Fabrication of poly(methyl methacrylate) capillary electrophoresis microchips by in situ surface polymerization†
Guoxi
Xu
a,
Joseph
Wang
b,
Yi
Chen
a,
Luyan
Zhang
a,
Derong
Wang
a and
Gang
Chen
*a
aDepartment of Chemistry, Fudan University, Shanghai, 200433, China. E-mail: gangchen@fudan.edu.cn; Fax: 86 21 65641740
bDepartments of Chemical & Materials Engineering and Chemistry, Arizona State University, Tempe, AZ 85287-5001, USA
First published on 30th November 2005
Abstract
A novel method based on in situ surface polymerization of methyl methacrylate (MMA) has been developed for the rapid fabrication of poly(methyl methacrylate) (PMMA) capillary electrophoresis (CE) microchips. MMA containing both thermal and ultraviolet (UV) initiators was allowed to prepolymerize in a water bath to form a fast curing molding solution that was subsequently sandwiched between a nickel template and a PMMA plate. The images of the raised microchannels on the nickel template were precisely replicated into the synthesized PMMA substrates during the UV-initiated polymerization of the molding solution within 30 min under ambient temperature. The attractive performances of the novel PMMA microchips have been demonstrated in connection with amperometric detection for the separation and detection of several model analytes. The new approach significantly simplifies the process for fabricating PMMA devices and could be applied to other materials that undergo light-initiated polymerization.
1. Introduction
In recent years, more and more attention has been paid to capillary electrophoresis (CE) microchips owing to their high degree of integration, portability, minimal solvent/reagent consumption, high performance and speed.1,2 A wide variety of polymer materials have been evaluated for fabricating microchips instead of glass because they can be produced with mass-replication technologies.3 Poly(dimethylsiloxane) (PDMS) and poly(methyl methacrylate) (PMMA) are the two commonly used polymers. However, the dimension variation of the microchannel due to the native elasticity of PDMS may limit its application.4 As a rigid polymer, PMMA has been particularly useful for microfluidic chips with the features of low price, excellent optic transparency, and excellent electric and mechanical properties.4,5 Because it can decompose into MMA at high temperature and can be reused, PMMA is an ideal material for preparing “green microchips”. It has been reported as the least hydrophobic of the commonly used plastic materials,6 and can directly generate stable electroosmotic flow (EOF) in the microchannels under the electrical field applied.7 Existing procedures for fabricating PMMA microchips include laser ablation,7 imprinting,8 injection molding,9etc. Recently, methods based on the in situ polymerization of methyl methacrylate (MMA) in molds have been developed for the fabrication of PMMA microchips with the aid of a UV10 or a thermal initiator.11 The bottleneck of the existing methods is the long polymerization time (4 to 12 h)10,11 that is not compatible with the mass production of CE microchips. To define the dimension of the PMMA channel plates, a rigid rectangle-shaped frame with a rectangular cavity was sandwiched between a hard plate and a silicon10 or stainless steel11 template to form a mold. The volume shrinkage of the prepolymerized MMA inside the cavity would result in bubble formation and additional molding solution usually had to be added to the cavity during the polymerization.10,11In this work, a fast curing molding solution has been developed based on the prepolymerization of MMA containing both thermal and UV initiators and employed in the rapid fabrication of PMMA CE microchip. The thermal initiator allowed MMA to prepolymerize under heat to produce the molding solution that could further polymerize to fabricate microchips between a nickel template and a PMMA plate with the aid of UV initiator. The times for prepolymerization, molding, demolding, and bonding were 15, 30, 10, and 10 min, respectively. Another novelty of this work was that the distance (400–500 µm) between the PMMA plate and the template decreased with the shrinkage of the molding solution so that the bubble formation could be eliminated. The ease, simplicity, versatility, and low cost of the new fabrication route thus make it extremely attractive for the mass production of PMMA microchips. The feasibility and performance of the PMMA microchips fabricated by the new method have been demonstrated in the following sections.
2. Experimental
2.1 Reagents
Methyl methacrylate (MMA), benzoin (BZI), and 2-2′-azo-bis-isobutyronitrile (AIBN), 1,4-phenyldiamine, and 2-methylaniline were all purchased from SinoPharm (Shanghai, China). Prior to use, the MMA needs to be washed with 5% NaOH aqueous solution and distilled under vacuum. AIBN was purified by recrystallization using hot methanol. Dopamine and catechol were both obtained from Sigma (St. Louis, MO, USA). The analysis of dopamine and catechol was performed with a 10 mM phosphate buffer (pH 6.5). The running buffer for the separations of aromatic amines was a 10 mM phosphate buffer (pH 4.5).2.2 Microchip layout and molding procedure
The newly fabricated PMMA chips (16 mm × 70 mm, Fig. 1A) had simple cross layouts, with the four-way injection cross-connected to the three reservoirs and the separation channel. The PMMA chip consisted of a 60 mm-long separation channel and a 5 mm-long injection channel. The two channels crossed each other halfway between the sample and the unused reservoirs, at 5 mm from the run buffer reservoir. The channel was approximately 40 µm deep and 50 µm wide (Fig. 1B). The nickel master template composed of a positive relief structure of nickel for the channels was made at the Research Institute of Micro/Nano Science and Technology in Shanghai Jiaotong University (Shanghai, China) by using an SU-8 (a negative photoresistor) based UV-LIGA technology. BZI (0.2% w/v, the UV initiator) and AIBN (0.2% w/v, the thermal initiator) were dissolved in MMA and the clear mixed solution was allowed to prepolymerize in a conical flask to generate a dense prepolymer molding solution in an 85 °C water bath for ∼15 min. Note that the molding solution can further polymerize under heat or UV light. The container for the molding solution should be wrapped with aluminum foil. The unused molding solution was stored in a 4 °C refrigerator and could be used for at least one month.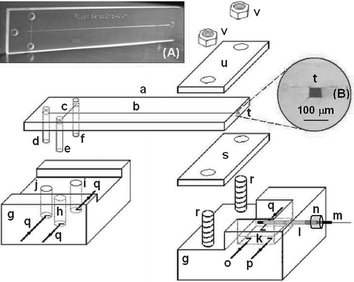 | ||
| Fig. 1 Microchip capillary electrophoretic system with electrochemical detection. Also shown are (A) a photograph of a typical PMMA microchip and (B) a microscopic image of the cross section of the channel in the complete PMMA microchips. (a) PMMA microchip, (b) separation channel, (c) injection channel, (d) pipette tip for buffer reservoir, (e) pipette tip for reservoir not used, (f) pipette tip for sample reservoir, (g) Plexiglas holders, (h) buffer reservoir not used, (i) sample reservoir, (j) buffer reservoir, (k) detection reservoir, (l) stainless-steel guiding tube, (m) capillary-based disc detection electrode, (n) silicon rubber holder, (o) auxiliary electrode, (p) reference electrode, (q) high voltage power electrodes, (r) screw bolts, (s) silicon rubber sheet, (t) channel outlet, (u) Plexiglas plate, (v) screw nuts. | ||
To fabricate the PMMA channel plate, an adequate amount of the molding solution (about 1.5 ml) was cast directly on the nickel template along the raised separation channel. Subsequently, the PMMA plate (70 mm × 16 mm × 1 mm) was carefully covered on it and pressed slightly until all the interspaces were filled by the molding solution. The excess molding solution could flow out and agglomerated along the edge of the PMMA plate on the nickel template and could prevent the ingress of air bubbles. Subsequently, the molding solution sandwiched between the PMMA plate and the nickel template was exposed to UV light (365 nm lamp, 20 W, Shanghai Jinguan Lamp Co. Ltd, Shanghai, China) through the PMMA plate. Complete polymerization of the solution under the UV light was accomplished within 30 min at 25 °C. Demolding was carried out by sonicating the mold in a 40 °C water bath for 10 min. The cover plate was fabricated in the same way as the PMMA channel plate except the nickel template was replaced by a glass slide (75 mm × 25 mm × 1 mm). Prior to demolding, the PMMA cover plate bonded to the glass slide was immersed in a 70 °C water bath for 1 min and flushed with tap water for 1 min to demold the cover plate from the glass slide.
Prior to sealing, 2 mm-diameter access holes were drilled at the ends of the channels to create reservoir ports. The channel plate and the cover plate were both cleaned by sonicating in water and isopropanol for 1 min each and were dried under a stream of nitrogen. Subsequently, both plates were quickly aligned and sandwiched between two glass slides (75 mm × 25 mm × 1 mm), clamped together using six binder clips (25 mm, Shanghai Stationery Co. Ltd.), and were placed in a 108 °C convection oven for 10 min. The bonded chip was then allowed to cool slowly to room temperature and removed from the glass slides.
2.3 Apparatus
Details of the microchip CE amperometric detection (AD) system were illustrated in Fig. 1. The detection reservoir port of the microchip was cut off to facilitate the end-column AD. The effective length of the separation channel was 55 mm (from cross-section to the channel outlet). A three-electrode AD system was fabricated in the detection reservoir (k). The 320 µm diameter carbon disc detection electrode12 (m) was placed opposite the channel outlet (t) through the guiding tube (l, 500 µm id × 800 µm od). The end of the guiding tube (l) outside the detection reservoir (k) was sealed by a small silicon-rubber holder (n), allowing the detection electrode to move back and forth. A piece of 2.5 mm thick high-elasticity silicon rubber sheet (s) was attached to the bottom of the microchip and subsequently sandwiched between a Plexiglas plate (u) and the Plexiglas holder (g) using the screws, allowing the microchip to be adjusted up and down more than 1 mm. With the aid of the two-dimensionally adjustable CE microchip (a) and the one-dimensionally adjustable disc detection electrode (m), the device shown in Fig. 1 facilitates the three-dimensional alignment between (t) and (m). The distance between (t) and (m) was ∼50 µm. Amperometric detection was performed with a CHI 830B electrochemical analyzer (Shanghai Chen-Hua Instruments Co., Shanghai, China). A scanning electron micrograph (SEM) of the cross section of the microchannels in the PMMA substrate was obtained with a PHILIPS XL 30 scanning electron microscope (Netherlands) to measure the width and the depth.2.4 Electrophoretic procedure
The channels of the PMMA chip were treated before use by rinsing with doubly distilled water and the running buffer for 10 min each. The “buffer” reservoirs (Fig. 1(h) and (j)) were filled with the corresponding CE running buffer, and the “sample” reservoir (Fig. 1(i)) with the sample mixture. A homemade high-voltage power supply had an adjustable voltage range between 0 and +4000 V. The injections were performed by applying a voltage of +1500 V between the “sample buffer” (Fig. 1(i)) and the “grounded-detection” reservoir (Fig. 1(k)) for 2 or 3 s while all other reservoirs were floating. Separations were performed by applying a voltage of +1500 V to the “running buffer” reservoir (Fig. 1 (j)) with the “detection” reservoir (Fig. 1(k)) grounded and all other reservoir floating.3. Results and discussion
In this report, a fast curing prepolymerized MMA solution containing both thermal and UV initiators has been developed for the rapid fabrication of PMMA microchips. The advantages of both the heat-initiated and the UV-initiated polymerizations of MMA had been taken in this work so that the total polymerization was significantly reduced to 15 + 30 min. Although the volume shrinkage of the molding solution was inevitable during molding, no bubble formation was found because no rigid spacer was sandwiched between the template and PMMA plate.The in situ polymerization on the surface of the PMMA plate method can ensure the images of the relief on the nickel template are precisely replicated into the synthesized PMMA layer with high fidelity. The microscopic image of the channel outlet after thermal bonding is displayed in Fig. 1B. No observable boundaries between the two plates along with the absence of air bubbles entrapped during the bonding process indicate the high quality of the microchannels. The structure transferred into the PMMA material is characterized by high precision of the master replication. The reproducibility of such replica molding was studied for eight separate chips, replicated from the same molding nickel master, and selected randomly from a pool of 50 different chips. The cross-sectional profile of the microchannel is rectangular with the measured bottom width and depth of 49.6 µm (1.2%) and 38.2 µm (3.7%), respectively. The surface-to-volume ratio of the rectangular channel was calculated to be 0.093 µm−1 while the typical values of the commonly used trapezoidal channels were 0.141 and 0.067 µm−1 for the 18 µm deep (top width, 101 µm; bottom width, 71 µm)10 and the 61.5 µm deep (top width, 169 µm; bottom width, 15.6 µm)8 channels in the PMMA substrates, respectively.
The UV-initiated polymerization was performed using MMA solutions containing different amounts of BZI when the content of AIBN was 0.2% w/v. When the amount of BZI, was 0.05, 0.1, 0.2, 0.3, and 0.4% w/v, the polymerization time of the molding solution was 95, 66, 28, 15, and 11 min, respectively. A 0.2% BZI level was proved to be optimal for the complete curing of the substrates within 30 min, while providing high-quality structures. Higher BZI levels resulted in faster curing, but led to uncontrolled polymerization and related excessive heating. The EOF properties of the newly fabricated device were evaluated for untreated surfaces, using the well-established baseline monitoring technique of Huang et al.13 The EOF value calculated at pH 6.5 (with a field strength of 272 V cm−1), 2.13 × 10−4 cm2 V−1 s−1, compares favorably with literature values for pure PMMA chips (1.2 × 10−4 − 2.6 × 10−4 cm2 V−1 s−1).4
The analytical performance of the molded PMMA microchips was demonstrated in connection to the separation of catecholamine analytes and two aromatic amines of environmental concern coupled to AD. As indicated from Fig. 2A, the PMMA microchip provides well-defined peaks for 200 µM dopamine and 300 µM catechol within 100 s. The number of theoretical plates for dopamine was determined to be greater than 6780 m−1. The half peak widths of dopamine and catechol are 6.2, and 5.9 s, respectively, with the corresponding sensitivities of 143.5 and 102.1 nA mM−1. The detection limits (3 × S/N) of dopamine and catechol are 0.21, and 0.29 µM, respectively. Fig. 2B displays the electropherograms for a mixture containing 200 µM 1,4-phenyldiamine and 2-methylaniline. The two aromatic amines could be separated on the baseline with well-defined peak shapes within 90 s, indicating the present PMMA CE microchip particularly attractive as an analysis tool in environmental monitoring.
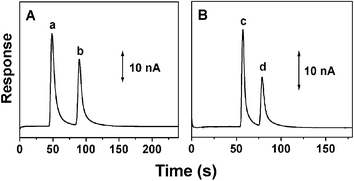 | ||
| Fig. 2 Electropherograms for a mixture (A) containing 200 µM dopamine (a) and 300 µM catechol (b) or (B) 200 µM 1,4-phenyldiamine (c) and 2-methylaniline (d). Conditions: separation and injection voltage, +1500 V; injection time, 3 (A) and 2 s (B); running buffer, 10 mM phosphate buffers (A, pH 6.5; B, pH 4.5); detection electrode, 300 µm diameter carbon disc electrode; detection potential, +0.80 V (vs. Ag/AgCl wire). | ||
Acknowledgements
This work was supported by the 863 Programme (2004AA639740), NSFC (20405002), Shanghai Science Committee (051107089 and 2004ZR140150212), and Education Ministry of China.References
- D. R. Reyes, D. Iossifidis, P. A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623 CrossRef CAS
.
- J. Wang, G. Chen, M. P. Chatrathi and M. Musameh, Anal. Chem., 2004, 76, 298 CrossRef CAS
.
- H. Becker and C. Gartner, Electrophoresis, 2000, 2, 12 CrossRef CAS
.
- S. A. Soper, S. M. Ford, S. Qi, R. L. McCarley, K. Kelly and M. C. Murphy, Anal. Chem., 2000, 72, 643A CAS
.
- B. Grass, A. Neyer, M. Jöhnck, D. Siepe, F. Eisenbeiß, G. Weber and R. Hergenröder, Sens. Actuators, B, 2001, 72, 249 CrossRef
.
- H. Bayer and H. Engelhardt, J. Microcolumn Sep., 1996, 8, 479 CrossRef CAS
.
- M. A. Roberts, J. S. Rossier, P. Bercier and H. Girault, Anal. Chem., 1997, 69, 2035 CrossRef CAS
.
- L. Martynova, L. E. Locascio, M. Gaitan, G. W. Kramer, R. G. Christensen and W. A. MacCrehan, Anal. Chem., 1997, 69, 4783 CrossRef CAS
.
- R. M. McCormick, R. J. Nelson, M. G. AlonsoAmigo, J. Benvegnu and H. H. Hooper, Anal. Chem., 1997, 69, 2626 CrossRef CAS
.
- A. Muck, J. Wang, M. Jacobs, G. Chen, M. P. Chatrathi, V. Jurka, Z. Vyborny, S. D. Spillman, G. Sridharan and M. J. Schoning, Anal. Chem., 2004, 76, 2290 CrossRef CAS
.
- Z. F. Chen, Y. H. Gao, R. G. Su, C. W. Li and J. M. Lin, Electrophoresis, 2003, 24, 3246 CrossRef CAS
.
- G. Chen, L. Y. Zhang and J. Wang, Talanta, 2004, 64, 1018 CrossRef CAS
.
- X. Huang, M. Gordon and R. N. Zare, Anal. Chem., 1988, 60, 1837 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Sandwich molding process and SEMs. See DOI: 10.1039/b515842g. |
| This journal is © The Royal Society of Chemistry 2006 |
