Assembly modes in the solid state structure of the complexes of melamine mono-cations with para-calix[4]arene sulfonic acid and calix[4]arene dihydroxyphosphonic acid
Adina N.
Lazar
a,
Oksana
Danylyuk
b,
Kinga
Suwinska
b and
Anthony W.
Coleman
*a
aInstitut de Biologie et Chimie des Protéines, CNRS UMR 5086, 7 passage du Vercors, F-69367 Lyon Cedex 07, France. E-mail: aw.coleman@ibcp.fr; Fax: +33-4-72-72-26-01
bInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka, 44/52, PL-01 224 Warszawa, Poland. Fax: 48 223433333
First published on 10th November 2005
Abstract
The crystal structures of the complexes between melamine mono-cations and para-calix[4]arene sulfonic acid and calix[4]arene dihydroxyphosphonic acid have been determined. In both cases the structure is based on a bilayer motif of calixarene molecules intercalated by motifs of melamine mono-cations. While in the complex with calix[4]arene dihydroxyphosphonic acid the motifs of melamine cations are parallel to the in-layer arrangement of the calixarene, for para-sulfonatocalix[4]arene there is 17.6° displacement between the axes of the two motifs generating a laminate structure.
Introduction
It is well known that non-covalent interactions play a determinant role in the functioning of natural macromolecules.1 Only relatively recently, however, have such interactions been exploited in the self-assembly of predefined supramolecular structures in the course of crystal engineering.2,3Melamine and its derivates have been widely used as precursors for building blocks in supramolecular chemistry, generating a wide range of structural motifs, including cyclic rosettes,4–6 linear or crinkled tapes7,8 and molecular strands or ribbons.9–13
Calix[4]arenes, such as para-calix[4]arene sulfonic acid and calix[4]arene dihydroxyphosphonic acid, have also shown a great capacity to generate a wide range of structural variations. A large body of studies exist on the solid structure of para-calix[4]arene sulfonic acid and its salts.14 The first structure, that of the sodium salt, was determined by Atwood in 1988; here the structure was a bilayer arrangement in which calixarene molecules alternated in orientation. The generation of bilayers with sulfonate groups facing into a hydrophilic environment containing water and the sodium cations included in a more hydrophobic core composed of the macrocycles, led to this structure being named an organic clay.15 A number of more complex structural motifs have been determined ranging from traversed bilayers,16 resembling bilayer membranes, through tubes17 to spheres18 and other geometrical solids.19
For calix[4]arene dihydroxyphosphonic acid, all of the observed structures have been based on interdigitation of aromatic rings producing an interpenetrating dimeric structural motif. A wide range of packing systems has been observed: in the complex with 1,6 diaminohexane,20 the dimeric calixarene motif is encapsulated in a cage; with 1,3-propanediamine the complex formed contains an aquapore.21 The dimeric calixarene motif forms cages holding π–π stacked dimers for the complex with phenanthroline.22 In the case of the complex with 2,2′-bipyridine, 4,4′-bipyridine and 1,2-bis(4-pyridyl)ethane23 1D ladder structures have been observed. Using melamine as a base and the above two calix[4]arenes as the acid, we present here the solid state structures of their complexes, showing subtle and interesting variations in the assembly mode.
Experimental section
To a solution of melamine (0.01 M) in water, a solution of para-calix[4]arene sulfonic acid (0.01 M) in methanol in the first case and of calix[4]arene dihydroxyphosphonic acid (0.01 M) in ethanol, in the second case, is added so as to form interfacial layers of the two solutions. Crystals were obtained by slow diffusion of the solvents at room temperature after several days.X-ray diffraction
Intensity data were collected at 100(2) K on a Nonius KappaCCD diffractometer using MoKα radiation (λ = 0.71073 Å). Data were corrected for Lorentz and polarisation effects but not for absorption. The structure was solved by direct methods and Fourier techniques (SHELXS-86). H-atoms were included in geometric positions and refined as ‘riding’ atoms (except those of hydroxyl groups which were located on Fourier difference maps with positional parameters refined) with isotropic thermal parameters based upon the corresponding bonding carbon atom [Uiso = 1.2 Ueq, Uiso = 1.5 Ueq for CH3 and OH hydrogens].†Results
In the complex between melamine mono-cations and para-calix[4]arene sulfonic acid (A), one molecule of calixarene is associated with four melamine cations and fifteen water molecules from which six have partial occupancies. For the complex between melamine mono-cations and calix[4]arene dihydroxyphosphonic acid (B), the unit cell comprises three melamine cations, one molecule of calixarene dihydroxyphosphonic acid, nine water molecules and one molecule of ethanol (all solvent molecules have full occupancies). Charge equilibrium in both structures is realized by protonation of one aromatic nitrogen atom of melamine.Both solid-state structures are generated by bilayer structural motifs of the calixarene molecules. The complex between para-calix[4]arene sulfonic acid and melamine mono-cations(A) is based on bilayers of calixarene molecules (see Fig. 1a) which are held together by π–π interactions between facing aromatic rings (3.62 Å and 3.43 Å, black) and by two hydrogen bonds of 2.88 (blue lines) and 3.30 Å (green) between the phenolic and the sulfonate groups of the same neighboring aromatic cycles.
![View of the molecular packing describing the two complexes: (a) planar bilayers of p-calix[4]arene sulfonic acid in complex with melamine mono-cations (A); (b) monolayer of calix[4]arene dihydroxyphosphonic acid dimers in the complex B.](/image/article/2006/NJ/b510232b/b510232b-f1.gif) | ||
| Fig. 1 View of the molecular packing describing the two complexes: (a) planar bilayers of p-calix[4]arene sulfonic acid in complex with melamine mono-cations (A); (b) monolayer of calix[4]arene dihydroxyphosphonic acid dimers in the complex B. | ||
In the case of complex B, the bilayers are formed of interpenetrated dimers of calix[4]arene dihydroxyphosphonic acid. The dimeric motif is described by the interdigitation of the phenolic rings, with the interdigitated para-carbon atom forming an angle of 153.4° with the two para-carbon atoms of the interpenetrating calixarene. One of the phosphonic acid groups forms a strong hydrogen bond with the neighbouring phosphonate group (PO–H⋯OP = 2.53 Å) (Fig. 1b) that further stabilizes the crystal motif in complex B. The bilayers are of about 10 Å thickness in A and of about 11 Å in B. The distance spanned by two layers of the calix[4]arene bilayer motifs changes from 18.29 Å to 14.89 Å, between complex A and B.
The set of interactions between the melamine mono-cations and their environment, in complex A, shows high diversity. In Fig. 2, the complete network of hydrogen bonds encountered by melamine mono-cations is presented. Normally, melamine may be considered as having three faces for non-covalent linking each of which can donate two hydrogen bonds from each amine groups and accept one on each aromatic nitrogen atom. However in the two current structures mono-protonation of one aromatic nitrogen atom occurs, meaning that it becomes also a hydrogen bond donor. There are four independent melamine mono-cations in the complex A arranged in a non-crystallographic pseudo-mirror symmetry (see in Fig. 2 lateral centro-symmetric molecules coloured in green and in turquoise). A set of five pairs of hydrogen bonds between neighbouring melamine mono-cations and four quintets of hydrogen bonds with solvent molecules or the sulfonate groups are further generated (for each of melamine cations). Even if there is only one calixarene in the unit cell of A, the large cavity of this macromolecule and the numerous polar groups that it carries seems to be the factor generating such a variation in hydrogen bond interactions: one of the melamine mono-cations shows hydrogen bonds with four water molecules and only one with a sulfonate group; all the three other melamine mono-cations interact, by hydrogen bonds, with two solvent molecules and with three sulfonate groups (distances in Fig. 2). It is worth mentioning that the protonated nitrogen forms bridges only with water molecules.
 | ||
| Fig. 2 View along Ob axis of the network of interactions encountered by melamine mono-cations in the complex A: interactions between melamine mono-cations in blue, melamine mono-cations–solvent in magenta and melamine mono-cations–calixarene in green. | ||
Although the melamine mono-cations lie in a plane, the orientation is not parallel to the molecules of para-calix[4]arene sulfonic acid. This shift of orientation of melamine sheets with respect to the alignment of calixarenes in the bilayer, (of a value of 17.6°) (Fig. 3), may be compared to a matrix of bi-directional laminates.24 Such packing in chains has been previously observed for melamine complexes;13 however the unusual element is the non-parallel arrangement, generally associated to a disorder in the structure.
 | ||
| Fig. 3 Representation of non-parallel laminate arrangement in complex A (lateral and top view along Oc axis, respectively). | ||
No less complex is the solid-state structure of B. The dimeric layers of calixarene alternate with sheets of parallel chains of melamine mono-cations (Fig. 4), two of which are facing the phosphonic groups (coloured in turquoise and designated 1) and another one intercalated between the two (coloured in green and designated 2). The melamine mono-cations are related by two elements of symmetry: mirror plus rotation, as represented by the dashed frame in Fig. 4 with melamine mono-cations coloured in different shades as a function of symmetry.
 | ||
| Fig. 4 View of melamine mono-cation parallel chains; in blue the ones facing the phosphonic groups and in green, the intercalated chain; the difference in colour shades and the dashed lines are illustrating the pseudo-symmetry elements characterizing melamine cations. | ||
The network of hydrogen bonds that characterizes chain 1 and its environment involves: three solvent molecules and two phosphonate groups per melamine mono-cation (see Fig. 5a). Chain 2 shows hydrogen bonds only with water molecules. Only one water molecule (indicated by an arrow in the figure) is involved in hydrogen bonds with melamine mono-cations from both of the chains, forming inter-chain bridges in the melamine mono-cations sheets. The other solvent molecules participate in intra-chain bridging on melamine mono-cations.
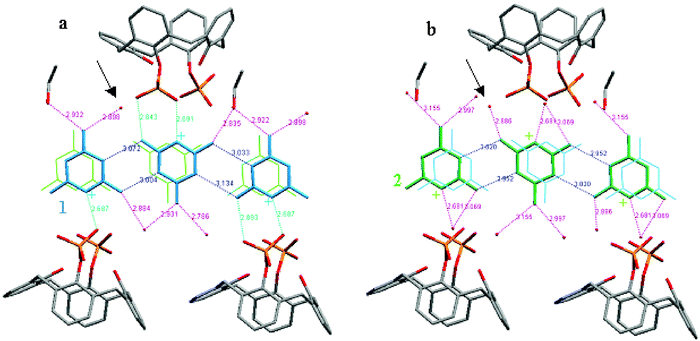 | ||
| Fig. 5 The network of interactions encountered by melamine molecules in the complex B: (a) for the chain 1; (b) for the chain 2; interactions between melamine mono-cations are coloured in blue, melamine cations–solvent in magenta and melamine cations–calixarene in green, respectively; the protonated aromatic nitrogens are symbolized with “+”. | ||
Double pair interactions between the melamine mono-cations are also present in complex B, for the two chains, but much weaker than in A (see Fig. 5 the hydrogen bonds between the melamine mono-cations marked in blue). Here, the protonated nitrogen atoms are involved in H-bonds with oxygens of the phosphonate groups (as in the case of two melamines mono-cations of the chain 1) as well as with water (for melamines mono-cations of chain 2).
These melamine mono-cations are still co-planar, as previously observed, but in contrast to A, they form tapes that are parallel to the dimeric layer of calixarene diphosphonate (Fig. 6, dashed frame). Perpendicular to those tapes, stabilized by the diverse set of hydrogen bonds, the melamine mono-cations generate continuous zig-zag chains in the complex B (Fig. 6). These chains of melamine mono-cations are characterised by shifted face-to-face aromatic interactions.
 | ||
| Fig. 6 Representation of melamine mono-cations sheets parallel to calixarene diphosphate dimers and of the zig-zag chains formed by shifted melamine mono-cations. | ||
Additional hydrogen bonds occurring in the two solid-state structures that play an important role in the rigidity of the complexes are presented in Tables 1 and 2, along with the angles of hydrogen bridging.
| Compound | C4S-Melamine | C4diP-Melamine |
|---|---|---|
| Formula | C40H66N24O28.3S4 | C39H68N18O20P2 |
| FW | 1467.41 | 1171.05 |
| Temperature, K | 100 (2) | 100 (2) |
| Colour | Colourless | Colourless |
| Crystal size, mm | 0.20 × 0.05 × 0.05 | 0.28 × 0.15 × 0.03 |
| Crystal system | Triclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/c |
| a/Å | 11.1991(6) | 22.1727(9) |
| b/Å | 15.293(1) | 12.4313(7) |
| c/Å | 19.008(1) | 19.508(1) |
| α/° | 75.286(4) | 90 |
| β/° | 86.453(4) | 103.281(3) |
| γ/° | 82.786(3) | 90 |
| V/Å3 | 3122.3(3) | 5233.3(5) |
| Z | 2 | 4 |
| ρ calc/mg m−3 | 1.561 | 1.486 |
| F(000) | 1532 | 2472 |
| μ (MoKα) mm−1 | 0.258 | 0.117 |
| θ range for data collection/° | 2.72 to 22.02 | 3.00 to 21.98 |
| Completeness to θ = 22.02 | 97.8% | 99.0% |
| Reflections collected/unique [Rint = 0.068] | 27 163/7510 | 55 429/6336 |
| Absorption correction | none | none |
| Data/restraints/parameters | 7510/0/896 | 6336/0/751 |
| Goodness-of-fit on F2 | 1.080 | 1.005 |
| Final R indices [I > 2σ(I)] | R = 0.069, wR = 0.148 | R = 0.056, wR = 0.098 |
| R indices (all data) | R = 0.106, wR = 0.116 | R = 0.101, wR = 0.110 |
| Extinction coefficient | 0.0022(4) | 0.0019(2) |
| Largest diff. peak and hole | 0.54 and −0.32 e Å−3 | 0.24 and −0.32 e Å−3 |
| Compound | C4S–Melamine | C4diP–Melamine |
|---|---|---|
| N–H+⋯A distance [Å] | 1.84; 1.84; 1.97; 1.92 | 1.83; 1.83; 1.84 |
| N–H+⋯A angle [°] | 168.2; 165.5; 146.4; 165 | 159.8; 163.6; 161.8 |
| S–O⋯O distance [Å] | 2.88; 2.75; 2.76; 2.98; 2.97 | |
| 2.9; 2.78; 2.84; 2.77 | ||
| 2.81; 2.79; 2.74 | ||
| 2.77; 2.76; 2.78; 2.73 | ||
| S–O⋯O angle [°] | 158.3; 149.6; 148.8; -; - | |
| 129.3; 166.4; 162.1; 143.4 | ||
| 165.7; 173.8; 148.4 | ||
| 169.4; 174.8; 152.2 | ||
| S–O⋯N distance [Å] | 2.91; 2.87 | |
| 2.99; 2.89 | ||
| 2.92; 3.01; 3.02 | ||
| 2.82; 3.05 | ||
| S–O⋯N angle [°] | 149.9; 168.3 | |
| 124.2; 136.5 | ||
| 137.1; 150; 123.8 | ||
| 146.3; 172.5 | ||
| P–O⋯O distance [Å] | 2.75; 2.77; 2.54; 2.75 | |
| 2.73; 2.78; 2.54; 2.94; 2.77 | ||
| P–O⋯O angle [°] | 153.5; 171.9; 165.9; 172.3 | |
| 164.8; 150.2; 166.4; 166.9; 153.4; | ||
| P–O⋯N distance [Å] | 2.69; 2.89 | |
| 2.69; 2.84 | ||
| P–O⋯N angle [°] | 161.8; 164.2 | |
| 168.8; 163.6 |
Thus double aromatic stacking is present in the system with diphosphonic acid as well as in the one with calixarene sulfonate. As seen in Fig. 7, the π–π stacking of melamine mono-cations is less compact in complex A (values between 3.64–3.79 Å) than in B (values in the range of 3.50–3.71 Å). The linear arrangement of melamine mono-cations involved in face-to-face aromatic interactions in the complex A as well as the zig-zag disposition of melamine mono-cations in the complex B are illustrated in Fig. 7.
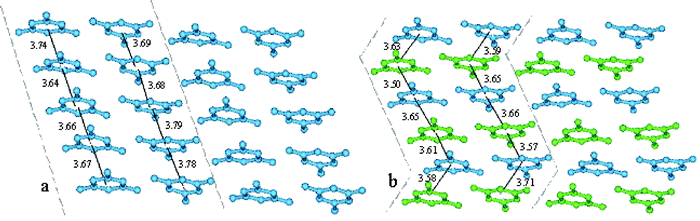 | ||
| Fig. 7 (a) Sheets of melamine mono-cations formed by linear chains of π–π stacked aromatic rings; (b) Sheets of melamine mono-cations formed by zig-zag chains of π–π stacked aromatic rings; the melamine cations are coloured similar to Figs. 4 and 5 in order to put into evidence the zig-zag step as function of their position with respect to calixarene matrix (in turquoise, the melamine cations interacting with the phosphonate group and in green the intercalated ones). | ||
Conclusion
The two solid-state systems show the role played by the disposition of the anionic groups of sulfonic acid and of dihydroxyphosphonic acid calixarene in their complexes with melamine mono-cations. Both architectures are based on the ubiquitous motif that characterizes the crystal structures of the two calixarene derivates, i.e. the bilayer arrangement in the case of calix[4]arene sulfonic acid and the dimeric layer specific to calix[4]arene dihydroxyphosphonic acid. Nevertheless the constructed geometries induced by complexation with melamine mono-cations prove to be highly complex and surprising. Both structures show a wide variety of interactions. The arrangement of melamine mono-cations presents remarkable specificities. Thus, chains of melamine mono-cations shifted from the calixarenic bilayer describe the complex of para-sulfonatocalix[4]arene, while, parallel chains of melamine cations intercalate layers of calix[4]arene dihydroxyphosphonic acid dimers, are seen in the second case.Aromatic–aromatic interactions exist in the two complexes, between the melamine mono-cations, but with a different orientation, depending on the complementary nature of the calixarene.
References
- A. Meyer, R. K. Castellano and F. Diederich, Angew. Chem., Int. Ed., 2003, 42, 1210–1250 CrossRef CAS.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS.
- J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3–32 CrossRef CAS.
- J. P. Mathias, C. T. Seto, E. E. Simanek and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 1725–1736 CrossRef CAS.
- J. P. Mathias, E. E. Simanek, J. A. Zerkowski, C. T. Seto and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 4316–4325 CrossRef CAS.
- J. P. Mathias, E. E. Simanek and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 4326–4340 CrossRef CAS.
- J. A. Zerkowski and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 4298–4304 CrossRef CAS.
- J. A. Zerkowski, J. P. Mathias and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 4305–4315 CrossRef CAS.
- R. Ahuja, P. L. Caruso, D. Mobius, W. Paulus, H. Ringsdorf and G. Wildburg, Angew. Chem., Int. Ed. Engl., 1993, 32, 1033–1036 CrossRef.
- P. V. Bernhardt, Inorg. Chem., 1999, 38, 3481–3483 CrossRef CAS.
- C. T. Seto and G. M. Whitesides, J. Am. Chem. Soc., 1993, 115, 905–916 CrossRef CAS.
- C. T. Seto, J. P. Mathias and G. M. Whitesides, J. Am. Chem. Soc., 1993, 115, 1321–1329 CrossRef CAS.
- J. P. Mathias, E. E. Simanek, C. T. Seto and G. M. Whitesides, Angew. Chem., Int. Ed. Engl., 1993, 32, 1766–1769 CrossRef.
- S. J. Dalgarno, M. J. Hardie and C. L. Raston, Cryst. Growth Des., 2004, 4(2), 227–234 CrossRef CAS.
- A. W. Coleman, S. G. Bott, S. D. Morley, C. M. Means, K. D. Robinson, H. Zhang and J. L. Atwood, Angew. Chem., 1988, 100, 1412–1413 CrossRef CAS.
- M. Selkti, A. W. Coleman, I. Nicolis, N. Douteau-Guevel, F. Villain, A. Tomas and C. Rango, Chem. Commun., 2000, 161–162 RSC.
- E. Da Silva, F. Nouar, M. Nierlich, B. Rather, M. J. Zaworotko, C. Barbey, A. Navaza and A. W. Coleman, Cryst. Eng., 2003, 6, 123–135 CrossRef CAS.
- G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049–1052 CrossRef.
- J. L. Atwood, L. J. Barbour, S. Dalgarno, C. L. Raston and H. R. Webb, J. Chem. Soc., Dalton Trans., 2002, 23, 4351–4356 RSC.
- B. Rather, B. Moulton, M. J. Zaworotko, F. Perret, N. Morel-Desrosiers, E. Da Silva and A. W. Coleman, Cryst. Eng., 2003, 6, 15–21 CrossRef CAS.
- A. W. Coleman, E. Da Silva, F. Nouar, M. Nierlich and A. Navaza, Chem. Commun., 2003, 7, 826–827 RSC.
- A. N. Lazar, A. Navaza and A. W. Coleman, Chem. Commun., 2004, 9, 1052–1053 RSC.
- A. N. Lazar, N. Dupont, A. Navasa and A. W. Coleman, Cryst. Growth Des., 2005 Search PubMed , in press.
- A. C. Neville, Biology of Fibrous Composites-Development beyond the Cell Membrane, Cambridge University Press, Cambridge, UK, 1993 Search PubMed.
Footnote |
| † CCDC reference numbers 278967 and 278968. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b510232b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2006 |
