolated from a Colombian Topsentia ophiraphidites.487
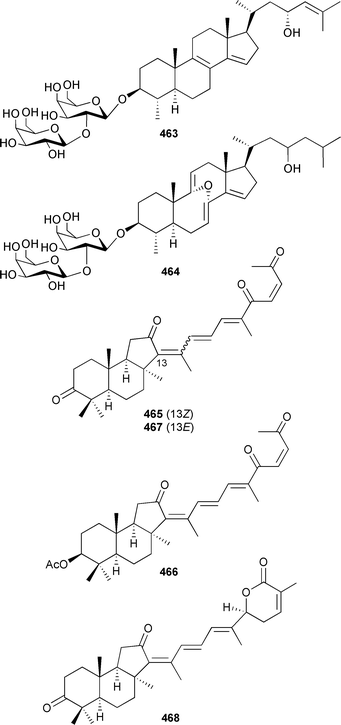
Two steroidal saponins, erylosides K 463 and L 464, were isolated from a Jordanian specimen of the same sponge, Erylus lendenfeldi, from which eryloside A was originally isolated.488,489Rhabdastrella aff. distincta (Hainan Is., China) provided four isomalabaricane-type triterpenoids isogeoditins A 465 and B 466, 13-(E)-isogeoditin A 467, and 22,23-dihydrostellettin B 468.490
8 Coelenterates
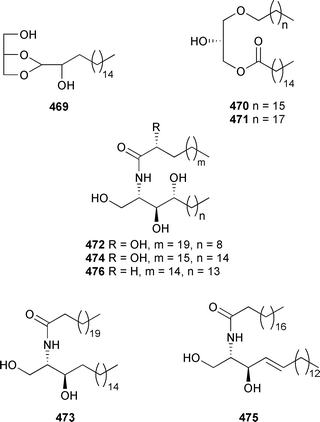
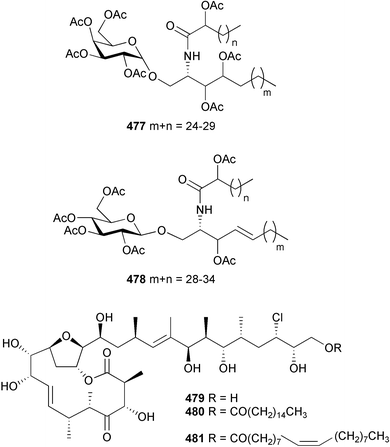
There was a slight increase in new chemistry identified from coelenterates in 2004 compared to the number reported in 2003. The glycerol derivative 469 was reported from a gorgonian Junceella juncea (South China Sea).491 The acyl glycerol ethers 470 and 471 and ceramide 472 were isolated from a Lobophytum species (Indian Ocean).492 The stereochemical assignment of 472 was achieved by analysis of derivatives of hydrolysis products. The sphingosines 473 and 474 were obtained as mildly antibacterial components of an Indian Ocean gorgonian Pseudopterogorgia australiensis.493 Structurally-related lipids 475494 and 476495 were isolated from Junceella juncea and a Lobophytum species respectively, with stereochemical assignments of 475 being made by analysis of hydrolysis products.An Alcyonium species (Senegal) was the source of glycolipids 477 and 478.496 The chloromacrolactones, lytophilippines A 479, B 480 and C 481, were isolated as bioactive constituents from a Red Sea hydroid Lytocarpus philippinus.497
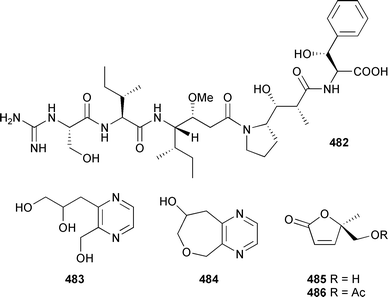
The absolute configuration of 479 was established based upon comprehensive derivatisation and chemical degradation studies in concert with J-based analysis and molecular modeling. The moderately cytotoxic pentapeptide gymnangiamide 482 was isolated from a Philippines hydroid Gymnangium regae.498 Chemical degradation and derivatisation, followed by HPLC analysis allowed the determination of the absolute configuration of 482. The related clavulazols, A 483 and B 484, are two new pyrazine derivatives from Clavularia viridis (Taiwan).499 Furanones 485 and 486 were obtained, in addition to a number of other compounds, from a Taiwanese soft coral Sinularia nanolobata.500
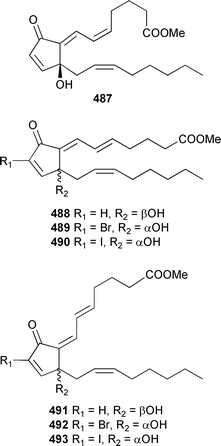
The absolute configuration of 485, and by association 486, was established by comparison with spectroscopic data previously reported for synthetically-derived 485. Prostanoids 487–493 were also isolated from a Taiwanese C. viridis.501
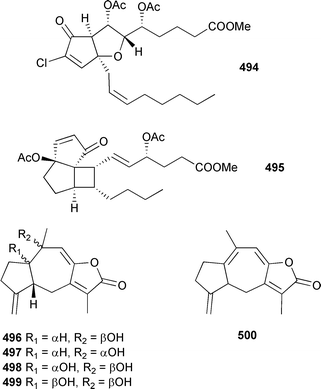
The absolute configurations of all compounds were assigned by comparison of CD spectra with that for clavulone I. 492 was the most potent cytotoxin in the series. The structurally-related punaglandins, originally isolated in 1985 from a Hawaiian Telesto riisei,502,503 inhibit ubiquitin isopeptidase activity504 while several members of a synthetically-derived library of punaglandin-related analogues exhibited similar cytotoxic potency to that observed for the natural product punaglandin 3.505 An unusual bicyclic prostanoid, carijenone 494, was obtained from an Eastern Pacific octocoral Carijoa multiflora.506 The absolute configuration of (+)-tricycloclavulone 495, from an Okinawan Clavularia viridis,507 has been established by enantioselective total synthesis.508 The guaiane-skeleton sesquiterpenes menverins A–D 496–499 were isolated from Menella verrucosa (South China Sea).509 The instability of the compounds was noted, particularly that of 497 which was observed to dehydrate to 500 in the NMR tube (CDCl3).
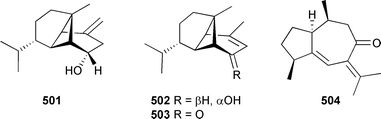
Bioassay-guided fractionation of a Taiwanese soft coral Lemnalia cervicorni yielded the known mildly cytotoxic sesquiterpene lemnalol as well as the inactive metabolites cervicol 501, isolemnalol 502 and the ketone 503.510 The ketone 503 was isomeric with 4-oxo-α-ylangene511 and exhibited identical 13C and NOESY NMR data consistent with an α-orientation of the isopropyl group. Sarcophyton glaucum (Kitungamwe Reef, Kenya) yielded, in addition to other known compounds, the mildly cytotoxic sesquiterpene guaiacophine 504.512
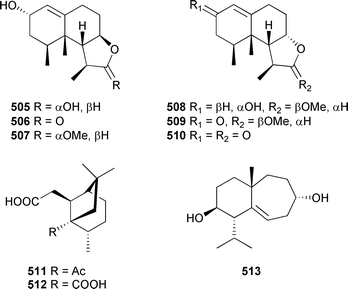
Five nardosinane-skeletoned sesquiterpenes, armatins A–E 505–509, were isolated from a Taiwanese Nephthea armata.513 Diketone 510 was also reported for the first time as a natural product. Isis hippuris (coast of Taiwan) was the source of isishippuric acids A 511 and B 512.514 Sesquiterpene 512 exhibited moderate in vitro cytotoxicity towards tumour cell lines. Cladidiol 513, a mild inhibitor of acetylcholinesterase, was obtained from a Cladiella species (Andaman Is., India).515
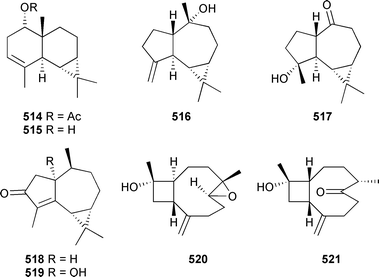
Sesquiterpenes 514–517 were isolated from Clavularia koellikeri (Okinawa).516 Mosher's method secured the absolute stereochemistry of 515, which on acetylation gave 514 securing the absolute configuration of this compound as well. The absolute configuration of 517, previously reported as a synthetic intermediate,516 followed from comparison of the [α]D with literature values.516 (+)-Cyclocolorenone 518, previously known as a terrestrial metabolite, and the 1α-OH analogue 519 were isolated from a soft coral Nephthea species (Andaman and Nicobar Islands, Indian Ocean).517 Nanonorcaryophyllenes A 520 and B 521 were obtained from Sinularia nanolobata (Taiwan).500
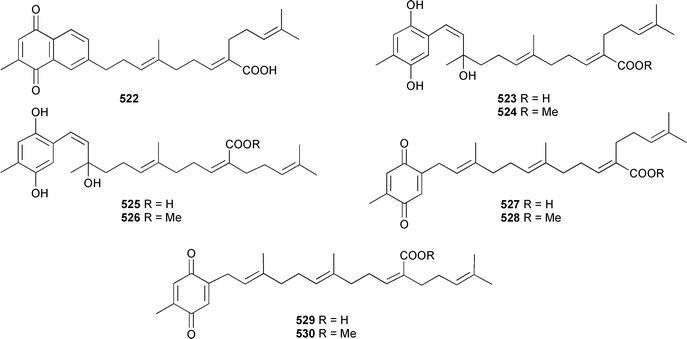
The absolute configuration of 520 was the opposite of a stereochemically-defined microbially-derived metabolite of caryophyllene. The structure of the sesquiterpene alcyopterosin N, from the sub-Antarctic soft coral Alcyonium paessleri,518 has been confirmed by total synthesis.519 Nine meroditerpenoids, chabrolonaphthoquinone A 522, chabrolohydroxybenzoquinones A–D 523–526, and chabrolobenzoquinones A–D 527–530, were isolated from a Taiwanese Nephthea chabrolii.520
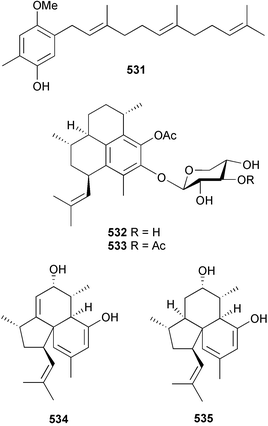
The merosesquiterpene 531, previously reported as a synthetic intermediate,521 has been obtained from a Scleronephthya species (South China Sea).522 Three publications in 2004, presenting new pterosin diterpene glycosides from the soft coral Pseudopterogorgia elisabethae, unfortunately led to duplication in structures and trivial names. Ata et al. reported structures of pseudopterosins P 532 and Q 533 in addition to elisabethins E 534 and F 535 from P. elisabethae (Bahamas).523
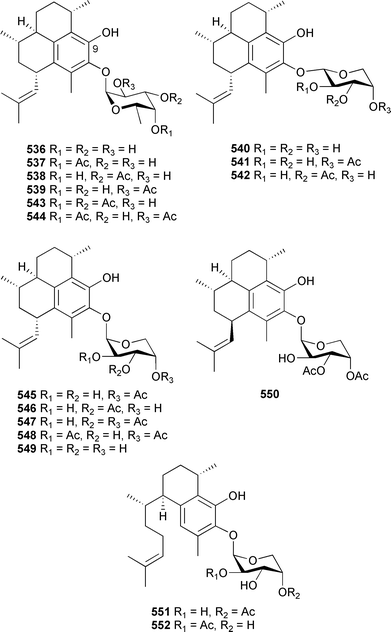
Hydrolysis and comparison of optical rotation data supported the presence of β-L-xylose in both 532 and 533. Both pseudopterosin P and Q, as well as previously reported analogues pseudopterosins A–E and K isolated from the same soft coral specimens, exhibited antibacterial activity towards Gram-positive bacteria, but not towards Gram-negative organisms. Elisabethins E 534 and F 535 were inactive in the same assays. Working with P. elisabethae (Colombian Caribbean), Duque et al. isolated seven diterpene glycosides, pseudopterosins P–V 536–542.524 The absolute configuration of the aglycone of 536 was established by phenolic O-benzylation at C-9, sugar removal by hydrolysis and comparison of NMR and chiroptical data reported for the known benzyl ether. In the case of 536–539, the sugar was identified as an appropriately acetylated α-L-fucopyranoside by hydrolysis, derivatisation and chromatographic analysis, while β-D-arabinopyranoside was the sugar moiety present in 540–542. The structures presented for pseudopterosins U 541 and V 542 are isomeric with those previously reported for pseudopterosins O and N respectively.525 Confusingly, in the original publication that reported pseudopterosins N and O,525 the structures were drawn as bearing xyloside sugar moieties, while the text suggested the structures contained D-arabinose sugars. The NMR data reported for pseudopterosins U and V are not coincident with the values previously reported for pseudopterosins O and N. Rodríguez et al., also working with P. elisabethae (Colombian Caribbean), isolated eleven diterpene glycosides that they named pseudopterosins P 537, Q 538, R 543, S 544, T 536, U–Z 545–550, and two seco-pseudopterosins, H 551 and I 552.526
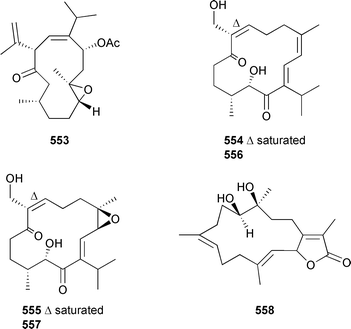
The absolute configuration of the sugars in 551 and 552 were not determined—they are arbitrarily represented in this review as D. Of the metabolites reported by Rodríguez et al., 543 and 544 represent unique diacetate derivatives. Whereas Duque et al. reported 540–542 claiming the presence of a β-D-arabinose sugar, Rodríguez et al. reported the apparently isomeric structures 549, 545 and 546 respectively as containing an α-D-arabinoside moiety, together with 547 and 548, also containing this moiety. The NMR and optical rotation data reported for the α-/β-anomer pairs 541/545 and 542/546 were identical (but different to pseudopterosins N and O, see above), indicating a lack of congruity between the structures and their stereochemistries. As of October 2005 these issues had not been addressed and resolved in the scientific literature. Diterpenes 537, 538, 545–548, 551 and 552 displayed activity in a number of biological assays including antiinflammatory, cytotoxicity and antiinfective evaluation.524,526 The relative stereochemistry of the cubitane-skeleton diterpene 553, from Eunicea laciniata (Barbados), was determined by use of a T-ROESY NMR experiment.527 Cembranes 7,8-dihydroflabellatene A 554 and B 555 were isolated from extracts of a sea pen Gyrophyllum sibogae (South Africa).528 The relative stereochemistry of 554 was secured by X-ray analysis (Mosher's method failed), while the absolute configurations of 554 and 555 were equated to those of the known sponge metabolites 556 and 557.529 Cembrane 554 exhibited potent antiproliferative activity towards tumour cell lines. Sarcophydiol 558 was isolated from a Sarcophyton species (Hainan Is., China).530
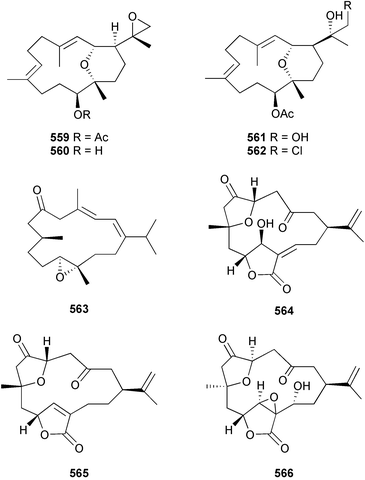
Cembradienes 559–563 were obtained from a Eunicea species (Colombian Caribbean).531 The structure and relative configuration of 559 was established by X-ray analysis, confirming that 559–562 contained the unusual C2–C12 ether linkage, while the inter-relationships between 559–562 were established by chemical conversion. Mild growth inhibition of Plasmodium falciparum was observed for 559, 560 and 562. Of three norditerpenoids, scabrolides E–G 564–566, from a Taiwanese Sinularia scabra, 564 was a modestly potent growth inhibitor against two tumour cell lines.532
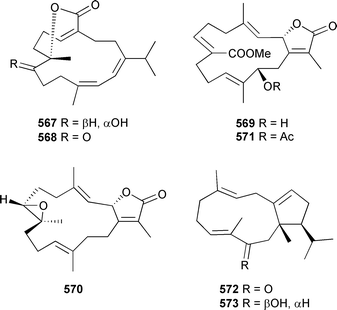
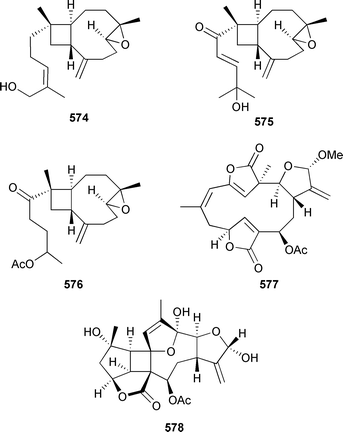
Cembratrienes 567 and 568, containing the rare (12Z) geometry, were isolated from a new species of Sarcophyton (Great Barrier Reef).533 The same study also led to the determination of the absolute configuration (MPA ester) of sarcoglaucol-16-one 569534 and, by comparison of CD spectra, sarcophine 570.535 The acetate derivative 571, also present in a Kenyan Sarcophyton glaucum, was identified as a natural product.512 A number of rearranged and halogenated semisynthetic derivatives of sarcophine have been prepared and evaluated in a range of biological assays.536,537 Neodolabellan-skeleton diterpenes 572 and the modestly cytotoxic 573 were isolated from an Okinawan soft coral Clavularia koellikeri.516
The structure and relative stereochemistry of 572 were established by X-ray analysis, while oxidation of 573 yielded 572. The absolute configuration of 573, and hence of 572, was secured (Mosher's). Nanolobatins A–C 574–576 were reported from Sinularia nanolobata (Taiwan).500 All three diterpenes exhibited modest cytotoxicity towards tumour cell lines. The structure and relative configuration of ciereszkolide 577, a rearranged cembrane from a Caribbean sea plume Pseudopterogorgia kallos, was established by X-ray analysis.538
Further examination of the same species from the same location also afforded the hexacyclic diterpene, bielschowskysin 578, secured by X-ray analysis.539 Modest biological activity towards Plasmodium falciparum and human tumour cell lines was observed. A number of new briarane-skeleton diterpenes were isolated from coelenterates in 2004. Fragilide A 579540 and junceellolide I 580541 were obtained in separate studies from the same collection of Junceella fragilis (southern coast of Taiwan). Another collection of J. fragilis, this time from the South China Sea, afforded junceellonoids A 581 and B 582,542 while extracts of J. juncea, (also South China Sea) yielded juncins O–Q 583–585.543
Briaexcavatolides W 586,544 and X–Z 587–589,545 were obtained from a Taiwanese Briareum excavatum. A sea whip Ellisella species (Okinawa) was the source of briaranes 590–593, of which 591 and 592 were inhibitors of cytokinesis.546 The same paper also reported 594–597 and a number of known diterpenes from a sea pen Pteroides species (Indonesia).
One of the known diterpenes, 12-O-desacetyl-12-O-benzoylpteroidine,547 demonstrated in vitro reversal of multi-drug resistance properties. The absolute stereochemistry of minabein-4 598 was established by X-ray analysis.548 From this it was concluded that the C-2/C-9 diastereomeric structure reported for nui-inoalide-D, isolated from Junceella gemmacea,549 which exhibits identical 13C NMR data to 598, was incorrect. Kitungolides A–C 599–601 are unusual coumarin-containing diterpenes from a new genus of Kenyan soft coral.550
Pregnanes 602 and 603, previously reported as synthetic intermediates,551,552 were isolated from a Scleronephthya species (South China Sea).522 The Δ20-pregnanes 604 and 605, from an Indopacific Carijoa species, were identified as moderately potent inhibitors of the mitochondrial respiratory chain.553Carijoa multiflora (Panama) gave the unusual chlorinated pregnanes 606 and 607.554 Pregnane 608, previously known as a synthetic intermediate,555 and the diacetoxy-analogue 609 were isolated from a Taiwanese Subergorgia mollis.556
The pregnane saponin 610, from a Panamanian Muricea cf purpurea, is unusual because it contains L-galactose linked to the aglycone by an α-glycosidic bond.557 The sugar configuration was determined by hydrolysis, derivatisation and comparison of CD spectra with the corresponding synthetically-prepared α-D and β-D-galactose derivatives. Interestingly, a saponin bearing the same overall structure and comparable [α]D value, but with a β-glycosidic linkage 611, was reported from Eunicea laciniata (Barbados).527
The natural product 612 was isolated from a Lobophytum species (Andaman and Nicobar Islands),558 while the 19-hydroxylated steroids acanthovagasteroids A–D 613–616 were reported from Acanthogorgia vagae (South China Sea).559
The 19-acetoxysterols armatinols A 617 and B 618 were isolated as mildly cytotoxic components from a Taiwanese Nephthea armata.513 Two new, 619 and 620, and three known sterols were obtained from Sarcophyton glaucum (Taiwan).560 Whilst 619 and 620 were inactive, two of the known sterols exhibited modest cytotoxicity towards a panel of mouse and human tumour cell lines. The modestly cytotoxic steroids 621–627 were isolated from an Antarctic octocoral Dasystenella acanthina.561
The absolute configuration at C-24 in 621 was established (Mosher's). Bioassay-directed fractionation of extracts of Dendronephthya gigantea (Green Is., Taiwan) yielded dendronesterones A–C 628–630 of which 628 exhibited mild cytotoxicity.510
In addition to the diterpenes reported above, the cholic acid derivatives 631 and 632 were obtained from an Eleutherobia species (Pohnpei, Micronesia).548 The structure of the first A-nor-hippuristanol steroid 633, a mildly cytotoxic constituent of a Taiwanese Isis hippuris, was established by X-ray analysis.514 Steroidal glycoside 634 was isolated from Junceella juncea (South China Sea).491
The structure of 24-methylene-cholesta-3β,5α,6β,19-tetraol, a mildly cytotoxic sterol from Nephthea albida and N. tiexieral verseveldt,562 has been confirmed by synthesis from stigmasterol.563 A number of steroids, from a range of coelenterates, exhibited significant inhibition of the 5α-reductase enzyme.564 The structures of three mildly cytotoxic biscembranoids, methyl tortuoates A 635 and B 636 from Sarcophyton tortuosum (Hainan Is.),565 and nyalolide 637, from a Kenyan Sarcophyton glaucum,512 were established by X-ray analyses of 635 and 637. The structures of all three are arbitrarily presented in this review as bearing the purported absolute configuration of the related metabolite methyl sartortuoate.566 Two unusual mixed-skeleton terpenoids were also reported from soft corals in 2004. Stolonilactone 638, putatively derived from condensation of a cembrane diterpenoid and trisnorsesquiterpene fragments, was obtained from an Okinawan Clavularia koellikeri.567 The structure of polymaxenolide 639, from a hybrid soft coral Sinularia maxima x S. polydactyla (Guam), was established by X-ray analysis,568 and represents the fusion of a cembrane diterpene with an africanane-type sesquiterpene.
9 Bryozoans
The number of new secondary metabolites reported from bryozoans in 2004 remains small. Six alkaloids, calibugulones A–F 640–645, were isolated from Calibugula intermis (Palau) with the structures of 641 and 642 being confirmed by chemical interconversion. All calibugulones displayed cytotoxicity against murine IC-2WT tumour cells in vitro with 644 being the most potent.569 Two syntheses of calibugulones employing similar methods from an isoquinoline dione,570 or 5-aminoisoquinoline571 were reported almost simultaneously. The potent and selective inhibition of the dual specificity phosphatase Cdc25B was also reported for these compounds.570 Larvae of Bugula neritina (Morehead, North Carolina) were the source of bryostatin 20 646, along with the known bryostatin 10572 and an as yet uncharacterised bryostatin.573Proof that Endobugula sertula, the bacterial symbiont of Bugula neritina, is the producer of the bryostatins was recently obtained.574 The bryostatins are concentrated in the bryozoan larvae and give protection against predation by fish, but the adults are unprotected. The bryostatins represent the first marine example of a microbial symbiont producing an antipredator defence for its host.574Flustra foliacea (“Steingrund”, North Sea) was the source of deformylflustrabromine B 647 along with a number of previously reported compounds. All compounds were tested in vitro for affinity towards the α4β2* and α7* subtype of the neuronal nicotinic acetylcholine receptor using radioligand binding assays, with deformylflustrabromine575 and deformylflustrabromine B 647 showing affinities in the lower micromolar range.576 Myriaporones are polyketide-derived compounds originally isolated from a bryozoan Myriapora truncata.577,578 The total syntheses of myriaporones 1 648, 3 649 and 4 650 have been reported by two groups allowing determination of the previously undetermined C-5 and C-6 configurations.579,580 The first enantioselective synthesis of the Flustra foliacea metabolite (−)-flustramine B581 has been accomplished, employing a cascade addition–cyclisation strategy.582
10 Molluscs
There was a decrease in new chemistry identified from molluscs in 2004. Bioassay-directed fractionation of extracts from Mytilus galloprovincialis (coast of Italy) afforded the cytotoxic chlorosulfolipid 651.583 The relative configurations were deduced from NMR data, while the absolute configuration was based upon comparison with related compounds from the same source. Collections of Mytilus edulis and Cerastoderma edule in the presence of a Dinophysis acuta bloom (coast of Norway) afforded PTX-12 652, a new member of the pectenotoxin family that occurs as a pair of C-36 equilibrating diastereoisomers.584 A brevetoxin analogue, brevetoxin B5 653, was isolated from a New Zealand cockle Austrovenus stutchburyi and also detected in two other mollusc species, Perna canaliculus and Crassostrea gigas.585 The structure was elucidated by comparison with related toxins brevetoxin B1 and PbTx-2 and confirmed by chemical conversion from PbTx-2. A sea hare Dolabella auricularia (Japan) afforded the cytotoxic depsipeptide aurilide 654.586 The absolute stereochemistry of 654 was determined by analysis of hydrolysis products and confirmed by enantioselective synthesis. Aurilide exhibits potent in vitro cytotoxicity, toxicity in in vivo evaluation and strongly stabilises microtubules, but with a different mechanism from that exhibited by taxol.The relative stereochemistry was established for the cytotoxic glycosidic macrolide dolastatin 19 655 from D. auricularia (Gulf of California).587 It has been demonstrated that mate attraction in sea hares of the genus Aplysia is mediated via attractin peptides that contain a conserved Ile-Glu-Glu-Cys-Lys-Thr-Ser heptapeptide sequence.588 Haterumaimides L 656 and M 657 and 3β-hydroxychlorolissoclimide 658 were obtained as cytotoxic components from Pleurobranchus albiguttatus and P. forskalii (Philippines).589
Structurally-related chlorinated diterpenes have been reported previously from Lissoclinum ascidians,590,591 supporting the assertion that the mollusc compounds are diet-derived. The isolation of a number of scalarane-skeleton metabolites, including 659 and 660 from a nudibranch Glossodoris rufomarginata (South China Sea), was proposed to be dietary-linked as the nudibranchs were found on an unidentified sponge which contained scalaradial.592 Tribromoimidazole 661 was reported for the first time as an antimicrobial natural product from the egg masses of Spanish and Chilean collections of Trunculariopsis trunculus, Ceratosoma erinaceum and Trophon geversianus, with the structure confirmed by synthesis.593 Sesquiterpenes 662–664 were isolated from a nudibranch Phyllidiella pustulosa, (Hainan Is., South China Sea).594
The absolute configuration of 662 was established by chemical conversion to a known diene. The absolute stereochemistry of oxazinin-3 665, from North Adriatic Sea mussels,595 was established by synthesis,596 while the structure and absolute stereochemistry of azaspiracid-1 666, originally obtained from Irish collections of Mytilus edulis,597 has been revised as a consequence of stereoselective total synthesis.598,599 The structure of cyercene A, a pyrone polypropionate from a Mediterranean Cyerce cristallina,600 has been confirmed by synthesis.601
Austrodoric acid 667, from an Antarctic nudibranch Austrodoris kerguelenensis,602 has been synthesised with comparison of CD spectra being used to establish the absolute configuration of the natural product.603 The structure proposed for onchidin,604 a cytotoxic depsipeptide from a pulmonate Onchidium species but possibly of cyanobacterial origin, has been synthesised and exhibited different spectroscopic data to that observed for the natural product.605 A full account of the stereoselective synthesis of (+)-brasilenyne, an antifeedant haloether from a sea hare Aplysia brasiliana,264 has been reported.266 Enantioselective syntheses of (−)-tochuinyl acetate 668 and (−)-dihydrotochuinyl acetate 669606 defined the absolute configurations of the sesquiterpenes that were isolated from a nudibranch Tochuina tetraquetra and its dietary soft coral Gersemia rubiformis.607
Dolastatin 18 670, from Dolabella auricularia,608 has been synthesized, confirming the original (R) stereochemistry of the N-Me-Phe unit.609 The absolute configuration of ulapualide A 671, the renowned bioactive macrolide from egg masses of a nudibranch Hexabranchus sanguineus,610 has been solved by X-ray analysis of a complex with G-actin.611 The sponge metabolite renieramycin M can be converted to jorumycin 672,612 a cytotoxic isoquinoline alkaloid comparable in potency to ecteinascidin 743, which was originally isolated from a nudibranch Jorunna funebris (Mandapam, India).613 The absolute configuration of (+)-norrisolide 673, from a nudibranch Chromodoris norrisi,614 has been established by stereoselective synthesis,615 while the biogenetically-proposed stereoisomer of acanthodoral 674, from a nudibranch Acanthodoris nanaimoensis,616 has also been synthesised.617
The de novo biosynthesis of aglajnes-1 and -3, linear and 4-hydroxy-α-pyrone polypropionate metabolites previously isolated from opistobranch molluscs Aglaja depicta618 and Bulla striata619 respectively, has been established by [1-14C]-propionate feeding experiments.620 Cell-free preparations of Oxynoe olivacea can convert the dietary algal metabolite caulerpenyne621 into oxytoxin-2,622 the main defensive metabolite of the mollusc.623 The biosynthesis of 3-alkylpyridine alkaloids haminol-1 and -2, from a Mediterranean Haminoea orbignyana,624 has been studied using [1,2-13C2]-acetate incorporation, establishing elongation with acetate of a nicotinic acid starter unit.625 Parguerol and isoparguerol, originally from Aplysia dactylomela,626 induced neurite outgrowth in PC-12 cells with modest potency.627 The shellfish biotoxin yessotoxin192 was a potent opener of the permeability transition pore on inner mitochondrial membranes.193
11 Tunicates (ascidians)
While the number of new metabolites isolated from ascidians has remained essentially static for each of 2002 and 2003, a slight decrease was reported in 2004. As with previous years, the majority of the metabolites reported from ascidians are derived from amino acids. (2S,3R)-2-Aminododecan-3-ol 675 was reported as an antifungal component of Clavelina oblonga (Brazil).628 The absolute stereochemistry was secured by preparation of the dibenzoyl derivative and comparison of CD data with structurally-defined model compounds. The first examples of acetylenic lipids from an organism of the phylum Chordata 676–679 were isolated from an unidentified ascidian of the family Polyclinidae (Spain).629 Biselides A 680 and B 681 were obtained from an Okinawan ascidian from the family Didemnidae.630Absolute stereochemistry was implied by comparison of NMR and CD data with those reported for the corrected absolute configuration of haterumalide NA.631,632 A stereoselective total synthesis633 has confirmed the structure and absolute configuration of bistramide A 682 from Lissoclinum bistratum.634 The absolute configurations of lepadins D635683, E635684 and H636685, from a Didemnum species635 and Aplidium tabascum,636 have been ascertained by stereoselective total synthesis.637 1,3-Dimethyl-8-oxoisoguanine 686 was isolated from an ascidian Pseudodistoma cereum (New Zealand).638
An Australian Atriolum robustum was the source of amino acid-derived metabolites 687–691.639 The absolute stereochemistries of 687 and 688 were established by degradative derivatisation studies while only the relative configurations of methylthioadenosine analogues 690 and 691 were determined.
Compound 690 was a relatively potent partial agonist of human A3 adenosine receptors. A Palaun Botrylloides tyreum afforded the weakly cytotoxic tyrosine analogues botryllamides E–H 692–695.640Cystodytes cf. violatinctus (Kenya) gave violatinctamine 696, which in solution was proposed to exist as a rapidly interconverting mixture of imino-phenol (shown) and amino quinone-methide tautomers.641 Lissoclibadin 1 697 was isolated as a mildly antibacterial component of an Indonesian Lissoclinum cf. badium.642
The relative orientations of the aromatic rings were based upon molecular modelling studies. Fascaplysin analogues 301 and 698–701 were obtained from a Didemnum species (Chuuk Atoll, Micronesia), while 301 was also isolated from a sponge Fascaplysinopsis reticulata (Fiji).376 Asymmetric syntheses of (+)-arborescidine A, (−)-arborescidine B and (−)-arborescidine C, antipodes of the natural products 702–704 from a New Caledonian Pseudodistoma arborescens,643 have been achieved via use of the Noyori catalytic asymmetric hydrogen transfer reaction.644
The synthetic arborescidines had opposite optical rotations to those of the natural products, establishing the absolute configurations as drawn and claimed originally,643 but opposite to those claimed in a subsequent revision.645 Three lamellarin alkaloids, lamellarins γ 705, α 706 and ε 707 were reported from Didemnum obscurum (Indian Ocean),646 while solid-phase syntheses of lamellarins Q and O have been reported.647 Four further ecteinascidins, ETs 731 708, 745b 709, 808 710 and 815 711 from Ecteinascidia turbinata were disclosed in a patent.648
Full accounts of the biomimetic synthesis of the dimeric prenylated quinone (−)-longithorone A649 from Aplidium longithorax,650,651 of the corrected structure of diazonamide A,652 and the isolation and synthesis of an endogenous sperm-activating and attracting factor from Ciona intestinalis653 have been reported. The structures of cyclic peptides bistratamides E, G and J,654,655 from Lissoclinum bistratum,656 botryllazine B,657 from a Spanish Botryllus leachi,658 and subarine,659 a potentially ring-opened pyridoacridine alkaloid from an unidentified Singaporean ascidian,660 have been confirmed by syntheses. Molecular dynamics simulations have modelled the interaction of didemnins A and B661,662 with human elongation factor eEF1A in the presence of GTP, an interaction that leads to inhibition of protein synthesis.663 Monomeric analogues of the heterodimeric antimicrobial peptide halocidin from Halocynthia aurantium664 have been prepared, leading to the identification of a more potent antimicrobial and less haemolytic variant.665 Further biological investigation of the meridianins, aminopyrimidineindole alkaloids originally from Aplidium meridianum,666 has revealed that they inhibit various protein kinases, prevent cell proliferation and induce apoptosis,667 while polycarpine, an alkaloid from Polycarpa clavata,668 and a dimethyl analogue both induced apoptosis via p53 and caspase-3 dependent pathways.669
12 Echinoderms
There was a slight increase in new compounds identified from echinoderms in 2004 compared to 2003. In addition to a number of known metabolites, xanthosine 712 was isolated from a starfish Asterias rollestoni (Yellow Sea, China).670 A ganglioside molecular assemblage, LMG-4, the major entity of which is represented by 713, was obtained from a Japanese starfish Luidia maculata.671 The ganglioside displayed neuritogenic activity against PC12 cells in the presence of nerve growth factor. A structurally more complex ganglioside molecular entity CJP4 714, also exhibiting neuritogenic activity, was isolated from a Japanese feather star Comanthus japonica.672The progesterone derivative 715 was obtained from a Russian starfish Lethasterias nanimensis chelifera,673 while the polyhydroxysterol 716 was isolated from a Pacific starfish Asterina pectinifera.674 Brine shrimp assay-directed fractionation of a starfish Certonardoa semiregularis, (Komun Is., Korea) extract, afforded twelve polyhydroxysterols 717–728 and two saponins 729 and 730.675
Side-chain configurations were determined either by Mosher analysis (certonardosterols Q2718, A3725, A4726, B4727 and certonardoside H4730) or biogenetic arguments (certonardosterols Q1717, Q3719, Q4720, Q5721, Q6722, Q7723, D5728 and certonardoside H3729). All of the compounds tested exhibited modest in vitro cytotoxicity towards a panel of human tumour cell lines. In a separate study of the same organism from the same location, a further eleven sterols 731–741 and eight saponins 742–749 were identified.676
A glycoside, phrygioside A 750 and the corresponding aglycone 751, were isolated from a starfish Hippasteria phrygiana (Sea of Okhotsk).677 The steroid-taurine conjugate, triseramide 752, was a mildly bioactive component of a Fijian starfish Astropecten triseriatus.678
The Far-Eastern starfish Henricia sanguinolenta and H. leviuscula leviuscula yielded the saponins sanguinosides A 753 and B 754.679 The C-24 configuration of 754 was based on biogenetic considerations. Further investigation of the side-chain absolute stereochemistries of henriciosides H1755, H2756 and H3757, originally isolated from a Far-Eastern starfish H. derjugini,680 (Mosher's) established that 755 is, in fact, identical to the previously reported steroid laevisculoside I,681 and that 756 has the (24R,25S) configuration while 757 is (24R,25R).682 Linckosides C–E 758–760, from the Okinawan starfish Linckia laevigata, induced neurite outgrowth in PC12 cells.683
The relative configurations of C-24 and C-28 in 759 were established, with the same configurations implied for 758. The triterpene aglycones, philinopgenins A–C 761–763, were obtained from the acid hydrolysate of the crude glycoside fraction from a sea cucumber Pentacta quadranglasis (South China Sea).684 The philinopgenins are possibly artefacts. Two saponins, thyonosides A 764 and B 765, were isolated from the sea cucumber Thyone aurea (Namibia).685
13 Miscellaneous
Two 21-residue antimicrobial peptides, arenicin-1 766 and arenicin-2 767 were obtained from a polychaete worm Arenicola marina (White Sea, Russia).686 Diatom-derived conjugated aldehydes such as (2E,4E)-decadienal continue to be of interest, exhibiting cytotoxicity towards a number of different organisms including bacteria, algae, fungi, echinoderms, molluscs and crustacea687 in addition to inhibiting fertilization success in broadcast-spawning invertebrates.688 The structure of the antipredatory chemical defence compound 2,3,4-tribromopyrrole from a hemichordate worm Saccoglossus kowalevskii,689 has been confirmed by synthesis.690 Mass spectrometric analysis of stable isotope incorporation experiments determined that Cypridina luciferin, an alkaloid utilised by the bioluminescent crustacean Cypridina (Vargula) hilgendorfii, is biosynthesised from L-tryptophan, L-arginine and L-isoleucine.691 Gymnorrhizol 768, an unusual macrocyclic polydisulfide possessing an unprecedented carbon skeleton, has been isolated from a mangrove Bruguiera gymnorrhiza (Guangxi Province, China). The structure was confirmed by X-ray analysis.692,69314 Conclusions
In this review, the structures of 768 compounds have been reported. The biological testing on, or biological properties of, 380 compounds is included. Using numbers extracted from MarinLit,79 the geographic origins of compounds reported in 2004 are compared with those up to 2003 (Fig. 1a). The geographical descriptors used in MarinLit are somewhat imprecise, ranging from exclusive to inclusive, but Australia, the Caribbean, the Indian Ocean, Japan, the Mediterranean and the Western Pacific clearly stand out as the sources for two-thirds of the reports on marine natural products, with Japan being the clear leader. What is most notable about the data for 2004 (Fig. 1b) is the marked increase in citations relating to the China Sea. Also shown in Fig. 1 is the relative proportion of articles for each region reporting bioactivity (“bioarticles”). These, taken collectively, suggest the possibility that greater attention is being paid to searching for, or commenting on, bioactive compounds (55% in 2004 vs. 49% up to 2003).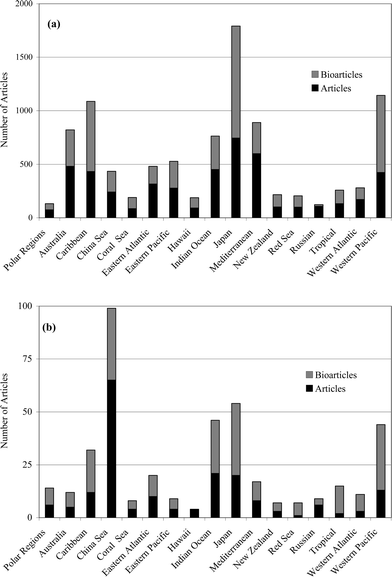 | ||
| Fig. 1 (a) Accumulated distribution of literature citations by geographic region to 2003. (b) Distribution in 2004. For clarity Polar Regions includes Antarctic and Arctic articles, North Sea and Baltic Sea records are included with Eastern Atlantic, while the Black Sea and Caspian Sea are placed in Russian. “Articles” and “Bioarticles” do not necessarily contain structural information. “Bioarticles” refer to citations that include comment on the biological properties of extracts or isolated compounds from that region. | ||
A second point of comparison is the type of biological activity reported which can grouped into eight areas—anticancer, antibiotic (which includes antifungal and antimalarial), antiinflammatory, antiviral, immunomodulatory, various, agricultural and methodology. Fig. 2 shows the types of the testing carried out up to, and including, 2004.
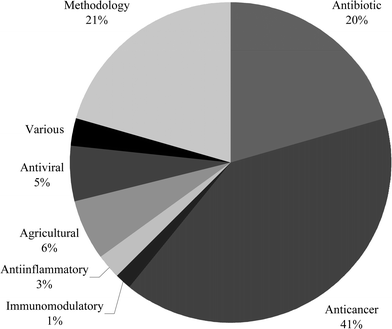 | ||
| Fig. 2 Reported distribution of biological testing carried out on marine natural product extracts and isolated compounds to 2004. The MarinLit database79 has 50 descriptors for key-wording biological testing. For convenience these have been grouped into eight, mainly disease-based, areas. Various includes neurological, blood pressure, fertility, allergy-based laxative, and enzyme activation assays, while Methodology covers bioassay methods, mechanism of action, structure–activity studies, other assays, radio immunoassays as well as the sea urchin egg and brine shrimp assay. | ||
The actual data for 2004 did not vary significantly from that reported cumulatively, but was suggestive that the “catch-all” category of methodology was increasing. Testing with an anticancer outcome remains far and away the most used assay, but increasingly results are appearing in the literature for antimalarial, antitubercular and antiinfective assays against drug-resistant microorganisms and in time these areas will stand out against the cumulative record. There can be no doubt of the urgent need for new therapeutics in those areas, but also in agricultural areas where resistance to the standard anthelmintics is becoming a serious problem.
15 Acknowledgements
We thank Dr Sunny Hu for the collection of data and diagrams for this review, and for proof reading.16 References
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2004, 21, 1 RSC.
- R. A. Hill, Annu. Rep. Prog. Chem., Sect. B, 2004, 100, 169 RSC.
- M. T. Davies-Coleman and D. R. Beukes, S. Afr. J. Sci., 2004, 100, 539 Search PubMed.
- N. Fusetani, J. Synth. Org. Chem. Jpn., 2004, 62, 1073 CAS.
- D. Laurent and F. Pietra, Chem. Biodiversity, 2004, 1, 539 Search PubMed.
- S.-S. Lee and W.-L. Chang, Curr. Med. Chem., 2004, 11, 1461 CAS.
- R. G. S. Berlinck, E. Hajdu, R. M. da Rocha, J. H. H. L. de Oliveira, I. L. C. Hernández, M. H. R. Seleghim, A. Claudia Granato, E. V. R. de Almeida, C. V. Nuñez, G. Muricy, S. Peixinho, C. Pessoa, M. O. Moraes, B. C. Cavalcanti, G. G. F. Nascimento, O. Thiemann, M. Silva, A. O. Souza, C. L. Silva and P. R. R. Minarini, J. Nat. Prod., 2004, 67, 510 CrossRef CAS.
- M. A. Vallim, J. C. De Paula, R. C. Pereira and V. L. Teixeira, Biochem. Syst. Ecol., 2004, 33, 1.
- D. J. Faulkner, D. J. Newman and G. M. Cragg, Nat. Prod. Rep., 2004, 21, 50 RSC.
- P. Bhadury and P. C. Wright, Planta, 2004, 219, 561 CAS.
- N. Fusetani, Nat. Prod. Rep., 2004, 21, 94 RSC.
- V. J. Paul and M. P. Puglisi, Nat. Prod. Rep., 2004, 21, 189 RSC.
- G. Pohnert, Top. Curr. Chem., 2004, 239, 179 CAS.
- E. Bachère, Y. Gueguen, M. Gonzalez, J. de Lorgeril, J. Garnier and B. Romestand, Immunol. Rev., 2004, 198, 149 CrossRef CAS.
- E. S. Loker, C. M. Adema, S. M. Zhang and T. B. Kepler, Immunol. Rev., 2004, 198, 10 CrossRef.
- M. Kasahara, T. Suzuki and L. Du Pasquier, Trends Immunol., 2004, 25, 105 Search PubMed.
- B. Rinkevich, Immunol. Rev., 2004, 198, 25 CrossRef.
- J. W. Daly, J. Nat. Prod., 2004, 67, 1211 CrossRef CAS.
- K. Fujiwara and A. Murai, Bull. Chem. Soc. Jpn., 2004, 77, 2129 CrossRef CAS.
- M. Inoue, Org. Biomol. Chem., 2004, 2, 1811 RSC.
- M. Sasaki and H. Fuwa, Synlett, 2004, 11, 1851 CrossRef.
- V. S. C. Yeh, Tetrahedron, 2004, 60, 11995 CrossRef CAS.
- V. Roussis, C. Vagias and L. A. Tziveleka, in Plants That Fight Cancer, ed. S. E. Spridon and M. G. Barberaki, CRC Press LLC, Boca Raton, FL, 2004, pp. 195, 274 Search PubMed.
- K. Matsubara, Curr. Med. Chem.: Cardiovasc. Hematol. Agents, 2004, 2, 13 Search PubMed.
- T. S. Bugni and C. M. Ireland, Nat. Prod. Rep., 2004, 21, 143 RSC.
- V. S. Bernan, M. Greenstein and G. T. Carter, Curr. Med. Chem.: Anti-Infect. Agents, 2004, 3, 181 Search PubMed.
- H. Katircioglu, B. S. Akin and T. Atici, Afr. J. Biotechnol., 2004, 3, 667 Search PubMed.
- C. Imada, Mar. Biotechnol., 2004, 6, 193 CAS.
- Z.-R. Zou, Y.-H. Yi, S.-Y. Zhang, D.-Z. Zhou and H.-F. Tang, Zhongguo Haiyang Yaowu, 2004, 23, 46 Search PubMed.
- A. J. Smit, J. Appl. Phycol., 2004, 16, 245 Search PubMed.
- L. Béress, J. Toxicol., Toxin Rev., 2004, 23, 451 Search PubMed.
- F. Rahm, P. Y. Hayes and W. Kitching, Heterocycles, 2004, 64, 523 CrossRef CAS.
- V. Costantino, E. Fattorusso, M. Menna and O. Taglialatela-Scafati, Curr. Med. Chem., 2004, 11, 1671 CAS.
- D. Rittschof and J. H. Cohen, Peptides (N. Y., NY, U. S.), 2004, 25, 1503 Search PubMed.
- P. Ciminiello and E. Fattorusso, Eur. J. Org. Chem., 2004, 2533 CrossRef CAS.
- P.-J. Sung, H.-H. Gwo, T.-Y. Fan, J.-J. Li, J. Dong, C.-C. Han, S.-L. Wu and L.-S. Fang, Biochem. Syst. Ecol., 2004, 32, 185 CrossRef CAS.
- J. Piel, Nat. Prod. Rep., 2004, 21, 519 RSC.
- A. M. S. Mayer and M. T. Hamann, Mar. Biotechnol., 2004, 6, 37 CrossRef CAS.
- C. E. Salomon, N. A. Magarvey and D. H. Sherman, Nat. Prod. Rep., 2004, 21, 105 RSC.
- A. Kijjoa and P. Sawangwong, Mar. Drugs, 2004, 2, 73 Search PubMed.
- M. Tulp and L. Bohlin, Drug Discovery Today, 2004, 9, 450 CrossRef CAS.
- D. D. Baker and K. A. Alvi, Curr. Opin. Biotechnol., 2004, 15, 576 CrossRef CAS.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2004, 67, 1216 CrossRef CAS.
- S. Aoki, Nat. Med. (N. Y.), 2004, 58, 251 Search PubMed.
- A. M. S. Mayer and K. R. Gustafson, Eur. J. Cancer, 2004, 40, 2676 CrossRef CAS.
- D. G. Nagle, Y. Zhou, F. D. Mora, K. A. Mohammed and Y. Kim, Curr. Med. Chem., 2004, 11, 1725 CAS.
- K. R. Gustafson, N. Oku and D. J. Milanowski, Curr. Med. Chem.: Anti-Infect. Agents, 2004, 3, 233 Search PubMed.
- T. F. Molinski, Curr. Med. Chem.: Anti-Infect. Agents, 2004, 3, 197 Search PubMed.
- A. L. Okunade, M. P. F. Elvin-Lewis and W. H. Lewis, Phytochemistry, 2004, 65, 1017 CrossRef CAS.
- J. A. Tincu and S. W. Taylor, Antimicrob. Agents Chemother., 2004, 48, 3645 CrossRef CAS.
- A. Aneiros and A. Garateix, J. Chromatogr. B, 2004, 803, 41 CrossRef CAS.
- T. Rezanka, M. Temina, A. G. Tolstikov and V. M. Dembitsky, Folia Microbiol. (Prague, Czech Repub.), 2004, 49, 339 Search PubMed.
- M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC.
- M. Kuramoto, H. Arimoto and D. Uemura, Mar. Drugs, 2004, 1, 39 Search PubMed.
- C. G. Yang, H. Huang and B. Jiang, Curr. Org. Chem., 2004, 8, 1691 CrossRef CAS.
- J. P. Michael, Nat. Prod. Rep., 2004, 21, 625 RSC.
- H. Rosemeyer, Chem. Biodiversity, 2004, 1, 361 Search PubMed.
- J. Li and Z. Chi, J. Ocean Univ. China, 2004, 3, 40 Search PubMed.
- D. Sica and D. Musumeci, Steroids, 2004, 69, 743 CrossRef CAS.
- V. M. Dembitsky, Chem. Biodiversity, 2004, 1, 673 Search PubMed.
- B. J. M. Jansen and A. de Groot, Nat. Prod. Rep., 2004, 21, 449 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2004, 21, 312 RSC.
- J. R. Hanson, Nat. Prod. Rep., 2004, 21, 785 RSC.
- G. W. Gribble, J. Chem. Educ., 2004, 81, 1441 CrossRef CAS.
- M. J. Garson and J. S. Simpson, Nat. Prod. Rep., 2004, 21, 164 RSC.
- A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef.
- M. V. Nora De Souza, TheScientificWorld, 2004, 4, 415 Search PubMed.
- P. Wipf, J. T. Reeves and B. W. Day, Curr. Pharm. Des., 2004, 10, 1417 Search PubMed.
- N. Nishi and H. Kawai, BIO Clin., 2004, 19, 1002 Search PubMed.
- J. Jimeno, G. Faircloth, J. M. Fernández Sousa-Faro, P. Scheuer and K. Rinehart, Mar. Drugs, 2004, 1, 14 Search PubMed.
- M. D'Incalci, M. Simone, M. Tavecchio, G. Damia, A. Garbi and E. Erba, J. Chemother. (Firenze, Italy), 2004, 16, 86 Search PubMed.
- J. Kobayashi and M. Tsuda, Nat. Prod. Rep., 2004, 21, 77 RSC.
- J. R. Williams and H. Gong, Lipids, 2004, 39, 795 CAS.
- C. Bailly, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 363 Search PubMed.
- T. Kawasaki, Meiji Yakka Daigaku Kenkyu Kiyo, 2004, 33, 13 Search PubMed.
- P. Proksch, R. Edrada and R. Ebel, BIOforum Eur., 2004, 8, 44 Search PubMed.
- G. Baggerman, P. Verleyen, E. Clynen, J. Huybrechts, A. De Loof and L. Schoofs, J. Chromatogr. B, 2004, 803, 3 CrossRef CAS.
- D. A. Volmer and L. Sleno, LCGC North Am., 2004, 22, 926 CAS.
- MarinLit database, Department of Chemistry, University of Canterbury: http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml.
- R. D. Charan, G. Schlingmann, J. Janso, V. Bernan, X. Feng and G. T. Carter, J. Nat. Prod., 2004, 67, 1431 CrossRef CAS.
- R. P. Maskey, M. Sevvana, I. Usón, E. Helmke and H. Laatsch, J. Antibiot., 2002, 55, 1031 CAS.
- R. P. Maskey, M. S. I. Usón, E. Helmke and H. Laatsch, Angew. Chem., Int. Ed., 2004, 43, 1281 CrossRef CAS.
- F. Tomita, T. Tamaoki, M. Morimoto and K. Fujimoto, J. Antibiot., 1981, 34, 1519 CAS.
- T. Tamaoki, K. Shirahata, T. Iida and F. Tomita, J. Antibiot., 1981, 34, 1525 CAS.
- K. Shirahata, T. Iida and N. Hirayama, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1981, 24, 199 Search PubMed.
- R. P. Maskey, E. Helmke, O. Kayser, H. H. Fiebig, A. Maier, A. Busche and H. Laatsch, J. Antibiot., 2004, 57, 771 CAS.
- I. L. C. Hernández, M. L. Macedo, R. G. S. Berlinck, A. G. Ferreira and M. J. L. Godinho, J. Braz. Chem. Soc., 2004, 15, 441 CAS.
- S. S. Mitchell, B. Nicholson, S. Teisan, K. S. Lam and B. C. M. Potts, J. Nat. Prod., 2004, 67, 1400 CrossRef CAS.
- K. Stritzke, S. Schulz, H. Laatsch, E. Helmke and W. Beil, J. Nat. Prod., 2004, 67, 395 CrossRef CAS.
- H. L. Zhang, H. M. Hua, Y. H. Pei and X. S. Yao, Chem. Pharm. Bull., 2004, 52, 1029 CrossRef CAS.
- K. Barbeau, G. Zhang, D. H. Live and A. Butler, J. Am. Chem. Soc., 2002, 124, 378 CrossRef CAS.
- R. J. Bergeron, G. Huang, R. E. Smith, N. Bharti, J. S. McManis and A. Butler, Tetrahedron, 2003, 59, 2007 CrossRef CAS.
- S. J. H. Hickford, F. C. Küpper, G. Zhang, C. J. Carrano, J. W. Blunt and A. Butler, J. Nat. Prod., 2004, 67, 1897 CrossRef CAS.
- B. Bister, D. Bischoff, M. Ströbele, J. Riedlinger, A. Reicke, F. Wolter, A. T. Bull, H. Zähner, H.-P. Fiedler and R. D. Süssmuth, Angew. Chem., Int. Ed., 2004, 43, 2574 CrossRef CAS.
- J. Riedlinger, A. Reicke, H. Zähner, B. Krismer, A. T. Bull, L. A. Maldonado, A. C. Ward, M. Goodfellow, B. Bister, D. Bischoff, R. D. Süssmuth and H.-P. Fiedler, J. Antibiot., 2004, 57, 271 CAS.
- R. N. Asolkar, D. Schröder, R. Heckmann, S. Lang, I. Wagner-Döbler and H. Laatsch, J. Antibiot., 2004, 57, 17 CAS.
- M. Mitova, G. Tommonaro, U. Hentschel, W. E. G. Müller and S. De Rosa, Mar. Biotechnol., 2004, 6, 95 CrossRef CAS.
- Y. Fusaoka, E. Ozeki, S. Kimura and Y. Imanishi, Int. J. Pept. Protein Res., 1989, 34, 104 CAS.
- T. Guedez, A. Nunez, E. Tineo and O. Nunez, J. Chem. Soc., Perkin Trans. 2, 2002, 2078 RSC.
- D. Hoffmeister, K. Ichinose, S. Domann, B. Faust, A. Trefzer, G. Dräger, A. Kirschning, C. Fischer, E. Künzel, D. W. Bearden, J. Rohr and A. Bechthold, Chem. Biol., 2000, 7, 821 CrossRef CAS.
- K. Ienaga, K. Nakamura and T. Goto, Tetrahedron Lett., 1987, 28, 1285 CrossRef CAS.
- M. Mitova, S. Popov and S. De Rosa, J. Nat. Prod., 2004, 67, 1178 CrossRef CAS.
- G. Bringmann, G. Lang, S. Steffens and K. Schaumann, J. Nat. Prod., 2004, 67, 311 CrossRef CAS.
- H. Wang, K. B. Gloer, J. B. Scott and D. Malloch, J. Nat. Prod., 2004, 67, 163.
- J. T. Gautschi, T. Amagata, A. Amagata, F. A. Valeriote, S. L. Mooberry and P. Crews, J. Nat. Prod., 2004, 67, 362 CrossRef CAS.
- H. Wang, K. B. Gloer, J. B. Scott and D. Malloch, J. Nat. Prod., 2004, 67, 1637 CrossRef CAS.
- J. T. Gautschi, T. Amagata, A. Amagata, F. A. Valeriote, S. L. Mooberry and P. Crews, J. Nat. Prod., 2004, 67, 1638 CrossRef CAS.
- M. Tsuda, Y. Kasai, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, Org. Lett., 2004, 6, 3087 CrossRef CAS.
- A. Numata, C. Takahashi, Y. Ito, T. Takada, K. Kawai, Y. Usami, E. Matsumura, M. Imachi, T. Ito and T. Hasegawa, Tetrahedron Lett., 1993, 34, 2355 CrossRef CAS.
- R. Jadulco, R. A. Edrada, R. Ebel, A. Berg, K. Schaumann, V. Wray, K. Staube and P. Proksch, J. Nat. Prod., 2004, 67, 78 CrossRef CAS.
- S. Tsukamoto, S. Miura, Y. Yamashita and T. Ohta, Bioorg. Med. Chem. Lett., 2004, 14, 417 CrossRef CAS.
- Y. Li, X. Li, S.-K. Kim, J. S. Kang, H. D. Choi, J. R. Rho and B. W. Son, Chem. Pharm. Bull., 2004, 52, 375 CrossRef CAS.
- A. Ogawa, C. Murakami, S. Kamisuki, I. Kuriyama, H. Yoshida, F. Sugawara and Y. Mizushina, Bioorg. Med. Chem. Lett., 2004, 14, 3539 CrossRef CAS.
- S. P. B. Ovenden, G. Sberna, R. M. Tait, H. G. Wildman, R. Patel, B. Li, K. Steffy, N. Nguyen and B. M. Meurer-Grimes, J. Nat. Prod., 2004, 67, 2093 CrossRef CAS.
- E. Jagers, E. Hillen-Maske, H. Schmidt, W. Steglich and E. Horak, Z. Naturforsch., B: Chem. Sci., 1987, 42, 1354 CAS.
- E. Jagers, E. Hillen-Maske and W. Steglich, Z. Naturforsch., B: Chem. Sci., 1987, 42, 1349.
- J. Hiort, K. Maksimenka, M. Reichert, S. Perovic-Ottstadt, W. H. Lin, V. Wray, K. Steube, K. Schaumann, H. Weber, P. Proksch, R. Ebel, W. E. G. Müller and G. Bringmann, J. Nat. Prod., 2004, 67, 1532 CrossRef CAS.
- T. Jiang, T. Li, J. Li, H.-Z. Fu, Y.-H. Pei and W.-H. Lin, J. Asian Nat. Prod. Res., 2004, 6, 249 Search PubMed.
- P. Vongvilai, M. Isaka, P. Kittakoop, P. Srikitikulchai, P. Kongsaeree and Y. Thebtaranonth, J. Nat. Prod., 2004, 67, 457 CrossRef CAS.
- Y. Usami, J. Yamaguchi and A. Numata, Heterocycles, 2004, 63, 1123 CrossRef CAS.
- S. S. Afiyatullov, T. A. Kuznetsova, V. V. Isakov, M. V. Pivkin, N. G. Prokof'eva and G. B. Elyakov, J. Nat. Prod., 2000, 63, 848 CrossRef CAS.
- S. S. Afiyatullov, A. I. Kalinovsky, T. A. Kuznetsova, M. V. Pivkin, N. G. Prokof'eva, P. S. Dmitrenok and G. B. Elyakov, J. Nat. Prod., 2004, 67, 1047 CrossRef CAS.
- A. Isogai, M. Washizu, K. Kondo, S. Murakoshi and A. Suzuki, Agric. Biol. Chem., 1984, 48, 2607 CAS.
- M. Varoglu and P. Crews, J. Nat. Prod., 2000, 63, 41 CrossRef CAS.
- C. Klemke, S. Kehraus, A. D. Wright and G. M. König, J. Nat. Prod., 2004, 67, 1058 CrossRef CAS.
- C. Takahashi, A. Numata, Y. Ito, E. Matsumura, H. Araki, H. Iwaki and K. Kushida, J. Chem. Soc., Perkin Trans. 1, 1994, 1859 RSC.
- T. Yamada, C. Iwamoto, N. Yamagaki, T. Yamanouchi, K. Minoura, S. Hagishita and A. Numata, Heterocycles, 2004, 63, 641 CrossRef CAS.
- H. Wei, T. Itoh, M. Kinoshita, Y. Nakai, M. Kurotaki and M. Kobayashi, Tetrahedron, 2004, 60, 6015 CrossRef CAS.
- R. X. Tan, P. R. Jensen, P. G. Williams and W. Fenical, J. Nat. Prod., 2004, 67, 1374 CrossRef CAS.
- T. Yamada, M. Iritani, M. Doi, K. Minoura, T. Ito and A. Numata, J. Chem. Soc., Perkin Trans. 1, 2001, 3046 RSC.
- T. Yamada, M. Iritani, K. Minoura, K. Kawai and A. Numata, Org. Biomol. Chem., 2004, 2, 2131 RSC.
- L. A. McDonald, L. R. Barbieri, V. S. Bernan, J. Janso, P. Lassota and G. T. Carter, J. Nat. Prod., 2004, 67, 1565 CrossRef CAS.
- K. Koyama, M. Ishino, K. Takatori, T. Sugita, K. Kinoshita and K. Takahashi, Tetrahedron Lett., 2004, 45, 6947 CrossRef CAS.
- X. Li, S.-K. Kim, J. S. Kang, H. D. Choi and B. W. Son, Bull. Korean Chem. Soc., 2004, 25, 607 CAS.
- W. P. D. Goldring and G. Pattenden, Org. Biomol. Chem., 2004, 2, 466 RSC.
- M. Sugano, A. Sato, Y. Iijima, K. Furuya, H. Kuwano and T. Hata, J. Antibiot., 1995, 48, 1188 CAS.
- H. Shigemori, Y. Kasai, K. Komatsu, M. Tsuda, Y. Mikami and J. Kobayashi, Mar. Drugs, 2004, 2, 164 Search PubMed.
- O. F. Smetanina, T. A. Kuznetsova, A. V. Gerasimenko, A. I. Kalinovsky, M. V. Pivkin, P. C. Dmitrenok and G. B. Elyakov, Russ. Chem. Bull., 2004, 53, 2643 CrossRef CAS.
- R. T. Williamson, B. L. Marquez, W. H. Gerwick and F. E. Koehn, Magn. Reson. Chem., 2001, 39, 544 CrossRef CAS.
- D. J. Edwards, B. L. Marquez, L. M. Nogle, K. McPhail, D. E. Goeger, M. A. Roberts and W. H. Gerwick, Chem. Biol., 2004, 11, 817 CrossRef CAS.
- R. T. Williamson, I. P. Singh and W. H. Gerwick, Tetrahedron, 2004, 60, 7025 CrossRef CAS.
- T. Okino, M. Wu, N. Sitachitta, L. M. Nogle, B. L. Marquez, R. T. Williamson, W. H. Gerwick, T. F. Murray, K. Colson, T. Asano, F. Yokohawa and T. Shioiri, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 2000, 42, 175 Search PubMed.
- F. Yokokawa, T. Asano, T. Okino, W. H. Gerwick and T. Shioiri, Tetrahedron, 2004, 60, 6859 CrossRef CAS.
- J. D. White, Q. Xu, C.-S. Lee and F. A. Valeriote, Org. Biomol. Chem., 2004, 2, 2092 RSC.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2004, 67, 49 CrossRef CAS.
- A. Ballio, Corsi Semin. Chim., 1968, 11, 140 Search PubMed.
- X.-H. Song, X.-H. Liu and Y.-C. Lin, Redai Haiyang Xuebao, 2004, 23, 66 Search PubMed.
- M. Kita, M. Kondo, T. Koyama, K. Yamada, T. Matsumoto, K.-H. Lee, J.-T. Woo and D. Uemura, J. Am. Chem. Soc., 2004, 126, 4794 CrossRef CAS.
- T. Kubota, Y. Sakuma, M. Tsuda and J. Kobayashi, Mar. Drugs, 2004, 2, 83 Search PubMed.
- J. Kobayashi, M. Ishibashi, H. Nakamura, Y. Ohizumi, T. Yamasu, T. Sasaki and Y. Hirata, Tetrahedron Lett., 1986, 27, 5755 CrossRef CAS.
- J. Kobayashi, M. Ishibashi and H. Hirota, J. Nat. Prod., 1991, 54, 1435 CrossRef CAS.
- B. M. Trost and P. E. Harrington, J. Am. Chem. Soc., 2004, 126, 5028 CrossRef CAS.
- J. Kobayashi, H. Shigemori, M. Ishibashi, T. Yamasu, H. Hirota and T. Sasaki, J. Org. Chem., 1991, 56, 5221 CrossRef CAS.
- S.-Y. Saito, J. Feng, A. Kira, J. Kobayashi and Y. Ohizumi, Biochem. Biophys. Res. Commun., 2004, 320, 961 CrossRef CAS.
- K. Shimbo, M. Tsuda, N. Izui and J. Kobayashi, J. Org. Chem., 2002, 67, 1020 CrossRef CAS.
- A. K. Ghosh and G. Gong, J. Am. Chem. Soc., 2004, 126, 3704 CrossRef CAS.
- M. Tsuda, N. Izui, K. Shimbo, M. Sato, E. Fukushi, J. Kawabata, K. Katsumata, T. Horiguchi and J. Kobayashi, J. Org. Chem., 2003, 68, 5339 CrossRef CAS.
- O. Lepage, E. Kattnig and A. Fürstner, J. Am. Chem. Soc., 2004, 126, 15970 CrossRef CAS.
- X.-C. Huang, D. Zhao, Y.-W. Guo, H.-M. Wu, L.-P. Lin, Z.-H. Wang, J. Ding and Y.-S. Lin, Bioorg. Med. Chem. Lett., 2004, 14, 3117 CAS.
- X.-C. Huang, D. Zhao, Y.-W. Guo, H.-M. Wu, E. Trivellone and G. Cimino, Tetrahedron Lett., 2004, 45, 5501 CrossRef CAS.
- M. Satake, A. Tubaro, J.-S. Lee and T. Yasumoto, Nat. Toxins, 1997, 5, 107 CrossRef CAS.
- M. Konishi, X. Yang, B. Li, C. R. Fairchild and Y. Shimizu, J. Nat. Prod., 2004, 67, 1309 CrossRef CAS.
- K.-I. Onodera, H. Nakamura, Y. Oba and M. Ojika, Tetrahedron, 2003, 59, 1067 CrossRef CAS.
- K.-I. Onodera, H. Nakamura, Y. Oba and M. Ojika, Biosci. Biotechnol. Biochem., 2004, 68, 955 CrossRef CAS.
- K.-I. Onodera, T. Fukatsu, N. Kawai, Y. Yoshioka, T. Okamoto, H. Nakamura and M. Ojika, Biosci. Biotechnol. Biochem., 2004, 68, 848 CrossRef CAS.
- A. J. Bourdelais, S. Campbell, H. Jacocks, J. Naar, J. L. C. Wright, J. Carsi and D. G. Baden, Cell. Mol. Neurobiol, 2004, 24, 553 CrossRef CAS.
- H.-N. Chou and Y. Shimizu, Tetrahedron Lett., 1982, 23, 5521 CrossRef CAS.
- J. M. Benson, F. F. Hahn, T. H. March, J. D. McDonald, M. L. Sopori, J. Seagrave, A. P. Gomez, A. J. Bourdelais, J. Naar, J. Zaias, G. D. Bossart and D. G. Baden, J. Toxicol. Environ. Health, Part A, 2004, 67, 1443 Search PubMed.
- Y.-Y. Lin, M. Risk, S. M. Ray, D. Van Engen, J. Clardy, J. Golik, J. C. James and K. Nakanishi, J. Am. Chem. Soc., 1981, 103, 6773 CrossRef CAS.
- J. L. Wong, R. Oesterlin and H. Rapoport, J. Am. Chem. Soc., 1971, 93, 7344 CrossRef CAS.
- N. V. Kulagina, T. J. O'Shaughnessy, W. Ma, J. S. Ramsdell and J. J. Pancrazio, Toxicon, 2004, 44, 669 CrossRef CAS.
- T. Igarashi, M. Satake and T. Yasumoto, J. Am. Chem. Soc., 1996, 118, 479 CrossRef CAS.
- T. Igarashi, M. Satake and T. Yasumoto, J. Am. Chem. Soc., 1999, 121, 8499 CrossRef CAS.
- M. Sasaki, M. Ebine, H. Takagi, H. Takakura, T. Shida, M. Satake, Y. Oshima, T. Igarashi and T. Yasumoto, Org. Lett., 2004, 6, 1501 CrossRef CAS.
- R. Engh, S. Konetschny-Rapp, H.-W. Krell, U. Martin and C. Tsaklakidis, PCT Int. Appl., 1997, WO97/21725 Search PubMed.
- S. Hanessian, M. Tremblay and J. F. W. Petersen, J. Am. Chem. Soc., 2004, 126, 6064 CrossRef CAS.
- N. Sitachitta and W. H. Gerwick, J. Nat. Prod., 1998, 61, 681 CrossRef CAS.
- J. R. Al Dulayymi, M. S. Baird and K. Jones, Tetrahedron, 2004, 60, 341 CrossRef CAS.
- R. A. Edrada, M. Heubes, G. Brauers, V. Wray, A. Berg, U. Gräfe, M. Wohlfarth, J. Mühlbacher, K. Schaumann, Sudarsono, G. Bringmann and P. Proksch, J. Nat. Prod., 2002, 65, 1598 CrossRef CAS.
- G. Bringmann, G. Lang, M. Michel and M. Heubes, Tetrahedron Lett., 2004, 45, 2829 CrossRef CAS.
- W.-Y. Tsui, G. A. Williams and G. D. Brown, Phytochemistry, 1996, 43, 1083 CrossRef CAS.
- S.-C. Lee and G. D. Brown, J. Nat. Prod., 1998, 61, 29 CrossRef CAS.
- G. D. Brown and H.-F. Wong, Tetrahedron, 2004, 60, 5439 CrossRef CAS.
- T. Barsby, M. T. Kelly and R. J. Andersen, J. Nat. Prod., 2002, 65, 1447 CrossRef CAS.
- D. J. Lipomi, N. F. Langille and J. S. Panek, Org. Lett., 2004, 6, 3533 CrossRef CAS.
- M. Namikoshi, H. Kobayashi, T. Yoshimoto and T. Hosoya, J. Antibiot., 1997, 50, 890 CAS.
- T. Suzuki, K. Usui, Y. Miyake, M. Namikoshi and M. Nakada, Org. Lett., 2004, 6, 553 CrossRef CAS.
- D. C. Rowley, S. Kelly, C. A. Kauffman, P. R. Jensen and W. Fenical, Bioorg. Med. Chem., 2003, 11, 4263 CrossRef CAS.
- D. C. Rowley, S. Kelly, P. Jensen and W. Fenical, Bioorg. Med. Chem., 2004, 12, 4929 CrossRef CAS.
- Y. Lin, X. Wu, S. Feng, G. Jiang, J. Luo, S. Zhou, L. L. P. Vrijmoed, E. B. G. Jones, K. Krohn, K. Steingröver and F. Zsila, J. Org. Chem., 2001, 66, 6252 CrossRef CAS.
- K. Krohn and M. Riaz, Tetrahedron Lett., 2004, 45, 293 CrossRef CAS.
- M. Murata, M. Kumagai, J. S. Lee and T. Yasumoto, Tetrahedron Lett., 1987, 28, 5869 CrossRef CAS.
- C. Bianchi, R. Fato, A. Angelin, F. Trombetti, V. Ventrella, A. R. Borgatti, E. Fattorusso, P. Ciminiello, P. Bernardi, G. Lenaz and G. P. Castelli, Biochim. Biophys. Acta, 2004, 1656, 139 CAS.
- J. Orjala, D. G. Nagle, V. L. Hsu and W. H. Gerwick, J. Am. Chem. Soc., 1995, 117, 8281 CrossRef CAS.
- W. I. Li, B. L. Marquez, T. Okino, F. Yokokawa, T. Shioiri, W. H. Gerwick and T. F. Murray, J. Nat. Prod., 2004, 67, 559 CrossRef CAS.
- G. Guella, F. Dini, A. Tomei and F. Pietra, J. Chem. Soc., Perkin Trans. 1, 1994, 161 RSC.
- D. Savoia, C. Avanzini, T. Allice, E. Callone, G. Guella and F. Dini, Antimicrob. Agents Chemother., 2004, 48, 3828 CrossRef CAS.
- S. Takamatsu, D. G. Nagle and W. H. Gerwick, Planta Med., 2004, 70, 127 CrossRef CAS.
- P. J. Proteau, PhD Dissertation, Oregon State University, 1993 Search PubMed.
- F. E. Koehn, R. E. Longley and J. K. Reed, J. Nat. Prod., 1992, 55, 613 CrossRef CAS.
- H. H. Sun, V. J. Paul and W. Fenical, Phytochemistry, 1983, 22, 743 CrossRef CAS.
- H. E. Högberg, R. H. Thomson and T. J. King, J. Chem. Soc., Perkin Trans. 1, 1976, 1696 RSC.
- W. H. Gerwick, A. Lopez, G. D. Van Duyne, J. Clardy, W. Ortiz and A. Baez, Tetrahedron Lett., 1986, 27, 1979 CrossRef CAS.
- W. H. Gerwick, J. Nat. Prod., 1989, 52, 252 CrossRef CAS.
- N. R. Ayyangar, R. A. Khan and V. H. Deshpande, Tetrahedron Lett., 1988, 29, 2347 CrossRef CAS.
- M. Sugano, A. Sato, Y. Iijima, T. Oshima, K. Furuya, H. Kuwano, T. Hata and H. Hanzawa, J. Am. Chem. Soc., 1991, 113, 5463 CrossRef CAS.
- M. Chu, I. Truumees, I. Gunnarsson, W. R. Bishop, W. Kreutner, A. C. Horan, M. G. Patel, V. P. Gullo and M. S. Puar, J. Antibiot., 1993, 46, 554 CAS.
- M. Sugano, A. Sato, Y. Iijima, K. Furuya, H. Haruyama, K. Yoda and T. Hata, J. Org. Chem., 1994, 59, 564 CrossRef CAS.
- T. Tokiwano, E. Fukushi, T. Endo and H. Oikawa, Chem. Commun., 2004, 1324 RSC.
- G. Massé, S. T. Belt and S. J. Rowland, Phytochemistry, 2004, 65, 1101 CrossRef CAS.
- M. P. Puglisi, L. T. Tan, P. R. Jensen and W. Fenical, Tetrahedron, 2004, 60, 7035 CrossRef CAS.
- Y. Fukuyama, M. Kodama, I. Miura, Z. Kinzyo, M. Kido, H. Mori, Y. Nakayama and M. Takahashi, Chem. Pharm. Bull., 1989, 37, 349 CAS.
- Y. Fukuyama, I. Miura, Z. Kinzyo, Y. Nakayama, M. Takahashi and M. Kido, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1983, 26, 126 Search PubMed.
- Y. Fukuyama, I. Miura, Z. Kinzyo, H. Mori, M. Kido, Y. Nakayama, M. Takahashi and M. Ochi, Chem. Lett., 1985, 739 CAS.
- Y. Okada, A. Ishimaru, R. Suzuki and T. Okuyama, J. Nat. Prod., 2004, 67, 103 CrossRef CAS.
- C. F. Cross, E. J. Bevan and J. F. Briggs, Chem. Ztg., 1907, 31, 725 Search PubMed.
- H. S. Kang, H. Y. Chung, J. H. Jung, B. W. Son and J. S. Choi, Chem. Pharm. Bull., 2003, 51, 1012 CrossRef CAS.
- Y. Fukuyama, M. Kodama, I. Miura, Z. Kinzyo, H. Mori, Y. Nakayama and M. Takahashi, Chem. Pharm. Bull., 1990, 38, 133 CAS.
- H. S. Kang, H. R. Kim, D. S. Byun, B. W. Son, T. J. Nam and J. S. Choi, Arch. Pharmacal. Res., 2004, 27, 1226 Search PubMed.
- G. Navarro, J. J. Fernández and M. Norte, J. Nat. Prod., 2004, 67, 495 CrossRef CAS.
- G. Culioli, A. Ortalo-Magné, M. Daoudi, H. Thomas-Guyon, R. Valls and L. Piovetti, Phytochemistry, 2004, 65, 2063 CrossRef CAS.
- K. Mori, T. Ooi, M. Hiraoka, N. Oka, H. Hamada, M. Tamura and T. Kusumi, Mar. Drugs, 2004, 2, 63 Search PubMed.
- P. Siamopoulou, A. Bimplakis, D. Iliopoulou, C. Vagias, P. Cos, D. Vanden Berghe and V. Roussis, Phytochemistry, 2004, 65, 2025 CrossRef CAS.
- M. S. Ali, M. K. Pervez, F. Ahmed and M. Saleem, Nat. Prod. Res., 2004, 18, 543 CrossRef CAS.
- C. Ireland and D. J. Faulkner, J. Org. Chem., 1977, 42, 3157 CrossRef CAS.
- J. P. Barbosa, R. C. Pereira, J. L. Abrantes, C. C. C. dos Santos, M. A. Rebello, I. C. P. P. Frugulhetti and V. L. Teixeira, Planta Med., 2004, 70, 856 CrossRef CAS.
- J. P. Barbosa, V. L. Teixeira and R. C. Pereira, Bot. Mar., 2004, 47, 147 Search PubMed.
- H. S. Pereira, L. R. Leão-Ferreira, N. Moussatché, V. L. Teixeira, D. N. Cavalcanti, L. J. Costa, R. Diaz and I. C. P. P. Frugulhetti, Antiviral Res., 2004, 64, 69 CrossRef CAS.
- J. F. Blount, R. W. Dunlop, K. L. Erickson and R. J. Wells, Aust. J. Chem., 1982, 35, 145 CAS.
- S.-H. Qi, S. Zhang, J.-S. Huang, Z.-H. Xiao, J. Wu and L.-J. Long, Chem. Pharm. Bull., 2004, 52, 986 CrossRef CAS.
- F. H. Song, X. Fan, X. L. Xu, J. L. Zhao, L. J. Han and J. G. Shi, Chin. Chem. Lett., 2004, 15, 316 CAS.
- F. Song, X. Fan, X. Xu, J. Zhao, Y. Yang and J. Shi, J. Nat. Prod., 2004, 67, 1644 CrossRef CAS.
- X. Xu, F. Song, S. Wang, S. Li, F. Xiao, J. Zhao, Y. Yang, S. Shang, L. Yang and J. Shi, J. Nat. Prod., 2004, 67, 1661 CrossRef CAS.
- X.-L. Xu, X. Fan, F.-H. Song, J.-L. Zhao, L.-J. Han, Y.-C. Yang and J.-G. Shi, J. Asian Nat. Prod. Res., 2004, 6, 217 Search PubMed.
- X. L. Xu, X. Fan, F. H. Song, J. L. Zhao, L. J. Han and J. G. Shi, Chin. Chem. Lett., 2004, 15, 661 CAS.
- K. Toume, M. Miyata, K. Egawa, K. Nose, M. Hayashi, K. Komiyama and M. Ishibashi, Nat. Med., 2004, 58, 79 CAS.
- S.-M. Li and K.-W. Glombitza, Phytochemistry, 1991, 30, 3417 CrossRef CAS.
- J. G. Urones, P. Basabe, I. S. Marcos, J. Pineda, A. M. Lithgow, R. F. Moro, F. M. S. Palma, A. M. E. M. Brito and M. D. G. Gravalos, Phytochemistry, 1992, 31, 179 CrossRef CAS.
- M. Danet, M. Normand-Bayle, J. Mahuteau, J. d'Angelo, G. Morgant and D. Desmaële, Eur. J. Org. Chem., 2004, 1911 CrossRef CAS.
- Y. Fukuyama, M. Kodama, I. Miura, Z. Kinzyo, H. Mori, Y. Nakayama and M. Takahashi, Chem. Pharm. Bull., 1989, 37, 2438 CAS.
- K.-W. Glombitza and G. Gerstberger, Phytochemistry, 1985, 24, 543 CrossRef CAS.
- M.-J. Ahn, K.-D. Yoon, S.-Y. Min, J. S. Lee, J. H. Kim, T. G. Kim, S. H. Kim, N.-G. Kim, H. Huh and J. Kim, Biol. Pharm. Bull., 2004, 27, 544 CrossRef CAS.
- I. Heilbron, R. F. Phipers and H. R. Wright, J. Chem. Soc. Abstr., 1934, 1572 Search PubMed.
- H. B. MacPhillamy, J. Am. Chem. Soc., 1942, 64, 1732 CrossRef CAS.
- Y. S. Lee, K. H. Shin, B.-K. Kim and S. Lee, Arch. Pharmacal. Res., 2004, 27, 1120 Search PubMed.
- E. Dorta, A. R. Díaz-Marrero, M. Cueto, L. D'Croz, J. L. Maté and J. Darias, Tetrahedron Lett., 2004, 45, 7065 CrossRef CAS.
- M. Suzuki, Y. Mizano, Y. Matsuo and M. Masuda, Phytochemistry, 1996, 43, 121 CrossRef CAS.
- Z. Aydoğmus, S. Imre, L. Ersoy and V. Wray, Nat. Prod. Res., 2004, 18, 43 CrossRef CAS.
- E. G. Lyakhova, A. I. Kalinovsky, S. A. Kolesnikova, V. E. Vaskovsky and V. A. Stonik, Phytochemistry, 2004, 65, 2527 CrossRef CAS.
- K. A. Mohammed, C. F. Hossain, L. Zhang, R. K. Bruick, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2004, 67, 2002 CrossRef CAS.
- Y. S. Roh, B. W. Son, K. S. Im and H. D. Choi, Saengyak Hakhoechi, 1994, 25, 117 Search PubMed.
- T. Ishii, T. Okino, M. Suzuki and Y. Machiguchi, J. Nat. Prod., 2004, 67, 1764 CrossRef CAS.
- S. V. Khotimchenko and V. E. Vas'kovsky, Russ. J. Bioorg. Chem., 2004, 30, 168 Search PubMed.
- K. L. McPhail, D. France, S. Cornell-Kennon and W. H. Gerwick, J. Nat. Prod., 2004, 67, 1010 CrossRef CAS.
- D. D. Weller, E. P. Stirchak and A. Yokoyama, J. Org. Chem., 1984, 49, 2061 CrossRef CAS.
- R. T. Borchardt, J. H. Huber and M. Houston, J. Med. Chem., 1982, 25, 258 CrossRef CAS.
- J. Zhao, X. Fan, S. Wang, S. Li, S. Shang, Y. Yang, N. Xu, Y. Lü and J. Shi, J. Nat. Prod., 2004, 67, 1032 CrossRef CAS.
- N. E. Awad, Phytother. Res., 2004, 18, 275 CrossRef CAS.
- F. Cafieri, P. Ciminiello, C. Santacroce and E. Fattorusso, Phytochemistry, 1983, 22, 1824 CrossRef CAS.
- J. Lee and J. Hong, J. Org. Chem., 2004, 69, 6433 CrossRef CAS.
- F. Cafieri, L. De Napoli, E. Fattorusso, M. Piattelli and S. Sciuto, Tetrahedron Lett., 1979, 963 CrossRef CAS.
- F. Cafieri, E. Fattorusso, B. Di Blasio and C. Pedone, Tetrahedron Lett., 1981, 22, 4123 CrossRef CAS.
- F. Cafieri, L. De Napoli, E. Fattorusso and C. Santacroce, Phytochemistry, 1987, 26, 471 CrossRef CAS.
- R. B. Kinnel, R. K. Dieter, J. Meinwald, D. Van Engen, J. Clardy, T. Eisner, M. O. Stallard and W. Fenical, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 3576 CAS.
- W. Fenical, H. L. Sleeper, V. J. Paul, M. O. Stallard and H. H. Sun, Pure Appl. Chem., 1979, 51, 1865 CrossRef CAS.
- S. E. Denmark and S.-M. Yang, J. Am. Chem. Soc., 2004, 126, 12432 CrossRef CAS.
- V. J. Paul and W. Fenical, Tetrahedron Lett., 1980, 21, 2787 CrossRef CAS.
- E. A. Couladouros and V. P. Vidali, Chem. Eur. J., 2004, 10, 3822 CrossRef CAS.
- V. H. Argandoña, J. Rovirosa, A. San-Martín, A. Riquelme, A. R. Díaz-Marrero, M. Cueto, J. Darias, O. Santana, A. Guadaño and A. González-Coloma, J. Agric. Food Chem., 2002, 50, 7029 CrossRef CAS.
- C. de Inés, V. H. Argandoña, J. Rovirosa, A. San-Martín, A. R. Díaz-Marrero, M. Cueto and A. González-Coloma, Z. Naturforsch., C: Biosci., 2004, 59, 339 CAS.
- T. Irie, M. Suzuki, E. Kurosawa and T. Masamune, Tetrahedron Lett., 1966, 1837 CrossRef CAS.
- T. Irie, M. Suzuki, E. Kurosawa and T. Masamune, Tetrahedron, 1970, 26, 3271 CrossRef CAS.
- R. Kazlauskas, P. T. Murphy, R. J. Quinn and R. J. Wells, Aust. J. Chem., 1976, 29, 2533 CAS.
- T. Ichiba and T. Higa, J. Org. Chem., 1986, 51, 3364 CrossRef CAS.
- G. T. Carter, K. L. Rinehart, L. H. Li, S. L. Kuentzel and J. L. Connor, Tetrahedron Lett., 1978, 4479 CrossRef CAS.
- C. S. Vairappan, T. Kawamoto, H. Miwa and M. Suzuki, Planta Med., 2004, 70, 1087 CrossRef CAS.
- J. N. Carter-Franklin and A. Butler, J. Am. Chem. Soc., 2004, 126, 15060 CrossRef CAS.
- N. M. Carballeira, H. Cruz, C. D. Kwong, B. Wan and S. Franzblau, Lipids, 2004, 39, 675 CrossRef CAS.
- A. B. Imbs and S. A. Rodkina, Chem. Phys. Lipids, 2004, 129, 173 CrossRef CAS.
- W.-J. Lan, J.-H. Yao, J.-Y. Su and L.-M. Zeng, Zhongshan Daxue Xuebao, Ziran Kexueban, 2004, 43, 115 Search PubMed.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, J. Org. Chem., 2004, 69, 1174 CrossRef CAS.
- R. G. Linington, M. Robertson, A. Gauthier, B. B. Finlay, R. van Soest and R. J. Andersen, Org. Lett., 2002, 4, 4089 CrossRef CAS.
- J. B. MacMillan, R. G. Linington, R. J. Andersen and T. F. Molinski, Angew. Chem., Int. Ed., 2004, 43, 5946 CrossRef CAS.
- K. Warabi, W. T. Zimmerman, J. Shen, A. Gauthier, M. Robertson, B. B. Finlay, R. van Soest and R. J. Andersen, Can. J. Chem., 2004, 82, 102 CrossRef CAS.
- Y. Nakao, T. Maki, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2004, 67, 1346 CrossRef CAS.
- M. J. Ortega, E. Zubía, M. C. Sánchez, J. Salvá and J. L. Carballo, Tetrahedron, 2004, 60, 2517 CrossRef CAS.
- T. A. Mansoor, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im, D. S. Lee and J. H. Jung, J. Nat. Prod., 2004, 67, 721 CrossRef CAS.
- R. J. Capon, C. Skene, E. H.-T. Liu, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, J. Nat. Prod., 2004, 67, 1277 CrossRef CAS.
- L.-Y. Li, Z.-W. Deng, J. Li, H.-Z. Fu and W.-H. Lin, Beijing Daxue Xuebao, Yixueban, 2004, 36, 12 Search PubMed.
- S. P. Gunasekera, R. A. Isbrucker, R. E. Longley, A. E. Wright, S. A. Pomponi and J. K. Reed, J. Nat. Prod., 2004, 67, 110 CrossRef CAS.
- G. R. Pettit, T. Nogawa, J. C. Knight, D. L. Doubek and J. N. A. Hooper, J. Nat. Prod., 2004, 67, 1611 CrossRef CAS.
- X.-H. Huang, R. van Soest, M. Roberge and R. J. Andersen, Org. Lett., 2004, 6, 75 CrossRef CAS.
- Z. Szilágyi, G. Pócsfalvi, V. Costantino, A. Mangoni, A. Malorni and E. Fattorusso, Rapid Commun. Mass Spectrom., 2004, 18, 2989 CrossRef CAS.
- J. S. Kim, K. S. Im, J. H. Jung, Y.-L. Kim, J. Kim, C. J. Shim and C.-O. Lee, Tetrahedron, 1998, 54, 3151 CrossRef CAS.
- H. J. Choi, S.-J. Bae, N. D. Kim, J. H. Jung and Y. H. Choi, Int. J. Mol. Med., 2004, 14, 1091 Search PubMed.
- S. Tsukamoto, S. Takeuchi, M. Ishibashi and J. Kobayashi, J. Org. Chem., 1994, 57, 5255.
- M. Perpelescu, M. Tsuda, M. Suzuki, S. Yoshida and J. Kobayashi, Nat. Med., 2004, 58, 86 CAS.
- N. Alam, B. H. Bae, J. Hong, C.-O. Lee, B. A. Shin, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 533 CrossRef CAS.
- E.-H. Lee, M.-R. Yun, W.-H. Wang, J. H. Jung and D.-S. Im, Acta Pharm. Sin., 2004, 25, 1521 Search PubMed.
- M. J. Ortega, E. Zubia, J. L. Carbalo and J. Salvá, Tetrahedron, 1997, 53, 331 CrossRef CAS.
- T. V. Hansen and L. Skattebøl, Tetrahedron Lett., 2004, 45, 2809 CrossRef CAS.
- N. M. Carballeira, V. Negrón and E. D. Reyes, Tetrahedron, 1992, 48, 1053 CrossRef CAS.
- B. A. Kulkarni, A. Sharma, S. Gamre and S. Chattopadhyay, Synthesis, 2004, 4, 595.
- F. Cafieri, E. Fattorusso, O. Taglialatela-Scafati, M. Di Rosa and A. Ianaro, Tetrahedron, 1999, 55, 13831 CrossRef CAS.
- P. Y. Hayes and W. Kitching, Heterocycles, 2004, 62, 173 CrossRef CAS.
- D. J. Gochfeld and M. T. Hamann, J. Nat. Prod., 2001, 64, 1477 CrossRef CAS.
- S. Kowashi, T. Ogamino, J. Kamei, Y. Ishikawa and S. Nishiyama, Tetrahedron Lett., 2004, 45, 4393 CrossRef CAS.
- Y. C. Shen, C. V. S. Prakash and J.-H. Guh, Tetrahedron Lett., 2004, 45, 2463 CrossRef CAS.
- R. H. Cichewicz, F. A. Valeriote and P. Crews, Org. Lett., 2004, 6, 1951 CrossRef CAS.
- G. R. Pettit, J.-P. Xu, J.-C. Chapuis, R. K. Pettit, L. P. Tackett, D. L. Doubek, J. N. A. Hooper and J. M. Schmidt, J. Med. Chem., 2004, 47, 1149 CrossRef CAS.
- E. J. Dumdei, J. W. Blunt, M. H. G. Munro and L. K. Pannell, J. Org. Chem., 1997, 62, 2636 CrossRef CAS.
- S. Matsunaga and N. Fusetani, Dev. Food Sci., 2004, 42, 131 CAS.
- J. Piel, D. Hui, G. Wen, D. Butzke, M. Platzer, N. Fusetani and S. Matsunaga, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16222 CrossRef CAS.
- S. J. Mickel, D. Niederer, R. Daeffler, A. Osmani, E. Kuesters, E. Scmid, K. Schaer, R. Gamboni, W. Chen, E. Loeser, F. R. Kinder, K. Konigsberger, K. Prasad, T. M. Ramsey, O. Repič, R.-M. Wang, G. Florence, I. Lyothier and I. Paterson, Org. Process Res. Dev., 2004, 8, 122 Search PubMed.
- S. P. Gunasekera, M. Gunasekera, R. E. Longley and G. K. Schulte, J. Org. Chem., 1990, 55, 4912 CrossRef CAS.
- A. R. Carroll, M. S. Buchanan, A. Edser, E. Hyde, M. Simpson and R. J. Quinn, J. Nat. Prod., 2004, 67, 1291 CrossRef CAS.
- R. N. Sonnenschein, J. J. Farias, K. Tenney, S. L. Mooberry, E. Lobkovsky, J. Clardy and P. Crews, Org. Lett., 2004, 6, 779 CrossRef CAS.
- C. Chevallier, A. D. Richardson, M. C. Edler, E. Hamel, M. K. Harper and C. M. Ireland, Org. Lett., 2003, 5, 3737 CrossRef CAS.
- P. Crews, J. J. Farias, R. Emrich and P. A. Keifer, J. Org. Chem., 1994, 59, 2932 CrossRef CAS.
- C. Liu, M. N. Masuno, J. B. MacMillan and T. F. Molinski, Angew. Chem., Int. Ed., 2004, 43, 5951 CrossRef CAS.
- E. W. Schmidt, C. Raventos-Suarez, M. Bifano, A. T. Menendez, C. R. Fairchild and D. J. Faulkner, J. Nat. Prod., 2004, 67, 475 CrossRef CAS.
- R. A. Davis, G. C. Mangalindan, Z. P. Bojo, R. R. Antemano, N. O. Rodriguez, G. P. Concepcion, S. C. Samson, D. de Guzman, L. J. Cruz, D. Tasdemir, M. K. Harper, X. Feng, G. T. Carter and C. M. Ireland, J. Org. Chem., 2004, 69, 4170 CrossRef CAS.
- N. Berer, A. Rudi, I. Goldberg, Y. Benayahu and Y. Kashman, Org. Lett., 2004, 6, 2543 CrossRef CAS.
- D. J. Milanowski, M. A. Rashid, K. R. Gustafson, B. R. O'Keefe, J. P. Nawrocki, L. K. Pannell and M. R. Boyd, J. Nat. Prod., 2004, 67, 441 CrossRef CAS.
- S. Aoki, L. Cao, K. Matsui, R. Rachmat, S.-I. Akiyama and M. Kobayashi, Tetrahedron, 2004, 60, 7053 CrossRef CAS.
- N. Oku, K. R. Gustafson, L. K. Cartner, J. A. Wilson, N. Shigematsu, S. Hess, L. K. Pannell, M. R. Boyd and J. B. McMahon, J. Nat. Prod., 2004, 67, 1407 CrossRef CAS.
- W.-L. Li, Y.-H. Yi, H.-M. Wu, Q.-Z. Xu, H.-F. Tang, D.-Z. Zhou, H.-W. Lin and Z.-H. Wang, J. Nat. Prod., 2003, 66, 146 CrossRef CAS.
- K. L. Greenman, D. M. Hach and D. L. Van Vranken, Org. Lett., 2004, 6, 1713 CrossRef CAS.
- G. R. Petit, R. Tan, D. L. Herald, R. L. Cerny and M. D. Williams, J. Org. Chem., 1994, 59, 1593 CrossRef CAS.
- Y. Nakao, M. Fujita, K. Warabi, S. Matsunaga and N. Fusetani, J. Am. Chem. Soc., 2000, 122, 10462 CrossRef CAS.
- N. Schaschke, Bioorg. Med. Chem. Lett., 2004, 14, 855 CrossRef CAS.
- A. Zampella, M. V. D'Auria, L. Gomez Paloma, A. Casapullo, L. Minale, C. Debitus and Y. Henin, J. Am. Chem. Soc., 1996, 118, 6202 CrossRef CAS.
- L. Trevisi, G. Cargnelli, G. Ceolotto, I. Papparella, A. Semplicini, A. Zampella, M. V. D'Auria and S. Luciani, Biochem. Pharmacol., 2004, 68, 1331 CrossRef CAS.
- J. Shin, H.-S. Lee, J.-Y. Kim, H. J. Shin, J.-W. Ahn and V. J. Paul, J. Nat. Prod., 2004, 67, 1889 CrossRef CAS.
- B. Vilozny, T. Amagata, S. L. Mooberry and P. Crews, J. Nat. Prod., 2004, 67, 1055 CrossRef CAS.
- Y. Kashman, A. Groweiss and U. Shmueli, Tetrahedron Lett., 1980, 21, 3629 CrossRef CAS.
- A. Grassia, I. Bruno, C. Debitus, S. Marzocco, A. Pinto, L. Gomez-Paloma and R. Riccio, Tetrahedron, 2001, 57, 6257 CrossRef CAS.
- J. Chen and C. J. Forsyth, Angew. Chem., Int. Ed., 2004, 43, 2148 CrossRef CAS.
- A. K. Ghosh and X. Xu, Org. Lett., 2004, 6, 2055 CrossRef CAS.
- G. R. Petit, Z. A. Cichacz, F. Gao, M. R. Boyd and J. M. Schmidt, J. Chem. Soc., Chem. Commun., 1994, 1111 RSC.
- I. Paterson, R. Britton, O. Delgado and A. E. Wright, Chem. Commun., 2004, 632 RSC.
- I. Paterson, R. Britton, O. Delgado, A. Meyer and K. G. Poullennec, Angew. Chem., Int. Ed., 2004, 43, 4629 CrossRef CAS.
- Y. Shin, J.-H. Fournier, Y. Fukui, A. M. Brückner and D. P. Curran, Angew. Chem., Int. Ed., 2004, 43, 4634 CrossRef CAS.
- A. E. Wright, Y. Chen, P. L. Winder, T. P. Pitts, S. A. Pomponi and R. E. Longley, J. Nat. Prod., 2004, 67, 1351 CrossRef CAS.
- D. E. Williams, M. Roberge, R. Van Soest and R. J. Andersen, J. Am. Chem. Soc., 2003, 125, 5296 CrossRef CAS.
- D. E. Williams, M. Lapawa, X. Feng, T. Tarling, M. Roberge and R. J. Andersen, Org. Lett., 2004, 6, 2607 CrossRef CAS.
- M. V. D'Auria, L. Gomez Paloma, L. Minale, A. Zampella, J.-F. Verbist, C. Roussakis, C. Debitus and J. Patissou, Tetrahedron, 1994, 50, 4829 CrossRef CAS.
- I. Paterson, R. Britton, K. Ashton, H. Knust and J. Stafford, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11986 CrossRef CAS.
- N. Fusetani, T. Sugawara, S. Matsunaga and H. Hirota, J. Org. Chem., 1991, 56, 4971 CrossRef CAS.
- S. Nishimura, S. Matsunaga, M. Yoshida, H. Hirota, S. Yokoyama and N. Fusetani, Bioorg. Med. Chem., 2004, 13, 449.
- H. Liu, M. Namikoshi, S. Meguro, H. Nagai, H. Kobayashi and X. Yao, J. Nat. Prod., 2004, 67, 472 CrossRef CAS.
- R. Sakai, K. Suzuki, K. Shimamoto and H. Kamiya, J. Org. Chem., 2004, 69, 1180 CrossRef CAS.
- K. Kobayashi, H. Shimogawa, A. Sakakura, T. Teruya, K. Suenaga and H. Kigoshi, Chem. Lett., 2004, 33, 1262 CrossRef CAS.
- W. Hassan, R. Edrada, R. Ebel, V. Wray and P. Proksch, Mar. Drugs, 2004, 2, 88 Search PubMed.
- R. J. Capon, C. Skene, E. H. Liu, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, Nat. Prod. Res., 2004, 18, 305 CrossRef CAS.
- I. Mancini, G. Guella, M. Sauvain, C. Debitus, A.-G. Duigou, F. Ausseil, J. L. Menou and F. Pietra, Org. Biomol. Chem., 2004, 2, 783 RSC.
- C. A. Volk and M. Köck, Org. Biomol. Chem., 2004, 2, 1827 RSC.
- C. A. Volk, H. Lippert, E. Lichte and M. Köck, Eur. J. Org. Chem., 2004, 3154 CrossRef CAS.
- N. Oku, K. Nagai, N. Shindoh, Y. Terada, R. W. M. van Soest, S. Matsunaga and N. Fusetani, Bioorg. Med. Chem. Lett., 2004, 14, 2617 CrossRef CAS.
- J. H. H. L. de Oliveira, A. Grube, M. Köck, R. G. S. Berlinck, M. L. Macedo, A. G. Ferreira and E. Hajdu, J. Nat. Prod., 2004, 67, 1685 CrossRef CAS.
- S. Matsunaga, Y. Miyata, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2004, 67, 1758 CrossRef CAS.
- H. Liu, Y. Mishima, T. Fujiwara, H. Nagai, A. Kitazawa, Y. Mine, H. Kobayashi, X. Yao, J. Yamada, T. Oda and M. Namikoshi, Mar. Drugs, 2004, 2, 154 Search PubMed.
- S. Tsukamoto, M. Takahashi, M. Matsunaga, N. Fusetani and R. W. M. Van Soest, J. Nat. Prod., 2000, 63, 682 CrossRef CAS.
- A. R. Carroll and P. J. Scheuer, Tetrahedron, 1990, 46, 6637 CrossRef CAS.
- S. P. Romeril, V. Lee and J. E. Baldwin, Tetrahedron Lett., 2004, 45, 3273 CrossRef CAS.
- M. Yousaf, N. L. Hammond, J. Peng, S. Wahyuono, K. A. McIntosh, W. N. Charman, A. M. S. Mayer and M. T. Hamann, J. Med. Chem., 2004, 47, 3512 CrossRef CAS.
- F. Borrelli, C. Campagnuolo, R. Capasso, E. Fattorusso and O. Taglialatela-Scafati, Eur. J. Org. Chem., 2004, 3227 CrossRef CAS.
- J. Cohen, G. K. Paul, S. P. Gunasekera, R. E. Longley and S. A. Pomponi, Pharm. Biol. (Lisse, Neth.), 2004, 42, 59 CrossRef CAS.
- M. Sjöegren, U. Göeransson, A.-L. Johnson, M. Dahlström, R. Andersson, J. Bergman, P. R. Jonsson and L. Bohlin, J. Nat. Prod., 2004, 67, 368 CrossRef.
- S. Sölter, R. Dieckmann, M. Blumenburg and W. Francke, Tetrahedron Lett., 2002, 43, 3385 CrossRef CAS.
- G. Lidgren, L. Bohlin and J. Bergman, Tetrahedron Lett., 1986, 27, 3283 CrossRef CAS.
- A.-L. Johnson, J. Bergman, M. Sjögren and L. Bohlin, Tetrahedron, 2004, 60, 961 CrossRef CAS.
- N. K. Garg, D. D. Caspi and B. M. Stoltz, J. Am. Chem. Soc., 2004, 126, 9552 CrossRef CAS.
- S. Pedpradab, R. Edrada, R. Ebel, V. Wray and P. Proksch, J. Nat. Prod., 2004, 67, 2113 CrossRef CAS.
- R. D. Charan, T. C. McKee and M. R. Boyd, Nat. Prod. Res., 2004, 18, 225 CrossRef CAS.
- N. L. Segraves, S. J. Robinson, D. Garcia, S. A. Said, X. Fu, F. J. Schmitz, H. Pietraszkiewicz, F. A. Valeriote and P. Crews, J. Nat. Prod., 2004, 67, 783 CrossRef CAS.
- N. K. Utkina, A. E. Makarchenko, V. A. Denisenko and P. S. Dmitrenok, Tetrahedron Lett., 2004, 45, 7491 CrossRef CAS.
- R. A. Keyzers, T. Samaai and M. T. Davies-Coleman, Tetrahedron Lett., 2004, 45, 9415 CrossRef CAS.
- E. M. Antunes, D. R. Beukes, M. Kelly, T. Samaai, L. R. Barrows, K. M. Marshall, C. Sincich and M. T. Davies-Coleman, J. Nat. Prod., 2004, 67, 1268 CrossRef CAS.
- F. Reyes, R. Martín, A. Rueda, R. Fernández, D. Montalvo, C. Gómez and J. M. Sánchez-Puelles, J. Nat. Prod., 2004, 67, 463 CrossRef CAS.
- E. M. Antunes, B. R. Copp, M. T. Davies-Coleman and T. Samaai, Nat. Prod. Rep., 2005, 22, 62 RSC.
- I. T. Sandoval, R. A. Davis, T. S. Bugni, G. P. Conception, M. K. Harper and C. M. Ireland, Nat. Prod. Res., 2004, 18, 89 CrossRef CAS.
- A. Herlt, L. Mander, W. Rombang, R. Rumampuk, S. Soemitro, W. Steglich, P. Tarigan and F. von Nussbaum, Tetrahedron, 2004, 60, 6101 CrossRef CAS.
- B. F. Bowden, B. J. McCool and R. H. Willis, J. Org. Chem., 2004, 69, 7791 CrossRef CAS.
- N. Kuwabara, H. Hayashi, N. Hiramatsu, T. Choshi, T. Kumemura, J. Nobuhiro and S. Hibino, Tetrahedron, 2004, 60, 2943 CrossRef.
- R. A. Edrada, P. Proksch, V. Wray, R. Christ, L. Witte and R. W. M. Van Soest, J. Nat. Prod., 1996, 59, 973 CrossRef CAS.
- N. Saito, C. Tanaka, T. Satomi, C. Oyama and A. Kubo, Chem. Pharm. Bull., 2004, 52, 282 CrossRef.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1249 CrossRef CAS.
- S. Nakahara and A. Kubo, Heterocycles, 2004, 63, 2355 CrossRef CAS.
- G. R. Petit, J. C. Collins, J. C. Knight, D. L. Herald, R. A. Nieman, M. D. Williams and R. K. Pettit, J. Nat. Prod., 2003, 66, 544 CrossRef CAS.
- J. M. Frincke and D. J. Faulkner, J. Am. Chem. Soc., 1982, 104, 265 CrossRef CAS.
- Y. Nakao, T. Shiroiwa, S. Murayama, S. Matsunaga, Y. Goto, Y. Matsumoto and N. Fusetani, Mar. Drugs, 2004, 2, 55 Search PubMed.
- P. Ralifo and P. Crews, J. Org. Chem., 2004, 69, 9025 CrossRef CAS.
- W. Hassan, R. Edrada, R. Ebel, V. Wray, A. Berg, R. van Soest, S. Wiryowidagdo and P. Proksch, J. Nat. Prod., 2004, 67, 817 CrossRef CAS.
- H.-M. Hua, J. Peng, F. R. Fronczek, M. Kelly and M. T. Hamann, Bioorg. Med. Chem., 2004, 12, 6461 CrossRef CAS.
- J. C. Braekman, D. Daloze, R. Tavares, E. Hajdu and R. W. M. Van Soest, J. Nat. Prod., 2000, 63, 193 CrossRef CAS.
- K. M. Meragelman, T. C. McKee and J. B. McMahon, J. Nat. Prod., 2004, 67, 1165 CrossRef CAS.
- J. T. Gautschi, S. Whitman, T. R. Holman and P. Crews, J. Nat. Prod., 2004, 67, 1256 CrossRef CAS.
- T. Endo, M. Tsuda, T. Okada, S. Mitsuhashi, H. Shima, K. Kikuchi, Y. Mikami, J. Fromont and J. Kobayashi, J. Nat. Prod., 2004, 67, 1262 CrossRef CAS.
- F. Cafieri, E. Fattorusso, A. Mangoni and O. Taglialatela-Scafati, Tetrahedron Lett., 1995, 36, 7893 CrossRef CAS.
- D. E. N. Jacquot, P. Mayer and T. Lindel, Chem. Eur. J., 2004, 10, 1141 CrossRef CAS.
- P. S. Baran, A. L. Zografos and D. P. O'Malley, J. Am. Chem. Soc., 2004, 126, 3726 CrossRef.
- V. B. Birman and X.-T. Jiang, Org. Lett., 2004, 6, 2369 CrossRef CAS.
- R. P. Walker, D. J. Faulkner, D. Van Engen and J. Clardy, J. Am. Chem. Soc., 1981, 103, 6772 CrossRef CAS.
- P. A. Keifer, R. E. Schwartz, M. E. S. Koker, R. G. Hughes, D. Rittschof and K. L. Rinehart, J. Org. Chem., 1991, 56, 2965 CrossRef CAS.
- P. S. Baran, D. P. O'Malley and A. L. Zografos, Angew. Chem., Int. Ed., 2004, 43, 2674 CrossRef CAS.
- S. K. Collins, A. I. McDonald, L. E. Overman and Y. H. Rhee, Org. Lett., 2004, 6, 1253 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero, C. Debitus, O. Ribes, J. Pusset, S. Leroy and F. Pietra, J. Chem. Soc., Chem. Commun., 1993, 1305 RSC.
- M. M. Domostoj, E. Irving, F. Scheinmann and K. J. Hale, Org. Lett., 2004, 6, 2615 CrossRef CAS.
- A. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carte, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westley and B. C. M. Potts, J. Org. Chem., 1995, 60, 1182 CrossRef CAS.
- J. Shimokawa, K. Shirai, A. Tanatani, Y. Hashimoto and K. Nagasawa, Angew. Chem., Int. Ed., 2004, 43, 1559 CrossRef CAS.
- E. A. Jares-Erijman, R. Sakai and K. L. Rinehart, J. Org. Chem., 1991, 56, 5712 CrossRef CAS.
- S. Aoki, D. Kong, K. Matsui and M. Kobayashi, Anticancer Res., 2004, 24, 2325 CAS.
- K. Takada, T. Uehara, Y. Nakao, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Am. Chem. Soc., 2004, 126, 187 CAS.
- S. Tilvi, C. Rodrigues, C. G. Naik, P. S. Parameswaran and S. Wahidhulla, Tetrahedron, 2004, 60, 10207 CrossRef CAS.
- M. N. Masuno, A. C. Hoepker, I. N. Pessah and T. F. Molinski, Mar. Drugs, 2004, 2, 176 Search PubMed.
- G. M. Nicholas, G. L. Newton, R. C. Fahey and C. A. Bewley, Org. Lett., 2001, 3, 1543 CrossRef CAS.
- A. S. Kende, J. Lan and J. Fan, Tetrahedron Lett., 2004, 45, 133 CrossRef CAS.
- B. Fetterolf and C. A. Bewley, Bioorg. Med. Chem. Lett., 2004, 14, 3785 CrossRef CAS.
- M. V. R. Reddy, M. K. Harper and D. J. Faulkner, Tetrahedron, 1998, 54, 10649 CrossRef.
- M. Ito, M. Yamanaka, N. Kutsumura and S. Nishiyama, Tetrahedron, 2004, 60, 5623 CrossRef CAS.
- A. Rudi, Y. Benayahu and Y. Kashman, Org. Lett., 2004, 6, 4013 CrossRef CAS.
- S. Cao, Z. Gao, S. J. Thomas, S. M. Hecht, J. S. Lazo and D. G. I. Kingston, J. Nat. Prod., 2004, 67, 1716 CrossRef CAS.
- S. Aoki, D. Kong, K. Matsui, R. Rachmat and M. Kobayashi, Chem. Pharm. Bull., 2004, 52, 935 CrossRef CAS.
- R. H. Cichewicz, V. A. Kenyon, S. Whitman, N. M. Morales, J. F. Arguello, T. R. Holman and P. Crews, J. Am. Chem. Soc., 2004, 126, 14910 CrossRef CAS.
- D. E. Williams, J.-B. Telliez, J. Liu, A. Tahir, R. van Soest and R. J. Andersen, J. Nat. Prod., 2004, 67, 2127 CrossRef CAS.
- K. Warabi, L. M. McHardy, L. Matainaho, R. Van Soest, C. D. Roskelley, M. Roberge and R. J. Andersen, J. Nat. Prod., 2004, 67, 1387 CrossRef CAS.
- N. G. Prokof'eva, N. K. Utkina, E. L. Chaikina and A. E. Makarchenko, Comp. Biochem. Physiol., B: Biochem. Mol. Biol., 2004, 139, 169 CrossRef.
- N. K. Utkina, A. E. Makarchenko, O. V. Shchelokova and M. V. Virovaya, Chem. Nat. Compd., 2004, 40, 373 CrossRef CAS.
- M.-L. Kondracki and M. Guyot, Tetrahedron Lett., 1987, 28, 5815 CrossRef CAS.
- S. Aoki, D. Kong, K. Matsui and M. Kobayashi, Anti-Cancer Drugs, 2004, 15, 363 CrossRef CAS.
- I. Erdogan-Orhan, B. Sener, S. de Rosa, J. Perez-Baz, O. Lozach, M. Leost, S. Rakhilin and L. Meijer, Nat. Prod. Res., 2004, 18, 1 CrossRef CAS.
- B. N. Ravi, H. P. Perzanowski, R. A. Ross, T. R. Erdman, P. J. Scheuer, J. Finer and J. Clardy, Pure Appl. Chem., 1979, 51, 1893 CrossRef CAS.
- M. E. Castro, M. González-Iriarte, A. F. Barrero, N. Salvador-Tormo, R. Muñoz-Chápuli, M. A. Medina and A. R. Quesada, Int. J. Cancer, 2004, 110, 31 CrossRef CAS.
- D. H. Hua, X. Huang, Y. Chen, S. K. Battina, M. Tamura, S. K. Noh, S. I. Koo, I. Namatame, H. Tomoda, E. M. Perchellet and J.-P. Perchellet, J. Org. Chem., 2004, 69, 6065 CrossRef CAS.
- T. H. Hoang, P. C. Nguyen, Q. H. Tran and M. H. Le, Tap Chi Duoc Hoc, 2004, 44, 20 Search PubMed.
- A. Brust and M. J. Garson, ACGC Chem. Res. Commun., 2004, 17, 33 Search PubMed.
- D. T. A. Youssef, A. N. B. Singab, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2004, 67, 1736 CrossRef CAS.
- D. E. Williams, B. O. Patrick, A. Tahir, R. Van Soest, M. Roberge and R. J. Andersen, J. Nat. Prod., 2004, 67, 1752 CrossRef.
- V. Satitpatipan and K. Suwanborirux, J. Nat. Prod., 2004, 67, 503 CrossRef CAS.
- H. Mitome, N. Shirato, H. Miyaoka, Y. Yamada and R. W. M. van Soest, J. Nat. Prod., 2004, 67, 833 CrossRef CAS.
- J. S. Simpson, A. Brust and M. J. Garson, Org. Biomol. Chem., 2004, 2, 949 RSC.
- K. D. Wellington, R. C. Cambie, P. S. Rutledge and P. R. Bergquist, J. Nat. Prod., 2000, 63, 79 CrossRef CAS.
- K. C. Nicolaou, D. L. F. Gray and J. Tae, J. Am. Chem. Soc., 2004, 126, 613 CrossRef CAS.
- B. W. Sullivan, D. J. Faulkner, K. T. Okamoto, M. H. M. Chen and J. Clardy, J. Org. Chem., 1986, 51, 5134 CrossRef CAS.
- T. Kochi and J. A. Ellman, J. Am. Chem. Soc., 2004, 126, 15652 CAS.
- B. Harrison and P. Crews, J. Org. Chem., 1997, 62, 2646 CrossRef CAS.
- S. K. Sabui and R. V. Venkateswaran, Tetrahedron Lett., 2004, 45, 9653 CrossRef.
- A. Rudi, H. Shalom, M. Schleyer, Y. Benayahu and Y. Kashman, J. Nat. Prod., 2004, 67, 106 CrossRef CAS.
- A. Rudi, M. Aknin, E. Gaydou and Y. Kashman, J. Nat. Prod., 2004, 67, 1932 CrossRef CAS.
- V. S. P. Chaturvedula, Z. Gao, S. H. Thomas, S. A. Hecht and D. G. I. Kingston, Tetrahedron, 2004, 60, 9991 CrossRef CAS.
- W. Rungprom, W. Chavasiri, U. Kokpol, A. Kotze and M. J. Garson, Mar. Drugs, 2004, 2, 101 Search PubMed.
- T. S. Bugni, M. P. Singh, L. Chen, D. A. Arias, M. K. Harper, M. Greenstein, W. M. Maiese, G. P. Conceptión, G. C. Mangalindan and C. M. Ireland, Tetrahedron, 2004, 60, 6981 CrossRef CAS.
- A. R. Díaz-Marrero, E. Dorta, M. Cueto, A. San-Martín and J. Darias, Tetrahedron, 2004, 60, 1073 CrossRef CAS.
- R. A. Keyzers, P. T. Northcote and O. A. Zubkov, Eur. J. Org. Chem., 2004, 419 CrossRef CAS.
- S. Ankisetty, C. D. Amsler, J. B. McClintock and B. J. Baker, J. Nat. Prod., 2004, 67, 1172 CrossRef CAS.
- J. S. Simpson and M. J. Garson, Org. Biomol. Chem., 2004, 2, 939 RSC.
- A. Patra, C. W. J. Chang, P. J. Scheuer, G. D. Van Duyne, G. K. Matsumoto and J. Clardy, J. Am. Chem. Soc., 1984, 106, 7981 CrossRef.
- R. D. White, G. F. Keaney, C. D. Slown and J. L. Wood, Org. Lett., 2004, 6, 1123 CrossRef CAS.
- D. T. A. Youssef, J. Nat. Prod., 2004, 67, 112 CrossRef CAS.
- E. Elkhayat, R. Edrada, R. Ebel, V. Wray, R. van Soest, S. Wiryowidagdo, M. H. Mohamed, W. E. G. Müller and P. Proksch, J. Nat. Prod., 2004, 67, 1809 CrossRef CAS.
- K. Choi, J. Hong, C.-O. Lee, D.-K. Kim, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2004, 67, 1186 CrossRef CAS.
- M. Namikoshi, S. Suzuki, S. Meguro, H. Nagai, Y. Koike, A. Kitazawa, H. Kobayashi, T. Oda and J. Yamada, Fish. Sci. (Carlton, Aust.), 2004, 70, 152 Search PubMed.
- S.-N. Wonganuchitmeta, S. Yuenyongsawad, N. Keawpradub and A. Plubrukarn, J. Nat. Prod., 2004, 67, 1767 CrossRef CAS.
- J.-R. Rho, H.-S. Lee, H. J. Shin, J.-W. Ahn, J.-Y. Kim, C. J. Sim and J. Shin, J. Nat. Prod., 2004, 67, 1748 CrossRef CAS.
- Y. Qiu, Z. Deng, Y. Pei, H. Fu, J. Li, P. Proksch and W. Lin, J. Nat. Prod., 2004, 67, 921 CrossRef CAS.
- P. Phuwapraisirisan, S. Matsunaga, R. W. M. van Soest and N. Fusetani, Tetrahedron Lett., 2004, 45, 2125 CrossRef CAS.
- X. Fu, M. L. G. Ferreira, F. J. Schmitz and M. Kelly, J. Nat. Prod., 1999, 62, 1190 CrossRef CAS.
- L. P. Ponomarenko, A. I. Kalinovsky and V. A. Stonik, J. Nat. Prod., 2004, 67, 1507 CrossRef CAS.
- L. Chill, A. Rudi, M. Aknin, S. Loya, A. Hizi and Y. Kashman, Tetrahedron, 2004, 60, 10619 CrossRef CAS.
- A. R. Díaz-Marrero, I. Brito, M. Cueto, A. San-Martín and J. Darias, Tetrahedron Lett., 2004, 45, 4707 CrossRef CAS.
- H.-S. Lee, J.-W. Ahn, Y.-H. Lee, J.-R. Rho and J. Shin, J. Nat. Prod., 2004, 67, 672 CrossRef CAS.
- A. Umeyama, N. Shoji, S. Arihara, Y. Ohizumi and J. Kobayashi, Aust. J. Chem., 1989, 42, 459 CAS.
- S. Rifai, A. Fassouane, A. Kijjoa and R. Van Soest, Mar. Drugs, 2004, 2, 147 Search PubMed.
- A. Randazzo, C. Debitus, L. Minale, P. García-Pastor, M. J. Alcazar, M. Payá and L. Gomez-Paloma, J. Nat. Prod., 1998, 61, 571 CrossRef CAS.
- M. C. Monti, A. Casapullo, R. Riccio and L. Gomez-Paloma, Bioorg. Med. Chem., 2004, 12, 1467 CrossRef CAS.
- R. Kazlauskas, P. T. Murphy, R. J. Quinn and R. J. Wells, Tetrahedron Lett., 1976, 2631 CrossRef.
- V. Ledroit, C. Debitus, F. Ausseil, R. Raux, J.-L. Menou and B. T. Hill, Pharm. Biol. (Lisse, Neth.), 2004, 42, 454 CrossRef CAS.
- S. De Rosa, A. Crispino, A. De Giulio, C. Iodice, R. Benrezzouk, M. C. Terenicio, M. L. Ferrándiz, M. J. Alcaraz and M. Payá, J. Nat. Prod., 1998, 61, 931 CrossRef CAS.
- A. K. Cheung, R. Murelli and M. L. Snapper, J. Org. Chem., 2004, 69, 5712 CrossRef CAS.
- D. J. Faulkner, Tetrahedron Lett., 1973, 3821 CrossRef.
- K. Takabe, H. Hashimoto, H. Sugimoto, M. Nomoto and H. Yoda, Tetrahedron: Asymmetry, 2004, 15, 909 CrossRef CAS.
- C. Funel, F. Berrué, C. Roussakis, R. F. Rodriguez and P. Amade, J. Nat. Prod., 2004, 67, 491 CrossRef CAS.
- M. N. Masuno, J. R. Pawlik and T. F. Molinski, J. Nat. Prod., 2004, 67, 731 CrossRef CAS.
- T. Teruya, S. Nakagawa, T. Koyama, H. Arimoto, M. Kita and D. Uemura, Tetrahedron, 2004, 60, 6989 CrossRef CAS.
- E. Fattorusso, O. Taglialatela-Scafati, F. Petrucci, G. Bavestrello, B. Calcinai, C. Cerrano, P. Di Meglio and A. Ianaro, Org. Lett., 2004, 6, 1633 CrossRef CAS.
- G. J. Calderón, L. Castellanos, C. Duque, S. Echigo, N. Hara and Y. Fujimoto, Steroids, 2004, 69, 93 CrossRef CAS.
- M. Fouad, K. Al-Trabeen, M. Badran, V. Wray, R. Edrada, P. Proksch and R. Ebel, Arkivoc, 2004, 13, 17.
- S. Carmely, M. Roll, Y. Loya and Y. Kashman, J. Nat. Prod., 1989, 52, 167 CrossRef CAS.
- F. Lv, Z. Deng, J. Li, H. Fu, R. W. M. van Soest, P. Proksch and W. Lin, J. Nat. Prod., 2004, 67, 2033 CrossRef.
- S.-H. Qi, S. Zhang, Z.-H. Xiao, J.-S. Huang, J. Wu and Q.-X. Li, Chem. Pharm. Bull., 2004, 52, 1476 CrossRef CAS.
- P. Radhika, V. L. Rao and H. Laatsch, Chem. Pharm. Bull., 2004, 52, 1345 CrossRef CAS.
- N. Krishna, P. Muralidhar, M. M. Kumar, D. V. Rao and C. B. Rao, J. Nat. Prod., 2004, 67, 1423 CrossRef CAS.
- N. Krishna, P. Muralidhar, M. M. K. Kumar, D. V. Rao and C. B. Rao, Nat. Prod. Res., 2004, 18, 551 CrossRef CAS.
- H. Liu, J.-Y. Su and L.-M. Zeng, Zhongshan Daxue Xuebao, Ziran Kexueban, 2004, 43, 124 Search PubMed.
- M. S. Diop and A. Samb, C. R. Chim., 2004, 7, 965 Search PubMed.
- T. Řezanka, L. O. Hanuš and V. M. Dembitsky, Tetrahedron, 2004, 60, 12191 CrossRef CAS.
- D. J. Milanowski, K. R. Gustafson, M. A. Rashid, L. K. Pannell, J. B. McMahon and M. R. Boyd, J. Org. Chem., 2004, 69, 3036 CrossRef CAS.
- Y.-C. Shen, L.-T. Wang, Y.-B. Cheng, A. T. Khalil, M.-H. Chen and Y.-C. Lin, J. Chin. Chem. Soc. (Taipei), 2004, 51, 1421 CAS.
- A. F. Ahmed, J.-H. Su, R.-T. Shiue, X.-J. Pan, C.-F. Dai, Y.-H. Kuo and J.-H. Sheu, J. Nat. Prod., 2004, 67, 592 CrossRef CAS.
- Y.-C. Shen, Y.-B. Cheng, Y.-C. Lin, J.-H. Guh, C.-M. Teng and C.-L. Ko, J. Nat. Prod., 2004, 67, 542 CrossRef CAS.
- B. J. Baker, R. K. Okuda, P. T. K. Yu and P. J. Scheuer, J. Am. Chem. Soc., 1985, 107, 2976 CrossRef CAS.
- B. J. Baker and P. J. Scheuer, J. Nat. Prod., 1994, 57, 1346 CrossRef CAS.
- S. M. Verbitski, J. E. Mullally, F. A. Fitzpatrick and C. M. Ireland, J. Med. Chem., 2004, 47, 2062 CrossRef CAS.
- C. Kuhn, E. Roulland, J.-C. Madelmont, C. Monneret and J.-C. Florent, Org. Biomol. Chem., 2004, 2, 2028 RSC.
- E. Dorta, A.-R. Díaz-Marrero, M. Cueto, L. D'Croz, J. L. Maté and J. Darias, Org. Lett., 2004, 6, 2229 CrossRef CAS.
- M. Iwashima, I. Terada, K. Okamoto and K. Iguchi, J. Org. Chem., 2002, 67, 2977 CrossRef CAS.
- H. Ito, M. Hasegawa, Y. Takenaka, T. Kobayashi and K. Iguchi, J. Am. Chem. Soc., 2004, 126, 4520 CrossRef CAS.
- W. Zhang, Y.-W. Guo, E. Mollo and G. Cimino, Helv. Chim. Acta, 2004, 87, 2919 CrossRef CAS.
- C.-Y. Duh, A. A. H. El-Gamal, P.-Y. Song, S.-K. Wang and C.-F. Dai, J. Nat. Prod., 2004, 67, 1650 CrossRef CAS.
- Y. Uchio, A. Matsuo, S. Eguchi, M. Nakayama and S. Hayashi, Tetrahedron Lett., 1977, 1191 CrossRef CAS.
- M. Feller, A. Rudi, N. Berer, I. Goldberg, Z. Stein, Y. Benayahu, M. Schleyer and Y. Kashman, J. Nat. Prod., 2004, 67, 1303 CrossRef CAS.
- A. A. H. El-Gamal, S.-K. Wang, C.-F. Dai and C.-Y. Duh, J. Nat. Prod., 2004, 67, 1455 CrossRef CAS.
- J.-H. Sheu, C.-H. Chao, G.-H. Wang, K.-C. Hung, C.-Y. Duh, M. Y. Chiang, Y.-C. Wu and C.-C. Wu, Tetrahedron Lett., 2004, 45, 6413 CrossRef CAS.
- A. Ata, J. Ackerman, A. Bayoud and P. Radhika, Helv. Chim. Acta, 2004, 87, 592 CrossRef CAS.
- K. Iguchi, T. Fukaya, A. Yasumoto and K. Watanabe, J. Nat. Prod., 2004, 67, 577 CrossRef CAS ; and references cited therein.
- C. B. Rao, V. C. Sekhar, B. Sarvani and D. V. Rao, Indian J. Chem., Sect. B, 2004, 43, 1329.
- J. A. Palermo, M. F. Rodríguez Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482 CrossRef CAS.
- Y. Nakao, Y. Hirata, S. Ishihara, S. Oda, T. Yukawa, E. Shirakawa and T. Hiyama, J. Am. Chem. Soc., 2004, 126, 15650 CAS.
- J.-H. Sheu, J.-H. Su, P.-J. Sung, G.-H. Wang and C.-F. Dai, J. Nat. Prod., 2004, 67, 2048 CrossRef CAS.
- A. G. Gonzalez, J. D. Martin and M. L. Rodriguez, An. Quim. (1968–1979), 1976, 72, 1004 Search PubMed.
- X.-H. Yan, Y.-W. Guo, X.-Z. Zhu, E. Mollo and G. Cimino, Youji Huaxue, 2004, 24, 1233 CAS.
- A. Ata, H. Y. Win, D. Holt, P. Holloway, E. P. Segstro and G. S. Jayatilake, Helv. Chim. Acta, 2004, 87, 1090 CrossRef CAS.
- C. Duque, M. Puyana, G. Narváez, O. Osorno, N. Hara and Y. Fujimoto, Tetrahedron, 2004, 60, 10627 CrossRef CAS.
- A. Ata, R. G. Kerr, C. E. Moya and R. S. Jacobs, Tetrahedron, 2003, 59, 4215 CrossRef CAS.
- I. I. Rodríguez, Y.-P. Shi, O. J. Garciá, A. D. Rodríguez, A. M. S. Mayer, J. A. Sánchez, E. Ortega-Barria and J. González, J. Nat. Prod., 2004, 67, 1672 CrossRef CAS.
- K. I. Marville, W. F. Reynolds, R. L. Sealy and W. F. Tinto, Heterocycles, 2004, 63, 107 CrossRef CAS.
- F. Reyes, A. Ardá, R. Martín, R. Fernández, A. Rueda, D. Montalvo, C. Gómez, C. Jiménez, J. Rodríguez and J. M. Sánchez-Puelles, J. Nat. Prod., 2004, 67, 1190 CrossRef CAS.
- A. Fontana, M. L. Ciavatta, P. Amodeo and G. Cimino, Tetrahedron, 1999, 55, 1143 CrossRef CAS.
- X.-Q. Ma, S.-J. Yan, J.-Y. Su and L.-M. Zeng, Gaodeng Xuexiao Huaxue Xuebao, 2004, 25, 479 CAS.
- X. Wei, A. D. Rodríguez, P. Baran, R. G. Raptis, J. A. Sánchez, E. Ortega-Barria and J. González, Tetrahedron, 2004, 60, 11813 CrossRef CAS.
- A. F. Ahmed, J.-H. Su, Y.-H. Kuo and J.-H. Sheu, J. Nat. Prod., 2004, 67, 2079 CrossRef CAS.
- H. Gross, A. D. Wright, W. Beil and G. M. König, Org. Biomol. Chem., 2004, 2, 1133 RSC.
- H. Gross, S. Kehraus, M. Nett, G. M. König, W. Beil and A. D. Wright, Org. Biomol. Chem., 2003, 1, 944 RSC.
- J. Bernstein, U. Shmeuli, E. Zadock, Y. Kashman and I. Néeman, Tetrahedron, 1974, 30, 2817 CrossRef.
- S. S. Sawant, P. W. Sylvester, M. A. Avery, P. Desai, D. T. A. Youssef and K. A. El Sayed, J. Nat. Prod., 2004, 67, 2017 CrossRef CAS.
- H. Fahmy, S. I. Khalifa, T. Konoshima and J. K. Zjawiony, Mar. Drugs, 2004, 2, 1 Search PubMed.
- J. Marrero, A. D. Rodríguez, P. Baran and R. G. Raptis, Eur. J. Org. Chem., 2004, 3909 CrossRef CAS.
- J. Marrero, A. D. Rodríguez, P. Baran, R. G. Raptis, J. A. Sánchez, E. Ortega-Barria and T. L. Capson, Org. Lett., 2004, 6, 1661 CrossRef CAS.
- P.-J. Sung, M.-R. Lin, W.-C. Chen, L.-S. Fang, C.-K. Lu and J.-H. Sheu, Bull. Chem. Soc. Jpn., 2004, 77, 1229 CrossRef CAS.
- P.-J. Sung, M.-R. Lin and L.-S. Fang, Chem. Pharm. Bull., 2004, 52, 1504 CrossRef CAS.
- W. Zhang, Y.-W. Guo, E. Mollo and G. Cimino, Helv. Chim. Acta, 2004, 87, 2341 CrossRef CAS.
- S.-H. Qi, S. Zhang, H. Huang, Z.-H. Xiao, J.-S. Huang and Q.-X. Li, J. Nat. Prod., 2004, 67, 1907 CrossRef CAS.
- S.-L. Wu, P.-J. Sung, J.-H. Su, G.-H. Wang and J.-H. Sheu, Heterocycles, 2004, 63, 895 CrossRef CAS.
- P.-J. Sung, W.-P. Hu, S.-L. Wu, J.-H. Su, L.-S. Fang, J.-J. Wang and J.-H. Sheu, Tetrahedron, 2004, 60, 8975 CrossRef CAS.
- C. Tanaka, Y. Yamamoto, M. Otsuka, J. Tanaka, T. Ichiba, G. Marriott, R. Rachmat and T. Higa, J. Nat. Prod., 2004, 67, 1368 CrossRef CAS.
- A. Clastres, A. Ahond, C. Poupat, P. Potier and S. K. Kan, J. Nat. Prod., 1984, 47, 155 CrossRef CAS.
- S. C. Lievens, H. Hope and T. F. Molinski, J. Nat. Prod., 2004, 67, 2130 CrossRef CAS.
- M. T. Hamann, K. N. Harrison, A. R. Carroll and P. J. Scheuer, Heterocycles, 1996, 42, 325 CrossRef CAS.
- L. Chill, A. Rudi, Y. Benayahu, M. Schleyer and Y. Kashman, Org. Lett., 2004, 6, 755 CrossRef CAS.
- M. D. Higgs and D. J. Faulkner, Steroids, 1977, 30, 379 CrossRef.
- P. M. Weintraub, J. Heterocycl. Chem., 1993, 30, 1635 Search PubMed.
- M. L. Ciavatta, M. P. L. Gresa, E. Manzo, M. Gavagnin, S. Wahidulla and G. Cimino, Tetrahedron Lett., 2004, 45, 7745 CrossRef CAS.
- E. Dorta, A. R. Díaz-Marrero, M. Cueto, L. D'Croz, J. L. Maté, A. San-Martín and J. Darias, Tetrahedron Lett., 2004, 45, 915 CrossRef CAS.
- P. L. Julian, E. W. Meyer and H. C. Printy, J. Am. Chem. Soc., 1948, 70, 887 CrossRef CAS.
- S.-L. Wu, G.-H. Wang, C.-F. Dai and J.-H. Sheu, J. Chin. Chem. Soc. (Taipei), 2004, 51, 205 CAS.
- M. Gutiérrez, T. Capson, H. M. Guzmán, E. Quiñoá and R. Riguera, Tetrahedron Lett., 2004, 45, 7833 CrossRef CAS.
- P. Radhika, R. N. Asolkar and H. Laatsch, Nat. Prod. Res., 2004, 18, 575 CrossRef CAS.
- W. Zhang, Y.-W. Guo, E. Mollo, A. Fontana and G. Cimino, J. Nat. Prod., 2004, 67, 2083 CrossRef CAS.
- G.-H. Wang, J.-H. Su, C.-T. Chen, C.-Y. Duh, C.-F. Dai and J.-H. Sheu, J. Chin. Chem. Soc. (Taipei), 2004, 51, 217 CAS.
- G. G. Mellado, E. Zubía, M. J. Ortega and P. J. López-González, Steroids, 2004, 69, 291 CrossRef CAS.
- G. Wang, F. Li, L. Zenf, L. Ma and G. Tu, Chem. J. Chin. Univ., 1992, 13, 623 Search PubMed.
- W. Lu, L. Zeng and J. Su, Steroids, 2004, 69, 445 CrossRef CAS.
- P. Radhika, M. Cabeza, E. Bratoeff and G. García, Steroids, 2004, 69, 439 CrossRef CAS.
- L.-M. Zeng, W.-J. Lan, J.-Y. Su, G.-W. Zhang, X.-L. Feng, Y.-J. Liang and X.-P. Yang, J. Nat. Prod., 2004, 67, 1915 CrossRef CAS.
- J. Y. Su, K. H. Long, T. S. Peng and L. M. Zeng, Sci. China, Ser. B, 1988, 31, 1172 Search PubMed.
- K. Iguchi, T. Fukaya, H. Takahashi and K. Watanabe, J. Org. Chem., 2004, 69, 4351 CrossRef CAS.
- H. N. Kamel, F. R. Fronczek, N. H. Fischer and M. Slattery, Tetrahedron Lett., 2004, 45, 1995 CrossRef CAS.
- D. J. Milanowski, K. R. Gustafson, J. A. Kelley and J. B. McMahon, J. Nat. Prod., 2004, 67, 70 CrossRef CAS.
- P. Wipf, B. Joo, T. Nguyen and J. S. Lazo, Org. Biomol. Chem., 2004, 2, 2173 RSC.
- D. Alagille, R. M. Baldwin and G. D. Tamagnan, Tetrahedron Lett., 2004, 45, 6179 CrossRef CAS.
- G. R. Pettit, Y. Kamano and C. L. Herald, J. Org. Chem., 1987, 52, 2848 CrossRef CAS.
- N. Lopanik, K. R. Gustafson and N. Lindquist, J. Nat. Prod., 2004, 67, 1412 CrossRef CAS.
- N. B. Lopanik, N. Lindquist and N. Targett, Oecologia, 2004, 139, 131 Search PubMed.
- L. Peters, G. M. König, H. Terlau and A. D. Wright, J. Nat. Prod., 2002, 65, 1633 CrossRef CAS.
- L. Peters, A. D. Wright, S. Kehraus, D. Gündisch, M. C. Tilotta and G. M. König, Planta Med., 2004, 70, 883 CrossRef CAS.
- K. L. Rinehart and K. Tachibana, J. Nat. Prod., 1995, 58, 344 CrossRef CAS.
- K. L. Rinehart, J.-F. Cheng and J.-S. Lee, US Pat., 1996, 5514708.
- M. Pérez, C. del Pozo, F. Reyes, A. Rodríguez, A. Francesch, A. M. Echavarren and C. Cuevas, Angew. Chem., Int. Ed., 2004, 43, 1724 CrossRef CAS.
- K. N. Fleming and R. E. Taylor, Angew. Chem., Int. Ed., 2004, 43, 1728 CrossRef CAS.
- J. S. Carlé and C. Christophersen, J. Am. Chem. Soc., 1979, 101, 4012 CrossRef CAS.
- J. F. Austin, S.-G. Kim, C. J. Sinz, W.-J. Xiao and D. W. C. MacMillan, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5482 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, P. Di Meglio, A. Ianaro and R. Poletti, Tetrahedron, 2004, 60, 7093 CrossRef CAS.
- C. O. Miles, A. L. Wilkins, I. A. Samdal, M. Sandvik, D. Petersen, M. A. Quilliam, L. J. Naustvoll, T. Rundberget, T. Torgersen, P. Hovgaard, D. J. Jensen and J. M. Cooney, Chem. Res. Toxicol., 2004, 17, 1423 CrossRef CAS.
- H. Ishida, A. Nozawa, H. Hamano, H. Naoki, T. Fujita, H. F. Kaspar and K. Tsuji, Tetrahedron Lett., 2004, 45, 29 CrossRef CAS.
- K. Suenaga, T. Mutou, T. Shibata, T. Itoh, T. Fujita, N. Takada, K. Hayamizu, M. Takagi, T. Irifune, H. Kigoshi and K. Yamada, Tetrahedron, 2004, 60, 8509 CrossRef CAS.
- G. R. Pettit, J.-P. Xu, D. L. Doubek, J.-C. Chapuis and J. M. Schmidt, J. Nat. Prod., 2004, 67, 1252 CrossRef CAS.
- S. F. Cummins, A. E. Nichols, K. Rajarathnam and G. T. Nagle, Peptides, 2004, 25, 185 CrossRef CAS.
- X. Fu, A. J. Palomar, E. P. Hong, F. J. Schmitz and F. A. Valeriote, J. Nat. Prod., 2004, 67, 1415 CrossRef CAS.
- Md. J. Uddin, S. Kokubo, K. Suenaga, K. Ueda and D. Uemura, Heterocycles, 2001, 54, 1039 CrossRef CAS.
- Md. J. Uddin, S. Kokubo, K. Ueda, K. Suenaga and D. Uemura, J. Nat. Prod., 2001, 64, 1169 CrossRef CAS.
- M. Gavagnin, E. Mollo, T. Docimo, Y.-W. Guo and G. Cimino, J. Nat. Prod., 2004, 67, 2104 CrossRef CAS.
- K. Benkendorff, R. Pillai and J. B. Bremner, Nat. Prod. Res., 2004, 18, 427 CrossRef CAS.
- E. Manzo, M. L. Ciavatta, M. Gavagnin, E. Mollo, Y.-W. Guo and G. Cimino, J. Nat. Prod., 2004, 67, 1701 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, A. Ianora and M. Di Rosa, Eur. J. Org. Chem., 2001, 49 CrossRef CAS.
- E. A. Couladouros, V. I. Moutsos and E. N. Pitsinos, Tetrahedron Lett., 2004, 45, 7779 CrossRef CAS.
- M. Satake, K. Ofuji, H. Naoki, K. J. James, A. Furey, T. McMahon, J. Silke and T. Yasumoto, J. Am. Chem. Soc., 1998, 120, 9967 CrossRef CAS.
- K. C. Nicolaou, S. Vyskocil, T. V. Koftis, Y. M. A. Yamada, T. Ling, D. Y.-K. Chen, W. Tang, G. Petrovic, M. O. Frederick, Y. Li and M. Satake, Angew. Chem., Int. Ed., 2004, 43, 4312 CrossRef CAS.
- K. C. Nicolaou, T. V. Koftis, S. Vyskocil, G. Petrovic, T. Ling, Y. M. A. Yamada, W. Tang and M. O. Frederick, Angew. Chem., Int. Ed., 2004, 43, 4318 CrossRef CAS.
- R. R. Vardaro, V. Di Marzo, A. Crispino and G. Cimino, Tetrahedron, 1991, 47, 5569 CrossRef CAS.
- J. E. Moses, J. E. Baldwin and R. M. Adlington, Tetrahedron Lett., 2004, 45, 6447 CrossRef CAS.
- M. Gavagnin, M. Carbone, E. Mollo and G. Cimino, Tetrahedron Lett., 2003, 44, 1495 CrossRef CAS.
- V. Kulcitki, N. Ungur, M. Gavagnin, M. Carbone and G. Cimino, Tetrahedron: Asymmetry, 2004, 15, 423 CrossRef CAS.
- J. Rodríguez, R. Fernández, E. Quiñoá, R. Riguera, C. Debitus and P. Bouchet, Tetrahedron Lett., 1994, 35, 9239 CrossRef CAS.
- Y. Peng, H. W. Pang and T. Ye, Org. Lett., 2004, 6, 3781 CrossRef CAS.
- S. Acherar, G. Audran, F. Cecchin and H. Monti, Tetrahedron, 2004, 60, 5907 CrossRef CAS.
- D. E. Williams and R. J. Andersen, Can. J. Chem., 1987, 65, 2244 CAS.
- G. R. Petit, J.-P. Xu, M. D. Williams, F. Hogan, J. M. Schmidt and R. L. Cerny, Bioorg. Med. Chem. Lett., 1997, 7, 827 CrossRef CAS.
- G. R. Pettit, F. Hogan and D. L. Herald, J. Org. Chem., 2004, 69, 4019 CrossRef CAS.
- J. A. Roesener and P. J. Scheuer, J. Am. Chem. Soc., 1986, 108, 846 CrossRef CAS.
- J. S. Allingham, J. Tanaka, G. Marriott and I. Rayment, Org. Lett., 2004, 6, 597 CrossRef CAS.
- N. Saito, C. Tanaka, Y.-I. Koizumi, K. Suwanborirux, S. Amnuoypol, S. Pummangura and A. Kubo, Tetrahedron, 2004, 60, 3873 CrossRef CAS.
- A. Fontana, P. Cavaliere, S. Wahidulla, C. G. Naik and G. Cimino, Tetrahedron, 2000, 56, 7305 CrossRef CAS.
- J. E. Hochlowski, D. J. Faulkner, G. K. Matsumoto and J. Clardy, J. Org. Chem., 1983, 48, 1141 CrossRef.
- T. P. Brady, S. H. Kim, K. Wen and E. A. Theodorakis, Angew. Chem., Int. Ed., 2004, 43, 739 CrossRef CAS.
- S. W. Ayer, R. J. Andersen, C. H. He and J. Clardy, J. Org. Chem., 1984, 49, 2653 CrossRef CAS.
- L. Zhang and M. Koreeda, Org. Lett., 2004, 6, 537 CrossRef CAS.
- G. Cimino, G. Sodano, A. Spinella and E. Trivellone, Tetrahedron Lett., 1985, 26, 3389 CrossRef CAS.
- G. Cimino, G. Sodano and A. Spinella, J. Org. Chem., 1987, 52, 5326 CrossRef CAS.
- A. Fontana, A. Cutignano, A. Giordano, A. D. Coll and G. Cimino, Tetrahedron Lett., 2004, 45, 6847 CrossRef CAS.
- V. Amico, G. Oriente, M. Piattelli, C. Tringali, E. Fattorusso, S. Magno and L. Mayol, Tetrahedron Lett., 1978, 3593 CrossRef CAS.
- G. Cimino, A. Crispino, V. Di Marzo, M. Gavagnin and J. D. Ros, Experientia, 1990, 46, 767 CAS.
- A. Cutignano, V. Notti, G. d'Ippolito, A. D. Coll, G. Cimino and A. Fontana, Org. Biomol. Chem., 2004, 2, 3167 RSC.
- A. Spinella, L. A. Alvarez, A. Passeggio and G. Cimino, Tetrahedron, 1993, 49, 1307 CrossRef CAS.
- A. Cutignano, G. Cimino, A. Giordano, G. d'Ippolito and A. Fontana, Tetrahedron Lett., 2004, 45, 2627 CrossRef CAS.
- F. J. Schmitz, D. P. Michaud and P. G. Schmidt, J. Am. Chem. Soc., 1982, 104, 6415 CrossRef.
- S. Tsukamoto, Y. Yamashita, T. Yoshida and T. Ohta, Mar. Drugs, 2004, 2, 170 Search PubMed.
- M. H. Kossuga, J. B. MacMillan, E. W. Rogers, T. F. Molinski, G. G. F. Nascimento, R. M. Rocha and R. G. S. Berlinck, J. Nat. Prod., 2004, 67, 1879 CrossRef CAS.
- M. Gavagnin, F. Castelluccio, A. Antonelli, J. Templado and G. Cimino, Lipids, 2004, 39, 681 CrossRef CAS.
- T. Teruya, H. Shimogawa, K. Suenaga and H. Kigoshi, Chem. Lett., 2004, 33, 1184 CrossRef CAS.
- N. Takada, H. Sato, K. Suenaga, H. Arimoto, K. Yamada, K. Ueda and D. Uemura, Tetrahedron Lett., 1999, 40, 6309 CrossRef CAS.
- H. Kigoshi, M. Kita, S. Ogawa, M. Itoh and D. Uemura, Org. Lett., 2003, 5, 957 CrossRef CAS.
- A. V. Statsuk, D. Liu and S. A. Kozmin, J. Am. Chem. Soc., 2004, 126, 9546 CrossRef CAS.
- M. P. Foster, C. L. Mayne, R. Dunkel, R. J. Pugmire, D. M. Grant, J.-M. Kornprobst, J.-F. Verbist, J.-F. Baird and C. M. Ireland, J. Am. Chem. Soc., 1992, 114, 1110 CrossRef CAS.
- A. D. Wright, E. Goclik, G. M. König and R. Kaminsky, J. Med. Chem., 2002, 45, 3067 CrossRef CAS.
- R. A. Davis, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 2002, 65, 454 CrossRef CAS.
- X. Pu and D. Ma, Angew. Chem., Int. Ed., 2004, 43, 4222 CrossRef CAS.
- D. R. Appleton, M. J. Page, G. Lambert and B. R. Copp, Nat. Prod. Res., 2004, 18, 39 CrossRef CAS.
- S. Kehraus, S. Gorzalka, C. Hallmen, J. Iqbal, C. E. Müller, A. D. Wright, M. Wiese and G. M. König, J. Med. Chem., 2004, 47, 2243 CrossRef CAS.
- M. R. Rao and D. J. Faulkner, J. Nat. Prod., 2004, 67, 1064 CrossRef CAS.
- L. Chill, A. Rudi, Y. Benayahu and Y. Kashman, Tetrahedron Lett., 2004, 45, 7925 CrossRef CAS.
- H. Liu, S. B. Pratasik, T. Nishikawa, T. Shida, K. Tachibana, T. Fujiwara, H. Nagai, H. Kobayashi and M. Namikoshi, Tetrahedron Lett., 2004, 45, 7015 CrossRef CAS.
- M. Chbani, M. Païs, J.-M. Delauneux and C. Debitus, J. Nat. Prod., 1993, 56, 99 CrossRef CAS.
- L. S. Santos, R. A. Pilli and V. H. Rawal, J. Org. Chem., 2004, 69, 1283 CrossRef CAS.
- F. D. Schubot, M. B. Hossain, D. van der Helm, M. Païs and C. Debitus, J. Chem. Crystallogr., 1998, 28, 23 CrossRef CAS.
- P. Krishnaiah, V. L. N. Reddy, G. Venkataramana, K. Ravinder, M. Srinivasulu, T. V. Raju, K. Ravikumar, D. Chandrasekar, S. Ramakrishna and Y. Venkateswarlu, J. Nat. Prod., 2004, 67, 1168 CrossRef CAS.
- M. Marfil, F. Albericio and M. Álvarez, Tetrahedron, 2004, 60, 8659 CrossRef CAS.
- K. L. Rinehart and R. Sakai, US Pat. Appl. Publ, 2004/0059112.
- C. A. Morales, M. E. Layton and M. D. Shair, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12036 CrossRef CAS.
- X. Fu, M. B. Hossain, D. van der Helm and F. J. Schmitz, J. Am. Chem. Soc., 1994, 116, 12125 CrossRef CAS.
- X. Fu, M. B. Hossain, D. van der Helm and F. J. Schmitz, J. Am. Chem. Soc., 1995, 117, 9381 CrossRef CAS.
- K. C. Nicolaou, D. Y. K. Chen, X. Huang, T. Ling, M. Bella and S. A. Snyder, J. Am. Chem. Soc., 2004, 126, 12888 CrossRef CAS.
- T. Oishi, H. Tsuchikawa, M. Murata, M. Yoshida and M. Morisawa, Tetrahedron, 2004, 60, 6971 CrossRef CAS.
- S.-L. You and J. W. Kelly, Chem. Eur. J., 2004, 10, 71 CrossRef CAS.
- C.-G. Shin, C. Abe and Y. Yonezawa, Chem. Lett., 2004, 33, 664 CrossRef CAS.
- L. J. Perez and D. J. Faulkner, J. Nat. Prod., 2003, 66, 247 CrossRef CAS.
- S. Mahboobi, A. Sellmer, T. Burgemeister, A. Lyssenko and D. Schollmeyer, Monatsh. Chem., 2004, 135, 333 CrossRef CAS.
- R. Durán, E. Zubía, M. J. Ortega, S. Naranjo and J. Salvá, Tetrahedron, 1999, 55, 13225 CrossRef CAS.
- L. Bijeire, L. Legentil, J. Bastide, F. Darro, C. Rochart and E. Delfourne, Eur. J. Org. Chem., 2004, 1891 CrossRef CAS.
- Nilar, P. J. Sidebottom, B. K. Carté and M. S. Butler, J. Nat. Prod., 2002, 65, 1198 CrossRef.
- K. L. Rinehart, J. B. Gloer, J. C. Cook, S. A. Mizsak and T. A. Scahill, J. Am. Chem. Soc., 1981, 103, 1857 CrossRef CAS.
- K. L. Rinehart, V. Kishore, S. Nagarajan, R. J. Lake, J. B. Gloer, F. A. Bozich, K.-M. Li, R. E. Maleczka, W. L. Todsen, M. H. G. Munro, D. W. Sullins and R. Sakai, J. Am. Chem. Soc., 1987, 109, 6846 CrossRef CAS.
- E. Marco, S. Martín-Santamaría, C. Cuevas and F. Gago, J. Med. Chem., 2004, 47, 4439 CrossRef CAS.
- W. S. Jang, K. N. Kim, Y. S. Lee, M. H. Nam and I. H. Lee, FEBS Lett., 2002, 521, 81 CrossRef CAS.
- X. Doisy, D. Ifrah and P. R. Hansen, Org. Biomol. Chem., 2004, 2, 2757 RSC.
- L. Hernández Franco, E. Bal de Kier Joffé, L. Puricelli, M. Tatian, A. M. Seldes and J. A. Palermo, J. Nat. Prod., 1998, 61, 1130 CrossRef CAS.
- M. Gompel, M. Leost, E. Bal de Kier Joffe, L. Puricelli, L. Hernandez Franco, J. Palermo and L. Meijer, Bioorg. Med. Chem. Lett., 2004, 14, 1703 CrossRef CAS.
- H. Kang and W. Fenical, Tetrahedron Lett., 1996, 37, 2369 CrossRef CAS.
- S. N. Fedorov, A. M. Bode, V. A. Stonik, I. A. Gorshkova, P. C. Schmid, O. S. Radchenko, E. V. Berdyshev and Z. Dong, Pharm. Res., 2004, 21, 2307 CrossRef CAS.
- G.-Q. Li, Z.-W. Deng, J. Li, H.-Z. Fu and W.-H. Lin, J. Chin. Pharm. Sci., 2004, 13, 81 Search PubMed.
- S. Kawatake, M. Inagaki, R. Isobe, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2004, 52, 1002 CrossRef CAS.
- K. Arao, M. Inagaki, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2004, 52, 1140 CrossRef CAS.
- A. A. Kicha, N. V. Ivanchina, A. I. Kalinovskii, P. S. Dmitrenok, S. O. Kainara, D. L. Aminin, I. G. Agafonova and V. A. Stonik, Russ. Chem. Bull., 2004, 53, 447 CrossRef CAS.
- L. Zhang, X. Fan, J.-G. Shi, R. Niu and X. Li, Zhongguo Haiyang Yaowu, 2004, 23, 6 Search PubMed.
- W. Wang, H. Jang, J. Hong, C.-O. Lee, K. S. Im, S.-J. Bae and J. H. Jung, J. Nat. Prod., 2004, 67, 1654 CrossRef CAS.
- W. Wang, J. Hong, C.-O. Lee, K. S. Im, J. S. Choi and J. H. Jung, J. Nat. Prod., 2004, 67, 584 CrossRef CAS.
- E. V. Levina, A. I. Kalinovskii, P. V. Andriyashchenko, P. S. Dmitrenok, E. V. Evtushenko and V. A. Stonik, Russ. Chem. Bull., 2004, 53, 2634 CrossRef CAS.
- E. V. Levina, A. I. Kalinovskii, P. S. Dmitrenok, N. G. Prokof'eva, P. V. Andriyashchenko and V. A. Stonik, Dokl. Biochem. Biophys., 2004, 396, 171 Search PubMed.
- A. I. Kalinovskii, E. V. Levina, V. A. Stonik, P. S. Dmitrenok and P. V. Andriyashchenko, Russ. J. Bioorg. Chem., 2004, 30, 191 Search PubMed.
- A. A. Kicha, A. I. Kalinovsky, N. V. Gorbach and V. A. Stonik, Khim. Prir. Soedin., 1993, 249 CAS.
- M. Iorizzi, F. de Riccardis, L. Minale and R. Riccio, J. Nat. Prod., 1993, 56, 2149 CrossRef CAS.
- N. V. Ivanchina, A. A. Kicha, A. I. Kalinovsky and V. A. Stonik, Russ. Chem. Bull., 2004, 53, 2639 CrossRef CAS.
- J. Qi, M. Ojika and Y. Sakagami, Bioorg. Med. Chem., 2004, 12, 4259 CrossRef CAS.
- S.-L. Zhang, L. Li, Y.-H. Yi, Z.-R. Zou and P. Sun, Mar. Drugs, 2004, 2, 185 Search PubMed.
- I. Bonnard and K. L. Rinehart, Tetrahedron, 2004, 60, 2987 CrossRef CAS.
- T. V. Ovchinnikova, G. M. Aleshina, S. V. Balandin, A. D. Krasnosdembskaya, M. L. Markelov, E. I. Frolova, Y. F. Leonova, A. A. Tagaev, E. G. Krasnodembsky and V. N. Kokryakov, FEBS Lett., 2004, 577, 209 CrossRef CAS.
- S. Adolph, S. Bach, M. Blondel, A. Cueff, M. Moreau, G. Pohnert, S. A. Poulet, T. Wichard and A. Zuccaro, J. Exp. Biol., 2004, 207, 2935 Search PubMed.
- G. S. Caldwell, M. G. Bentley and P. J. W. Olive, Mar. Ecol.: Prog. Ser, 2004, 273, 97 CrossRef CAS.
- C. E. Kicklighter, J. Kubanek and M. E. Hay, Limnol. Oceanogr., 2004, 49, 430 CAS.
- E. A. John, P. Pollet, L. Gelbaum and J. Kubanek, J. Nat. Prod., 2004, 67, 1929 CrossRef CAS.
- S.-I. Kato, Y. Oba, M. Ojika and S. Inouye, Tetrahedron, 2004, 60, 11427 CrossRef CAS.
- Y.-Q. Sun and Y.-W. Guo, Tetrahedron Lett., 2004, 45, 5533 CrossRef CAS.
- Y.-Q. Sun, G. Zahn and Y.-W. Guo, Z. Kristallogr., New Cryst. Struct., 2004, 219, 121 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |