DOI:
10.1039/B513437B
(Paper)
New J. Chem., 2006,
30, 32-43
Detailed nomenclature for dendritic molecules
Received
(in Montpellier, France)
20th September 2005
, Accepted 7th November 2005
First published on 30th November 2005
Abstract
A systematical and detailed nomenclature for dendritic species (“cascadane-nomenclature”) is developed which is based on the rules of naming dendrimers introduced by G. R. Newkome in 1993. As the importance and versatility of dendritic species in organic chemistry is increasing, it is to distinguish between different dendritic architectures such as dendrons and dendrimers. We attach importance to the swift conveyance of the molecular architecture and its building units by such name-fragments as expressions in brackets, characteristic exponents and indices, as in previously successful naming methods for such molecular structure-classes as polycycles, phanes, crown compounds, podands and mechanically linked molecules, e.g. catenanes. Furthermore, the new modular nomenclature incorporates as many analogies to the IUPAC-nomenclature as possible.
Introduction
The IUPAC-nomenclature enables chemists to name molecules in an exact way. However, with the increasing complexity of molecular structures the names sometimes become less clear. Therefore, separate nomenclatures for special classes of substances (e.g. cyclophanes,1 podands,2 rotaxanes, catenanes, and molecular knots3) were established to enlarge the classic IUPAC-nomenclature.
The IUPAC- and the nodal-nomenclature4 can also be applied to dendritic structures.5 The nodal-nomenclature reduces atoms to spots which are identified in a second step. But, as a result of the high degree of branching and the large number of chemically equivalent groups, the structure of the names is not easy to recognize. That is why Newkome et al. proposed a useful specific nomenclature to describe the structural properties of dendritic species.6,7 Although the name dendrimer is somewhat misleading it was established instead of names like molecular trees8 or arborols. In many cases, the IUPAC-nomenclature is enlarged to get definite and clear names for special molecules and structures. However, some of the existing IUPAC-rules are revoked. In dendritic structures it is less important to look for the longest chain because it is almost impossible to allow conclusions to be drawn therefrom about the structural moieties of molecules.
The increasing complexity and variability of dendritic structures synthesized in recent years demand further refinements of the Newkome-nomenclature used so far so that even more diverse dendritic structures can clearly be named by standardized rules. Vice versa, the names found should be easily translated into the formula.
 |
| | Fig. 1 Simplified structure of a dendrimer (on the left) and a dendron (on the right). | |
The rules of the Newkome-nomenclature can be applied to complete molecules (called dendrimers), but only in a lesser way to smaller dendritic structures (e.g. dendrons). Nowadays, dendrons are becoming more important as educts in synthesis, intermediate products and as substituents.
Therefore, we intend to improve the Newkome-nomenclature in such a way that the names provide detailed information about the structure of dendritic molecules. The name of a dendritic structure should already show the difference between a dendron and a dendrimer. Furthermore, the number of generations should be included in the name. Finally, it should be possible to locate individual branches and structural defects in the dendritic framework.
Cascadane-nomenclature rules
The Cascadane-nomenclature for dendritic molecules which is presented here starts with the definition of a dendritic structure followed by the detailed rules.
1.
Dendritic structures contain self-resembling structural units, called fractals. These are geometrical elements which are repetitions on a different scale.
The fractal geometry of a dendrimer is shown by the Sierpinski-triangle9 (Fig. 2 top), which can be considered as an example for a dendrimer consisting of (1 → 3)-branching units.
 |
| | Fig. 2 Construction of the Sierpinski-triangle. | |
Starting with an equilateral triangle the spots in the middles of the sides are connected with each other to form four congruent triangles. Next, the triangle located in the centre is removed. The procedure described above is applied to the newly generated triangles. They are exact and reduced copies of the triangles existing in the step before. We get another graph of the Sierpinski-triangle if the spots in the middles of the triangles that are removed step by step are connected to each other. The branches of the tree shown at the bottom in Fig. 2 are growing and become longer in total from generation to generation.
A dendritic structure contains at least one unit which is based on the principle of self-resemblance. Fig. 3 shows two examples.
 |
| | Fig. 3 Examples of dendritic structures. | |
The schematic compound 1 in Fig. 3 symbolizes a highly symmetrical dendrimer. The general case is shown by compound 2 in Fig. 3: a dendritic substituent, which is called a dendron or dendryl substituent,10 is bound to a core-molecule.
2.
A dendritic structure consists of a core connected to a dendryl substituent (Fig. 3, compound 2). The core is the centre unit of the dendritic structure. It carries the dendryl substituent without being part of it.
The core unit itself can be part of larger system (Fig. 4, molecule 6). The different colours of the dendritic molecules shown in Fig. 4 symbolize various functional groups.
 |
| | Fig. 4 Examples of dendritic structures. | |
3.
To name compounds, different patterns of substitution have to be considered (Fig. 5).
 |
| | Fig. 5 Various dendritic structures. | |
Each of the molecules 7, 8 and 9 consists of a core unit that is fixed to at least two dendryl substituents of the same size and structure. In these cases the names of the molecules end in the suffix
The core unit is the structural unit of the molecule which connects the similar dendritic units in a direct way.
As is shown in compound 10, the core unit can carry different dendryl units, which are treated as substituents. Their names end in the suffix
The classification scheme in Fig. 6 illustrates the way to find the name for a dendritic structure.
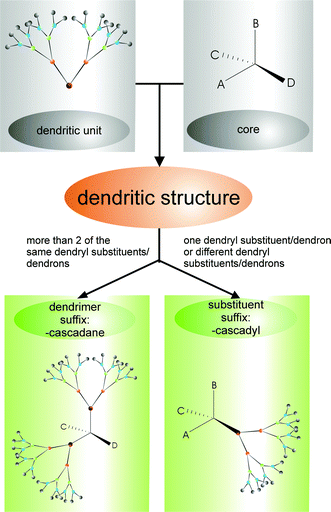 |
| | Fig. 6 Classification scheme to find names for dendritic molecules and to distinguish between cascadane and cascadyl. | |
If there are more than two dendryl substituents of a different kind in one molecule, the cascadane is named by those dendryl substituents which appear in a larger number in the molecule. If it is impossible to decide by this criterion, the dendrons with the higher mass define the cascadane according to the rules of Cahn, Ingold and Prelog.11 The remaining dendrons are treated as cascadyl-substituents.
4.
The parts of the dendryl substituent from which branchings start are called “generations”.12 This is illustrated in Fig. 7.
 |
| | Fig. 7 Dendrimer with generations 0 to 4. | |
The core is termed “generation 0” or the “0th generation”. Each generation ends at the next generation fork (see the Sierpinski-triangle, Fig. 2). The terminal groups or end groups do not represent a further generation. Molecule 11 symbolizes a dendrimer of the 4th generation carrying 48 end groups.
5.
Dendritic substituents in one molecule are listed in order of increasing length of the chain in the first generation. If a molecule consists both of non-dendritic and dendritic substituents, the non-dendritic substituents are arranged according to the rules of the IUPAC-nomenclature. The dendritic substituents follow after the non-dendritic substituents.
6.
The name of a dendritic structure consists of the name of the core unit and the names of the dendryl substituents. First, the core unit is named, then the dendryl substituents. The name of the core unit in dendrimers (cascadanes)13 and dendrons (cascadyl) is determined in accordance with the rules of the IUPAC-nomenclature. The shortest chain connecting two or more of the equal dendrons names the core unit. The name is followed by an expression written in parentheses, which contains the position of the connections to the dendrons.
 |
| | Fig. 8 Diagram of a dendritically substituted dendrimer. | |
Compound 12 consists of three dendryl substituents. Appearing twice, dendron D1 defines the molecule as a cascadane, i.e. the molecule is a dendrimer. The dendrons D1 are connected to a six-membered chain which is called hexane(1,6). The dendryl substituent D2 is treated as a cascadyl-substituent. Hence, the name for the core unit is:
| 5-(A-methyl)-3-B-1-(C-methyl)-2-(D2-cascadyl)-hexane(1,6). |
In accordance with rule 5, the dendritic substituent is put at in the end. In dendron D2 the
methyl group functions as a core unit and is mentioned in the name of this dendron.
If dendrimer and dendron have the same core unit, “0” is put in front of the name of the dendron to symbolize the shared core unit and to avoid repetition of names.
Fig. 9, shows a dendrimer (cascadane) consisting of a dendron (cascadyl-residue D2) which does not have a core unit of its own. The name is derived later (see “Example of application”).
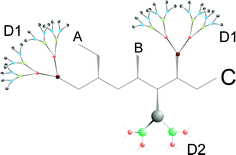 |
| | Fig. 9 Dendrimer with dendron, which has no core unit of its own. | |
7.
The enumeration of the building blocks in the dendryl substituent begins at the connection to the core unit. From there it proceeds to the groups at the end of the dendritic scaffold. The atoms of the branching units are numbered in the same way. The chain starts with the first atom after the inner branching and ends at the next branching. If the next branches are connected to different atoms the chain ends with the branching atom which is furthest away from the beginning of this chain.
 |
| | Fig. 10 Examples of the way in which the atoms are numbered. | |
The name is based not on the longest chain but on the chain which ends with the last branching when proceeding outwards from the core.
Fig. 11 shows two examples. The names of the red parts of the molecules are:
| molecule 15: 4-aza-butyl(4,4) |
| molecule 16: 4-aza-3-ethyl-butyl(4,4). |
At the end of the names of all branches there are parentheses, which contain the positions of the branchings (here: 4,4).
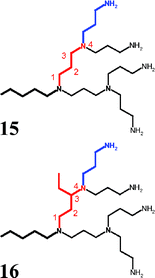 |
| | Fig. 11 Fragments of polyamine-structures. | |
8.
The individual subunits, branches or generations which connect two branching units occur in curly brackets. After the name in the term surrounded by the curly brackets there is another term in parentheses containing the positions of the branchings (see procedure for naming the core unit). Each term in curly brackets is followed by an exponent which stands for the ordinal number of the generation formed by the subunits and by an index expressing the number of subunits per generation. In the exponent “G” stands for generation. The index is completed by an “×” for “number”. The different names of the subunits are separated from each other and from the core unit by colons. The terminal groups or end groups are separated from the preceding generation by colons, too. The number of end groups is given by an index after the name. End groups are named as substituents. The atom that connects the dendritic unit does not belong to the end group.
Hence, the name of the schematic molecule 17 in Fig. 12 is:
| core(1,1):{branch A(A,A)}G12×:{branch B(B,B)}G24×:end8-cascadane. |
 |
| | Fig. 12 Simplified picture of a dendritic unit; end = end group. | |
When it is not possibile to write indices and exponents in a matrix-like scheme they might be written one after the other like this: {branch}G1,G21×,2×.
9.
If there are repetitions of a subunit in different generations, the numbers of the generations are indicated in the exponent and separated by commas. The number of subunits is mentioned in the same way. The “×” which follows the number of repetitions per generation avoids a misinterpretation of the numbers as decimal numbers. Fig. 13, shows a simplified structure named according to the rules described above:
| core (1,1):{branch A(A,A)}G1,G22×,4×:end8-cascadane. |
 |
| | Fig. 13 Variation compared with 17: branch A = branch B. | |
Repetitions of the same subunits in generations which do not follow each other directly (molecule 19) are indicated by a list of the generations in the exponent after the curly brackets. The generations are separated by commas. The number of units per generation is given in the index in the same order and separated by commas as well.
| Core(1,1):{branch A(A,A)}G1,G32×,8×:{branch B(B,B)}G2,G44×,16×: end32-cascadane. |
 |
| | Fig. 14 A simple dendritic scaffold; A = branch A, B = branch B, end = end group. | |
10.
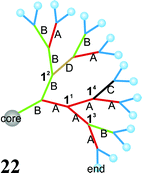
Different branches in one generation are written in angle brackets and arranged according to the increasing length of the chains. The whole term for the generation is put into curly brackets. Each term in angle brackets is followed by an index presenting the number of branches in this generation. In the index after the curly brackets the number of all subunits is given followed by a “×”. Concerning the structure of dendritic units there are two different patterns of substitution: a symmetrical pattern (e.g. molecule 20) and an unsymmetrical (e.g. molecules 21 and 22) one.
If the dendritic unit is constructed in a symmetrical way (molecule 20) the different branches are sorted according to the increasing length of the chains and indices indicating the number of repetitions are added.
 |
| | Fig. 15 Dendritic structure with different branches in one generation. | |
| Core(1,1):{branch A(1,1)}G1,G32×,8×:{branch B(1,1)}G24×:{〈branch B(1,1)〉14×〈branch C(1,1)〉2×}G416×: end32-cascadane. |
The indices after the curly brackets stand for the number of subunits in the corresponding generation.
If the branches in one generation are arranged in an unsymmetrical way, the molecule has to be cut into smaller fragments until the cascadyl-substituents are symmetric again. The slight modification is shown in molecule 21.
 |
| | Fig. 16 Dendritic structure with modified substitution pattern in comparison with the dendrimer presented in Fig. 15. | |
The name of the molecule 21 is:
| (A(1,1):{branch A(1,1)}G24×:{branch B(1,1)}G1,G32×,8×:end16-cascadyl)-(A(1,1):{branch B(1,1)}G12×:{branch A(1,1)}G24×:{〈branch B(1,1)〉6×
〈branch C(1,1)〉2×}G38×:end16-cascadyl)-core |
As the C-branches in the left dendron are distributed symmetrically, the name exactly describes the structure of molecule
21 analogous to the name of molecule
20.
If it is impossible to name a molecule because of the unsymmetrical distribution of its branches, it is also necessary to characterize the branches to make them unique. The exact location of the branches is indicated by exponents after the description of the positions of the branchings. If branching units occur repeatedly, the branching positions are listed, separated by obliques and written in parentheses. The branching unit with the highest priority (CIP-nomenclature) in the lowest generation gets the smallest exponent. The numbering is to be continued in outer generations if it is necessary.
The branches which are fixed to the numbered branching units are marked as well. The number of the branching position of the previous generation is put in front of them. The example below shows the name of dendron 22:
| core:{branch B(1,1)}:{〈branch A(11,11)〉〈branch B(12,12)〉}G12×:{11,11〈branch A(13,13/14,14)〉2×12〈branch B(14,14)〉12〈branch D(1,1)〉}G24×:{13,14,15,16〈branch A(1,1)〉4×13,15,16〈branch B(1,1)〉3×14〈branch C(1,1)}G38×:end16-cascadyl. |
 |
| | Fig. 17 Example of an unsymmetrical dendron. | |
11.
The numbering of atoms in a branch containing cyclic systems is shown in Fig. 18. The cyclic system, here a phenyl ring, is treated—according to the rules of the nodal-nomenclature—as one member of the chain. The numbering of the ring-atoms starts at the atom connected to the part of the dendritic scaffold which is next to the core unit. Following rules of the IUPAC-nomenclature, the remaining atoms are numbered. To avoid confusion, the numbers of the ring atoms are marked with a prime.
 |
| | Fig. 18 Dendron consisting of an arene-unit; core = core unit of the dendron. | |
In dendron 23 the self-resembling framework is formed by a four-membered chain and a phenyl ring. The core unit of the dendron consists of a methylene group. For the dendron which is situated next to the cones and written according to the structure formula the name is:
| (methyl(1):{5-(phenyl-3′,5′-diyl)-pentyl(3′,5′)}G1,G21×,2×:(pentyl)4)-cascadyl |
In molecule 24 the branchings are situated at the end of the chain (position 8), while the fifth member of the chain consists of an aromatic system (benzene ring). The name of the molecule is:
| (methyl(1):{5-(phenyl-3′-yl)octyl(7,7)}G1,G21×,2×:ethyl4)-cascadyl |
 |
| | Fig. 19 Dendron containing an aromatic unit, which is not a branching unit; core = core unit of the dendron. | |
Summary
1.
Dendritic structures consist of self-resembling units.
2.
A dendritic structure is made up of a core unit and a dendryl substituent.
3.
If a molecule contains only one dendryl substituent (dendron) or only different ones, the dendrons are treated as substituents. The names of the substituents are completed by the suffix “-cascadyl”. If there are at least two dendrons of the same species, a suffix identifies the molecule as “-cascadane”.
4.
Each branching defines a generation. The end groups do not form a generation.
5.
The enumeration of different dendrons starts with the dendron which consists of the shortest chain in the first generation. In the case of equality the next generation has to be observed.
6.
The name of the core unit in dendrimers is derived from the shortest chain which connects the dendrons of the same species. The positions at which the dendrons are connected to the core unit are added in parentheses directly after the name of the core unit, separated by commas from each other.
7.
The atoms in the branches of the scaffold are numbered from the inside to the outside excluding the inner branching. The chain ends at the outer branching in the self-resembling branch.
8.
The name of the core unit is followed by the name of the framework units. They are put into curly brackets. After the term in curly brackets there is an exponent consisting of a “G” and the ordinal number of the generation. Furthermore, there is an index giving the number of framework units per generation followed by an “×”. The different generations are separated by colons, both from each other and from the end groups. The number of end groups is indicated by an index.
9.
If there are repetitions of scaffold units in several generations, they are listed in the exponent after the curly brackets and separated by commas. The number of units per generation is indicated in the corresponding generation in the same way. This is also done if generations do not directly follow each other. Generations which complete the dendritic framework are listed at the end of the formula.
10.
If different scaffold units are distributed symmetrically in a generation, the term which is written in curly brackets gives their names put into angle brackets. They are sorted according to the increasing length of chains. After the angle brackets an index shows the number of this unit followed by an “×”. The index after the curly brackets gives the total number of repetitions of the units in this generation. If the framework units are distributed unsymmetrically in the generation the scaffold has to be divided into smaller dendrons. If it is necessary to know the exact location of the framework unit, exponents are added to the branching positions. Equal branchings are separated by obliques. These branching positions are put in front of the names of the framework units of the next generation. If the same branching unit occurs several times, all branching positions of the preceding generation are listed in front of the name. The number of the branches followed by a “×” is added as an index after the angle brackets.
11.
Following the nodal-nomenclature, arene units are taken as one member of the chain. The ring-atoms are numbered according to the rules of the IUPAC-nomenclature.
The formula is written in this way:
| core(a,b):{〈branch A(c,d)〉〈branch B(e,f)}G12×:{c,d,e〈branch C(g,h)〉×f〈branch D(x,x)×〉}G24×:end8-cascadane |
or
| (core(a,b):{〈branch A(c,d)〉〈branch B(e,f)}G12×:{c,d,e〈branch C(g,h)〉×f〈branch D(x,x)×〉}G24×:end8-cascadyl). |
Application of the cascadane-nomenclature compared to the Newkome-nomenclature
The following examples illustrate the differences.
(b).
Name according to the cascadane-nomenclature:
| hexane(1,1,1):{2-oxapentyl(3,3,3)}G13×:{1-oxo-2-azapropyl(3,3,3)}G29×:hydroxymethyl27-cascadane. |
Name according to the Newkome-nomenclature:
| 27-cascade:hexane[3-1,1,1]:(4-oxapentylidene):(3-oxo-2azapropylidene):methanol. |
(c).
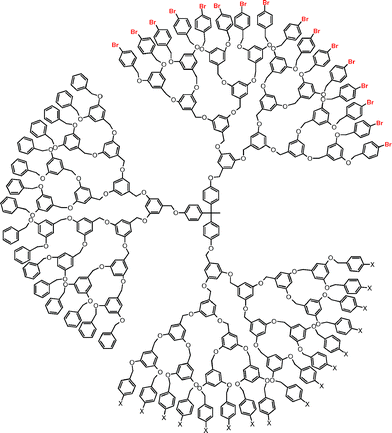
Names according to the cascadane-nomenclature:
for X = H
| 1-(phenyl(4):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-4′-bromobenzyl)16-cascadyl)-ethane(1,1)-phenyl(4):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-benzyl)32-cascadane |
for X = Br
| 1-(phenyl(4):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-benzyl)16-cascadyl)-ethane(1,1)-phenyl(4):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-4′-bromobenzyl)32-cascadane |
for X = methyl
| 1-(phenyl(4):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-benzyl)16-cascadyl)-1-(phenyl(4′):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-4-bromobenzyl)16-cascadyl)-1-(phenyl(4″):{1-oxa-3-(phenyl-3′,5′-diyl)propyl(3′,5′)}G1,G2,G3,G41×,2×,4×,8×:(1-oxa-4-methylbenzyl)16-cascadyl)-ethane. |
Names according to the Newkome-nomenclature:
for X = H
| 48-cascade:ethane[3-1,1,1]:bis[(5-(2- |
| -phenyl-2-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
| :(2-oxaethyl)benzene]-[(5-(2- |
| -phenyl-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
for X = Br
| 48-cascade:ethane[3-1,1,1]:[(5-(2- |
| -phenyl-2-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
| :(2-oxaethyl)benzene]-bis[(5-(2- |
| -phenyl-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
for X = methyl
| 48-cascade:ethane[3-1,1,1]:[(5-(2- |
| -phenyl-2-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
| :(2-oxaethyl)benzene]-[(5-(2- |
| -phenyl-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
| :4-(2-oxaethyl)-1-bromobenzene]-[(5-(2- |
| -phenyl-oxaethyl)-1,3-phenylene):(5-(2-oxaethyl)-1,3-phenylene) |
(d).
Name according to the cascadane-nomenclature:
| thiophosphor(V)(1,1,1):{1-oxa-2-phenyl(4′-yl)-2-en-3-aza-4-(methyl)aza-5-(thio)phospha-hexyl(5,5)}G1,G2,G33×,2×,2×: R24-cascadane. |
Name according to the Newkome-nomenclature:
| 24-cascade:thiophosphor(V)[3]:(1-thiophosphor(V)-2-methyl-2,3-diaza-3-en-6-oxa-5-phenyl(4′-yl)-hexylidene)3:R |
Comparison of Cascadane- and the Newkome-nomenclature
| |
Newkome-nomenclature |
Cascadane-nomenclature |
| General name |
Cascade (prefix) |
-Cascadane (suffix): more than 2 equal substituents |
| -Cascadyl (suffix): single substituent |
| |
|
Core unit
|
| Name |
According to IUPAC |
IUPAC |
| Numbering system |
IUPAC |
IUPAC |
| Multiplicity of branching |
In angle brackets (e.g. [3]) |
|
| Positions of branching |
Separated from the multiplicity of branching by hyphen |
In parentheses after the name |
| |
|
Framework
|
| Numbering of branch atoms |
From the outer branching (included) to the inner branching (excluded) |
From the inner branching (excluded) to the outer branching (included) |
| Multiplicity of branching |
In the name of the branch |
Indicated by the number of branching atoms |
| Naming branching positions |
Automatically: first atom of branch |
In parentheses after the name of branch |
| Naming of generations |
No |
Exponent after the term containing the branches |
| Repetitions of branching |
Exponent behind the term containing the branches (only possible when direct sequence of equal branches) |
List of generations in the exponent behind the term in curly brackets |
| Branching units per generation |
|
Index (number +
“×”) behind the term in curved brackets |
| Repetitions of branches |
Colon |
Colon |
| |
|
End groups
|
| Number |
In front of the name of molecule |
Index after the end groups |
| Name |
IUPAC, end group treated as free molecule |
IUPAC |
Example of application
For further explanation the rules are applied to some compounds. In Fig. 9 is presented a dendrimer (cascadane) carrying a dendron (cascadyl substituent) at position 2 of its core unit. This dendron does not have a core unit of its own. So the core unit of the dendrimer is used twice: on the one hand it is the core unit of the dendrimer, on the other hand it is the core unit of the dendron. The name of the core unit is:
| 5-(A-methyl)-3-B-1-(C-methyl)-2-(0:D2-cascadyl)-hexane(1,6). |
In this formula, “0” stands for the core unit of the cascadyl-substituent D2 demonstrating the absence of a further core unit.
Furthermore, the procedure for naming dendrons is illustrated in a concrete form by the molecules in Fig. 20. Both molecules consist of a focal spot ( ) and four dendrons.
) and four dendrons.
 |
| | Fig. 20 Examples of the explanation of names. | |
The points of interest in both cases are the dendrons on the right sides of the molecules, those with their structural formulae displayed. Generally, all dendrons are different ( ≠
≠ ≠
≠ displayed structural formula). For that reason, all dendrons have to be treated as cascadyl-substituents of the core unit (
displayed structural formula). For that reason, all dendrons have to be treated as cascadyl-substituents of the core unit ( ). In the case of Fig. 20a the dendron is fixed to the focal unit (
). In the case of Fig. 20a the dendron is fixed to the focal unit ( ) via a methylene bridge (m) which functions as the core unit for the dendron. The name for the dendron is:
) via a methylene bridge (m) which functions as the core unit for the dendron. The name for the dendron is:
| (methyl):{5-phenyl(3′,5′)-pentyl(3′,5′)} |
In the molecule shown in Fig. 20b, the dendron shares the core unit ( ) with the other dendrons. The name is now:
) with the other dendrons. The name is now:
| X-(0:{5-phenyl(3′,5′)-pentyl(3′,5′)}G1,G21×,2×:pentyl5-cascadyl). |
“X” indicates the position of the core unit (

) to which the dendron is connected. The “0” in front of the name of the dendron underlines the fact that there exists a higher structural unit.
Simplified names for an overview of the structural properties of dendritic molecules
Naming dendrimers of highly complex structures or higher generations is difficult and rather time-consuming because there are various rules which have to be obeyed. Therefore, it seems to be reasonable to use short names (simplified names) as they are usually used in the daily conversations in the laboratory. We do not want to introduce trivial names which differ widely from the existing nomenclature. We think of simplified names in which specific parts of the exact names of the molecules are left out. Molecules could roughly be described even if it is not possible to name each structural subunit or assign each scaffold unit to the dendritic structure. In discussions about dendritic molecules the types and numbers of end groups or generations are very important.
That is why we propose arranging defined building blocks in a logical sequence to name dendritic structures. A simple scheme is shown in Fig. 21. The rectangles contain only available data (e.g. core, branching units, end groups, etc.).
 |
| | Fig. 21 Simplified scheme to name dendritic molecules. | |
In many cases it is easy to put these data into the scheme. Other details may be left out for the moment if it is difficult to name those structural units according to the rules of nomenclature. The name might be fragmentary because it does not supply all details, but it is clear enough to be used in everyday life. Simplified names have been used in chemistry for years, but not in the consequent way that is proposed in this paper.
The modular construction system described above may possibly be used to develop a “universal nomenclature” for all classes of chemical substances. Structural units which are difficult to name or are not yet classified may simply be left out. Provided that there is a name for the body of the compound, communication may be simplified because the most important properties of compounds can directly be derived from these names. Dividing complete names into fragments may support electronic storage and research for chemical compounds.
Finally, we want to present the complete procedure, including the reduced one, for naming the most popular and commercially available dendritic molecules.
Fig. 22 shows a dendrimer of the poly(propylene amine)amine type (short: POPAM-dendrimer or PPI, commercially available14 as “DAB-Am-16, Polypropylenimine hexadecaamine Dendrimer, Generation 3.0”).
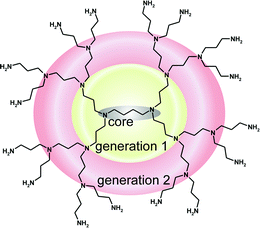 |
| | Fig. 22 POPAM-dendrimer of the 2nd generation. | |
The complete name according to the Newkome-nomenclature is:
| 16-cascade:1,4-diaminobutane[4-N,N,N′,N′]:(1-azabutylidyne)2:aminopropane. |
Following the cascadane-nomenclature the name is:
| 1,4-diaminobutane[N,N,N′,N′]:{4-azabutyl(4,4)}G1,G24×,8×:3-aminopropyl16-cascadane. |
Sometimes it is not necessary to provide all structural details to describe a dendritic scaffold. In all these cases simplified names are helpful because they contain only the most significant data. Different ways reducing a description are illustrated in Fig. 23. Complexity and completeness are decreasing from top to bottom.
 |
| | Fig. 23 Simplifying the name of a POPAM-dendrimer (2nd generation). | |
In the same way the name of a dendrimer of the poly(amido)amine-type (short: PAMAM-dendrimer, Fig. 24, commercially available as “PAMAM-50% C12 Dendrimer, Generation 3 Solution”14) can be determined and simplified.
 |
| | Fig. 24 2nd generation of a PAMAM-dendrimer. | |
The Newkome-nomenclature defines the name as follows:
| 16-cascade:1,4-diaminobutane[4-N,N,N′,N′]:(1,4-diaza-5-oxo-heptylidyne)2:aminoethane. |
The complete name according to the rules of the new cascadane-nomenclature is:
| 1,4-diaminobutane[N,N,N′,N′]:{4,7-diaza-3-oxo-heptyl(7,7)}G1,G24×,8×:3-aminoethyl16-cascadane. |
 |
| | Fig. 25 Simplifying the name of a PAMAM-dendrimer (2nd generation). | |
Simplified names allow chemists to compare different compounds without knowing each detail concerning their structures.
Conclusion
The nomenclature for dendrimers, for the first time introduced by Newkome,6,7 can successfully be applied to highly symmetrical dendrimers. However, new synthetic methods lead to a large diversity of structures which can only ponderously be named by the Newkome-nomenclature.
Improving the existing nomenclature, the new cascadane-nomenclature enables chemists to treat dendritic structures either as dendrimers or as dendrons. This is already expressed in the names, and is quite useful in everyday life. The fact that atoms in a dendritic framework are numbered in a consequent order (always from the inside to the outside) underlines the radial structure which begins in the centre, focal spot or core unit. By including the number of generations in the names of the dendritic structures it becomes obvious that these molecules are shaped in the form of a continuously peeled apple-skin. The indices and positions of branchings express the multiplicity of bonding. We avoided square brackets because they normally indicate polycyclic compounds. Multiplicities of bonding are given by multiplying the quantity of branching positions together with the number of scaffold units per generation. They occur in the indices after the curly brackets. Avoiding numbers which do not represent the regular function (e.g. multiplicity of bonding) leads to very clear names. If we locate functional groups, branching units and branches and combine those numbers, it is possible to describe unsymmetrically constructed dendritic scaffolds and structural defects.
In most cases it is not necessary to give the complete names. Here, the cascadane-nomenclature offers the opportunity to simplify the name to provide information about the most important structural properties of the dendritic part of the molecule. So it is much easier for chemists to compare different dendrimers consisting of a similar framework or having the same number of end groups.
Perspective
There is rapid progress in the research on dendrimers, and it is not easy to predict its development. However, there will be better methods of synthesis, and the variability of structures will increase as well, and dendritic molecules will fulfil new functions. Therefore, it is absolutely necessary to improve the nomenclature to organize the complex research on dendrimers.
In supramolecular chemistry dendrimers will also become more and more important. The host–guest-function, for example, may be integrated into the cascadane-nomenclature presented in this paper with the help of the signs introduced by J.-M. Lehn.15 According to J.-M. Lehn the sign “⊂” symbolizes inclusion while “∩” stands for penetration. The scheme shown in Fig. 21 may be extended by a host–guest-connection and so it would be possible to name supramolecular systems, too. The host would stand in front of the sign resembling a bracket. This symbolizes a cavity as is known from cryptand-complexes.
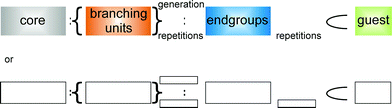 |
| | Fig. 26 Extended scheme to name supramolecular dendritic structures. | |
As these systems are very dynamic, localizing hosts and determining the stoichiometrical properties of dendritic host–guest complexes will be a big challenge for chemists. If it is possible to locate the docking positions of guests in dendritic hosts, these positions might be marked analogically to the branchings in a dendritic framework.
In principle it would be reasonable to provide information about the purity (dispersity, other impurities) of dendrimers and other multiply-branched substances produced in bulk. But this is not the purpose of systematic nomenclatures because they give molecules with single names. Impure compounds are mixtures of different species. However, in daily use, it is important to inform customers about the purity of a sample or compound as it is usual for analytical or pharmaceutical materials. This should be done separately by adding the degree of purity (e.g. in percent), by a list of identified impurities which still remain in the compound or by a specification such as p.a., techn., puriss., purum, etc. This is not only a problem for dendrimers, it is a problem for each chemical substance as well.
Acknowledgements
We thank Dr Uwe Hahn (Strasbourg) and Gero Paul (Königswinter) for stimulating discussions.
References
-
F. Vögtle, Cyclophane Chemistry, Wiley & Sons, Chichester, 1993 Search PubMed.
- E. Weber and F. Vögtle, Inorg. Chim. Acta, 1980, 45, L45–L67 CrossRef.
- O. Safarowsky, B. Windisch, A. Mohry and F. Vögtle, J. Prakt. Chem. (Weinheim, Ger.), 2000, 342(5), 337–342 Search PubMed.
- N. Lozac’h, A. L. Goodson and W. H. Powell, Angew. Chem., 1979, 91(12), 951–1032 CrossRef.
-
(a)
G. R. Newkome, C. N. Moorefield and F. Vögtle, Dendritic Molecules: Concepts, Synthesis, Perspectives, VCH-Verlag, Weinheim, 1996 Search PubMed;
(b)
G. R. Newkome, C. N. Moorefield and F. Vögtle, Dendrimers and Dendrons, Wiley-VCH, Weinheim, 2001 Search PubMed;
(c)
M. J. Fréchet and D. A. Tomalia, Dendrimers and other Dendritic Polymers, Wiley & Sons, New York, 2001 Search PubMed.
- G. R. Newkome, G. R. Baker, J. K. Young and J. G. Traynham, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 641 CrossRef CAS.
- G. R. Newkome and G. R. Baker, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1994, 35, 6.
- N. Ardoin and D. Astruc, The molecular Trees: from Syntheses towards Applications, Bull. Soc. Chim. Fr., 1995, 132, 875 CAS.
- W. Sierpinski, C. R. Hebd. Seances Acad. Sci., 1915, 160, 302–305 Search PubMed.
- F. Vögtle, M. Plevoets, G. Nachtsheim and U. Wörsdörfer, J. Prakt. Chem./Chem.-Ztg., 1998, 340, 112.
- R. S. Cahn, C. K. Ingold and V. Prelog, Angew. Chem., 1966, 78, 413–447 CrossRef.
- D. A. Tomalia, H. Baker, J. Deweld, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J. (Tokyo), 1985, 17, 117 Search PubMed.
- According to the pioneer work of E. Buhleier, W. Wehner and F. Vögtle, Synthesis, 1978, 155–158 Search PubMed.
- SigmaAldrich, Handbuch 2005–2006, Deutschland/Österreich, p. 816, Aldrich Cat. No. 595861.
-
J.-M. Lehn, Supramolecular Chemistry, VCH, Weinheim, 1995 Search PubMed.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2006 |
Click here to see how this site uses Cookies. View our privacy policy here. 


















 -5-
-5- -pentyl(5,4)}G1,G31×,4×:{1-
-pentyl(5,4)}G1,G31×,4×:{1- -5-
-5- -pentyl(5,5)}G2,G42×,8×:end16-cascadane.
-pentyl(5,5)}G2,G42×,8×:end16-cascadane.
 -butylidene):(1-
-butylidene):(1- -5-
-5- -pentylidene:1-
-pentylidene:1- -5-
-5- -6-
-6- -hexylidene))2:end16.
-hexylidene))2:end16. ) and four dendrons.
) and four dendrons.
 ≠
≠ ≠
≠ displayed structural formula). For that reason, all dendrons have to be treated as cascadyl-substituents of the core unit (
displayed structural formula). For that reason, all dendrons have to be treated as cascadyl-substituents of the core unit ( ). In the case of Fig. 20a the dendron is fixed to the focal unit (
). In the case of Fig. 20a the dendron is fixed to the focal unit ( ) via a methylene bridge (m) which functions as the core unit for the dendron. The name for the dendron is:
) via a methylene bridge (m) which functions as the core unit for the dendron. The name for the dendron is:  ) with the other dendrons. The name is now:
) with the other dendrons. The name is now:  ) to which the dendron is connected. The “0” in front of the name of the dendron underlines the fact that there exists a higher structural unit.
) to which the dendron is connected. The “0” in front of the name of the dendron underlines the fact that there exists a higher structural unit.