Magnetism and microfluidics
Nicole
Pamme
National Institute for Materials Science (NIMS), International Centre for Young Scientists (ICYS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: pamme.nicole@nims.go.jp; Fax: +81-29-860-4706
First published on 28th November 2005
Abstract
Magnetic forces are now being utilised in an amazing variety of microfluidic applications. Magnetohydrodynamic flow has been applied to the pumping of fluids through microchannels. Magnetic materials such as ferrofluids or magnetically doped PDMS have been used as valves. Magnetic microparticles have been employed for mixing of fluid streams. Magnetic particles have also been used as solid supports for bioreactions in microchannels. Trapping and transport of single cells are being investigated and recently, advances have been made towards the detection of magnetic material on-chip. The aim of this review is to introduce and discuss the various developments within the field of magnetism and microfluidics.
 Nicole Pamme | Nicole Pamme obtained a Diploma in Chemistry from the University of Marburg, Germany, in 1999. For her PhD she went to Imperial College London, where she worked on single particle analysis in microfluidic chips until 2004. Since then she has been living and working in Tsukuba, Japan as an independent research fellow in the International Centre for Young Scientists (ICYS) at the National Institute for Materials Science (NIMS). Her current research interests include analysis of biomagnetic particles/cells in microfluidic devices. From December 2005, she will be joining the University of Hull, UK, as a lecturer in Analytical Chemistry. |
Introduction
Magnetism and microfluidics; neither of these two concepts are new, yet it has only been in recent years that they have been combined. Electric fields have long been utilised in microfluidic applications, such as capillary electrophoretic separations, electroosmotic pumping and dielectrophoretic trapping.1–3 Magnetic fields however were initially employed relatively rarely, despite the great advantages they could offer. For example, objects inside a microfluidic channel can be manipulated by an external magnet that is not in direct contact with the fluid. Biomolecules can be isolated from a sample by attaching them to small magnetic particles and then recovered using an external magnetic field. In contrast to electric manipulation, magnetic interactions are generally not affected by surface charges, pH, ionic concentrations or temperature.What has prevented researchers from utilising magnetic forces? There are a number of reasons for this. MEMS techniques for fabrication of miniaturised magnets have only recently been combined with microfluidic fabrication. Microfluidic researchers have been somewhat reluctant to place objects such as magnetic particles into microchannels.4 Furthermore, magnetic microparticles functionalised with antibodies or other biomolecules have only become off the shelf products in the last few years.
Today we can see magnetic forces being combined with microfluidics in an amazing variety of ways. Magnetic forces can not only be utilised to manipulate magnetic objects such as magnetic particles, magnetically labelled cells or plugs of ferrofluids inside a microchannel; they can also be used to manipulate non-magnetic, i.e. diamagnetic objects. Magnetic fields are usually applied from outside the microchannel, sometimes by means of sophisticated microfabricated electromagnets and sometimes by an approach as low tech as a conventional benchtop stirrer plate. Applications include pumping and mixing of fluids, as well as the incorporation of switches and valves into lab-on-a-chip devices. Magnetic forces are used for transport, positioning, separation and sorting of magnetic as well as non-magnetic objects. Bio-assays have been performed on the surface of magnetic particles trapped inside a microchannel. More recently, on-chip detection techniques based on magnetic forces have been investigated and basic research of magnetic behaviour, not possible on the large scale, has been undertaken in the confined space of microchannels. The objective of this review paper is to give the reader a summary of the developments of magnetism in microfluidics.
Magnetic theory
Magnetic field lines can reach a certain density within a material, quantified by the material's magnetic permeability, µ. The magnetic flux density, B (in Tesla or T) describes the number of field lines per unit area. The flux density decreases quickly with increasing distance from the magnet surface (Fig. 1(a)). If a magnetic material, such as soft iron, is placed in a magnetic field, the magnetic field lines are redirected through that material to take advantage of its greater permeability (Fig. 1(b)).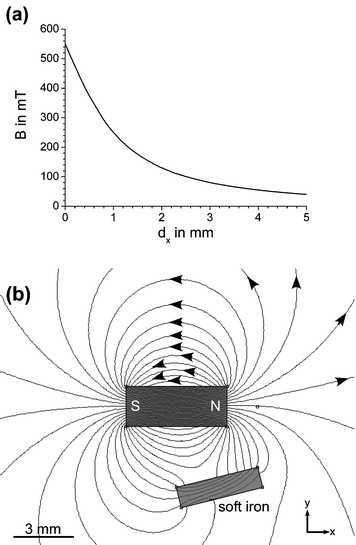 | ||
| Fig. 1 (a) The magnetic flux decreases rapidly with distance from the magnet surface, as plotted here for a NdFeB magnet. (b) Influence of a magnetically permeable material on the magnetic field lines. | ||
By combining magnets into arrays, complex magnetic field patterns can be achieved even on the micron-size scale. In this context it is important to differentiate between homogeneous and inhomogeneous magnetic fields. In a homogeneous magnetic field, the density of flux lines is constant over a distance x, there is no gradient in the flux density. In an inhomogeneous magnetic field however, there is a gradient in the density of flux lines over a distance x. Homogeneous fields are required for NMR spectroscopy and magnetohydro-dynamic pumping. To achieve this, magnets of rather large size with respect to the fluidic volume are often employed (Fig. 2(a)). Inhomogeneous fields with high magnetic field gradients are desired, when the aim is to trap particles or transport materials within a fluid volume (see eqn (1) below). To this end, tapered magnets (Fig. 2(b)) or even layered structures (Fig. 2(c)) can be used.
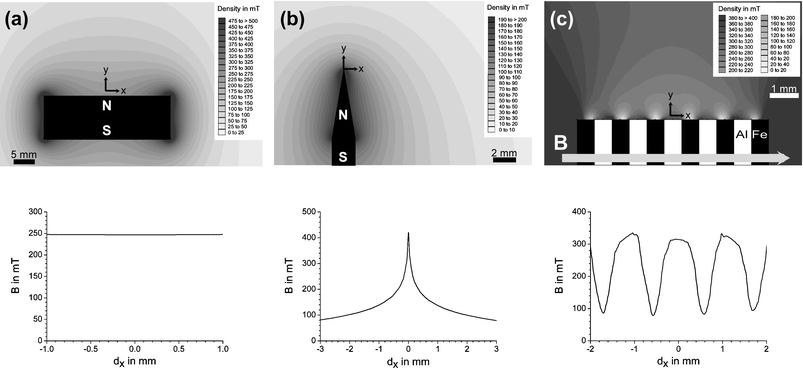 | ||
| Fig. 2 Magnetic fields from permanent NdFeB magnets modelled with FEMM-freeware (http://www.femm.foster-miller.net): (a) A homogeneous field at a distance of 1 mm along the surface of a large magnet. (b) An inhomogeneous field 100 µm above the surface of a tapered magnet. (c) A magnetic field with local minima and maxima at 100 µm distance above a stack of alternating iron and aluminium blocks. | ||
Magnetic field design is often accompanied by computer simulations. As a basic option, the freely available software FEMM (http://www.femm.foster-miller.net) can be employed. More dedicated packages include MagNet (http://www.infolytica.com) or FEMLAB (http://www.comsol.com).
Permanent magnets retain their magnetic properties once any external magnetising field has been removed. Materials that exhibit this behaviour include iron, nickel and cobalt. Some of the strongest magnetic fields can be achieved with alloys such as samarium cobalt (SmCo) or neodymium iron boron (NdFeB). Electromagnetic fields are generated around any current carrying wire. Such fields can be switched on and off and tuned depending on the applied current. Furthermore, time-varying fields can be obtained with alternating currents (ac). Care has to be taken to insulate the wires and to avoid excessive heating at high currents. Strong magnetic fields can be achieved by using an electromagnet with a highly permeable core material such as soft iron or µMetal.
According to their magnetic susceptibility, χ, materials are classified as diamagnetic, paramagnetic and ferromagnetic. Diamagnetic materials (χ < 0) are repelled from magnetic fields, i.e. they are forced towards minima of magnetic field strength. Most materials are weakly diamagnetic, including water, proteins, DNA, cells, polymers, wood and glass. Often such materials are simply called non-magnetic. Paramagnetic materials (χ > 0) align in a magnetic field and experience a small force towards magnetic field maxima, i.e. they are attracted to magnetic fields. Examples of paramagnetic materials include oxygen, platinum and manganese(II) salts. Ferromagnetic materials such as iron, cobalt and nickel have χ ≫ 0 and are strongly attracted to magnetic fields. Another special case of paramagnetism is superparamagnetism. Superparamagnetic particles have a core of small iron oxide crystals encased by a polymer shell. The particles are magnetised in a magnetic field. However, they have no magnetic memory. Once the external field is removed, the particles redisperse and behave like a non-magnetic material.
Force on a magnetic particle
The force on a magnetic particle inside a magnetic field depends on the volume of the particle (V), the difference in magnetic susceptibilities, Δχ , between the particle (χp) and surrounding buffer medium (χm), as well as the strength and gradient of the applied magnetic field: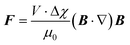 | (1) |
Note that for a homogeneous field, i.e. for a field with ∇B = 0, the force on the particle is zero. In this case, a particle would merely be magnetised in the field but not be pulled into any direction. Typically, forces on magnetic particles range from a few pN to a few tens of pN.
The term Δχ = χp − χm is the difference in magnetic susceptibility between the magnetic particle, χp, and its surrounding buffer or medium, χm. For diamagnetic objects (χp < 0) in a diamagnetic medium (χm < 0), the term Δχ can be positive or negative, i.e. the particle can be repelled from or attracted to the magnetic field. Since the magnetic susceptibilities of a particle or cell and the surrounding material are generally very close to each other, the term Δχ is rather small, i.e. the force on the particle is almost negligible. The buffer medium however, can also be made paramagnetic, for example by adding Mn2+ ions5,6 or Gd3+ ions.7 When a diamagnetic object (χp < 0) is placed into a paramagnetic medium (χm > 0), then the term Δχ is always negative and thus the diamagnetic object is repelled from the magnetic field and pushed towards field minima. The larger χm, the stronger the repelling force. At the same time, a paramagnetic particle (χp > 0) can be made to act like a diamagnetic material by placing it into a strongly paramagnetic medium, (χm > χp > 0). In this case, Δχ is also negative and hence the paramagnetic particle is repelled from the magnetic field.
Magnet selection and fabrication
It can be rather challenging to achieve the desired magnetic field pattern, strength and gradient over the confined space of a microfluidic channel, especially when keeping in mind how quickly the flux density decreases with distance from the magnet surface (Fig. 1(a)). For manipulation in microfluidic channels, two approaches can be taken: (i) conventional permanent magnets or electromagnets are placed outside the microchip, or (ii) microfabricated permanent or electromagnets are incorporated into the microchip. Approach (i) has the obvious advantage of ease of fabrication, and low cost. Approach (ii) allows for a much more confined spatial control of field strength and pattern and also often allows for closer proximity of the magnet to the microchannel.(i) Conventional magnets
Permanent magnets used in microfluidic applications are usually small neodymium iron boron (NdFeB) magnets featuring magnetic flux densities of up to 500 mT at the pole surface. This allows for manipulation of magnetic particles or cells inside a microchannel even when the magnet is placed at several mm distance from the channel. Depending on the application, large or small magnets are required. For magneto-hydrodynamic pumping, the entire channel area must be subjected to a homogeneous magnetic field, i.e. large magnets are required that are several cm long.8 For trapping magnetic particles into a small plug, local magnetic field maxima are necessary and magnets with diameters of only a few mm are used.9 Rotation of magnetic material inside a microchannel can be achieved by a rotating external magnet for example with a conventional stirrer plate.10–12Electromagnets frequently feature tapered cores in order to achieve high field gradients13–15 (see Fig. 2(b)). Electro-magnets have not been as popular as permanent magnets, although they can be switched on and off and their field strength can be tuned. Even when using a large number of windings and high currents, it is difficult to build a small electromagnet with a field strength comparable to that of a small NdFeB magnet. Bulkiness and Joule heating quickly become problematic.
(ii) Microfabricated magnets
Integrating microfabricated magnets into microfluidic devices involves a considerable number of fabrication steps, not to mention cost. On the other hand, small integrated magnets permit very precise control of the magnetic field. When positioned in close proximity to the microchannel, the requirements for field strength are also reduced (Fig. 1(a)).The techniques for micromagnet fabrication resemble those employed for microfluidic chip fabrication (Fig. 3). Metal layers are obtained either by sputtering,16 electroplating,17 evaporation18 or vapour deposition.19 Lithography and photoresists are used for patterning in combination with etching and lift-off techniques. Substrates are either silicon20–22 or glass.23–26 In many cases a seed layer is required to obtain good adhesion between the desired metal and the substrate. Electrical insulation is realised by spin-coating with polyimide18 or by putting down a layer of silicon oxide.18,21,22
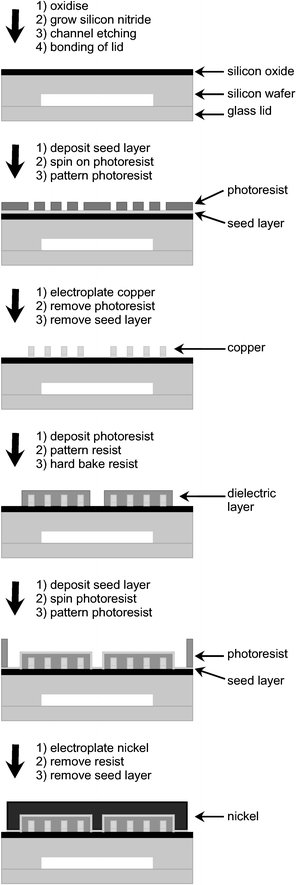 | ||
| Fig. 3 Fabrication steps for a micro-electromagnet with wires and core, redrawn with permission from ref. 30. | ||
Ferromagnetic materials such as permalloy17,26,27 or nickel16 are used for permanent magnets or as cores for electromagnets. Planar electromagnets consisting simply of a current carrying wire, can be fabricated easily by depositing a conducting material, such as copper,20,23 gold18,25,28,29 or aluminium.19,24 Joule heating, wire insulation and connection to an external power supply must all be considered when designing an electromagnet. More complex magnets with a core and a wire wound around it, require further fabrication steps, often with sacrificial layers.25,31–33 Choi and Ahn have been pioneering in this field. In order to trap magnetic particles in flow they developed several integrated electromagnets including a meandering permalloy-core, copper-wire design,31 as well as serpentine27 and spiral26 copper-wires with a semi-encapsulated permalloy. Integrated electromagnets were also fabricated as coils for NMR on chip.22–25,34
Another approach to obtain precise control and strong gradients in a microchannel is to micropattern magnetically susceptible features in close proximity to the microchannel and to magnetise these with an external magnet.28,35 For example, Deng et al. fabricated Ni posts at the bottom of a PDMS channel36 and Inglis et al. patterned narrow nickel strips on the bottom of a microchamber.16 These features were then magnetised with external NdFeB magnets.
Manipulated material
Magnetic materials
In some applications, small bars of steel37 or permalloy11,17 have been used inside microfluidic chambers. Most commonly, however, magnetic particles are employed, as reviewed recently.38,39 These range in size from a few nm to many µm. The majority of these particles are superparamagnetic, i.e. they have no magnetic memory. Although much is now commercially available, the development of new types of magnetic particles and their surface modification are still an area of ongoing research.40–43Superparamagnetic particles are available with carboxyl groups or amino groups on their surface. Biomolecules such as DNA strands or antibodies can then easily be attached to the particle surface. Many companies offer particles with the desired biomolecule already immobilised (Fig. 4). Popular suppliers of such particles include Dynal (http://www.dynalbiotech.com), Micromod (http://www.micromod.de), Bangs Laboratories (http://www.bangslabs.com), Polysciences (http://www.polysciences.com), Seradyn (http://www.seradyn.com) and Estapor (http://www.estapor.com). Dynal's Dynabeads are by far the most commonly used superparamagnetic particles, due to their spherical shape and highly uniform size distribution. Two sizes of Dynabeads are available, 2.8 µm and 4.5 µm diameter. The magnetic core comprises only a small fraction of the particle volume.44 Particles with a higher magnetite content have a stronger interaction with applied magnetic fields.13 Best are Ni30Fe7045 or NdFeBLa46 particles, however, these uncoated metal particles are not biocompatible.
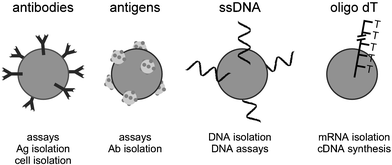 | ||
| Fig. 4 Surface functionalised particles can be used for a wide variety of biochemical reactions. | ||
Two types of cells are naturally magnetic:47 red blood cells because of their paramagnetic haemoglobin and magnetotactic bacteria which synthesise intracellular chains of magnetic nanoparticles and use these to orient themselves along the geomagnetic field. All other types of cells must be labelled to render them magnetic. External magnetisation with micro-particles is most common, although internal magnetisation with nanoparticles48 is also possible. Cells have also been labelled with paramagnetic lanthanide ions,49 such as Er3+.
Magnetic fluids or ferrofluids are another class of material that can be used in microfluidic devices. A ferrofluid is a stable suspension of magnetic nanoparticles in a carrier liquid such as water, or an organic solvent. The particles are coated with a surfactant in order to prevent aggregation. A ferrofluid can be moved through channels and adopt any geometry. Even in intense magnetic fields it retains fluidity. Since the suspension is composed of nanometer sized particles, ferrofluids are superparamagnetic. Ferrofluidic foams, i.e. ferrofluids with air bubbles, have also been investigated.50
Diamagnetic objects
As mentioned above (eqn (1)), it is possible to manipulate diamagnetic objects with magnetic fields. Such objects experience a force towards magnetic field minima, allowing for levitation and trapping. This was impressively shown by Simon and Geim when they levitated a frog.51 Examples on the microfluidic scale include the trapping of polystyrene particles,6 the patterning of cells7 and the levitation of droplets.52Pumps
Magnetohydrodynamic (MHD) pumps
MHD pumps are an alternative to pressure driven or electroosmotic flows. MHD flow is generated when an electric field and a magnetic field are applied perpendicular to each other across the height and width of a microchannel filled with a conducting buffer solution. A Lorentz force acts in the direction of the channel length and induces fluid movement. MHD pumping is suitable for any conducting liquid and does not require any moving parts. Pumping can be achieved in straight channels as well as in circular channels. The flow velocity can be adjusted by changing the applied current and magnetic field or the ionic concentration of the buffer.MHD micropumps operating with dc and homogeneous magnetic fields are relatively easy to realise. For example, seawater was pumped at velocities up to 2.5 mm s−1 in a straight silicon channel53 using aluminium electrodes, deposited above and below the channel in combination with a NdFeB magnet (440 mT). Bau's group pumped mercury slugs, saline solutions and deionised water through toroidal channels at flow rates of up to 200, 12 and 0.5 mm s−1, respectively.54 However, electrolysis can be problematic for dc-MHD pumps. A high current density dc-MHD micropump8 transported buffer solutions at up to 1 mm s−1. To avoid bubble formation in the pumping channel, electrodes were located in a neighbouring channel which was connected to the main pumping channel via small channels.
Electrolysis can be avoided altogether with ac-MHD pumps. With simultaneous switching of the electric and magnetic fields, the Lorentz force always points into the same direction. However, well-designed switching circuits are required. Lemoff and Lee deposited platinum electrodes on the side of a looped silicon channel and generated a magnetic field with small commercial electromagnets.55 Flow velocities between 0.3 mm s−1 and 1.5 mm s−1 were obtained for NaCl solutions with concentrations between 0.01 M and 1 M. PBS buffer was pumped at 0.16 mm s−1. West et al. combined circular MHD pumping with on-chip polymerase chain reactions (PCR) using concentrated buffer solutions.56 Pt electrodes were deposited on the vertical channel walls and a custom made electromagnet generated a flux density of 11 mT across the channel. Eijkel et al. designed a pump for circular chromatography57,58 with gold electrodes on the channel side walls. The magnetic field was generated by five custom made electromagnets, each 100 mT, that covered about 40% of the channel circumference. Maximum flow rates obtained were 40 µm s−1 for a 1 M solution of KNO3, unfortunately too slow for the anticipated chromatography application.
Ferrofluidic pumps
Plugs of ferrofluid, transported by external magnetic fields, can be used for pumping liquids through channels. Such pumps can be easy and cheap to realise. Ferrofluid and pumped liquid must be immiscible, hence hydrophobic ferrofluids are used for the pumping of aqueous solutions.Hatch et al. fabricated a circular ferrofluidic micropump (Fig. 5) based on two Nd magnets and two plugs of ferrofluid.59 One plug was held in place between the inlet and outlet channel, acting as a closed valve. A second plug was moved around a circle by a rotating external magnet. This plug pulled and pushed liquid into and out of the circular channel. Water was pumped at 4 to 8 rpm, corresponding to 2.1 to 4.2 mm s−1. Choi and Ahn investigated pumping of small volumes (7 to 60 nL) by rotating an external magnet (340 mT) with steps of 18° above a plug of ferrofluid.60 Hartshorne et al. could pump both air and water, in a T-shaped microfluidic network containing three plugs of ferrofluid.61 Two plugs were used as valves. To close the valve, ferrofluid was pulled with an external magnet from a side pocket into the main channel. Another plug was used as a piston. By switching the valves in the main channel and moving the piston in the vertical channel, air could be pumped from the atmosphere to an off-chip pressure sensor. Due to the high viscosity of the ferrofluid, the piston could only be moved at slow speeds of 30 to 50 µm s−1. Thus, each cycle took about 30 min and allowed for pumping of 300 nL of air. For pumping of water, the channel surface was coated with hydrophobic silane in order to prevent leakage around the ferrofluid plugs. Numerical simulations on how an array of micro-electromagnets could be used to induce flow of ferrofluids in microchannels62,63 were also reported.
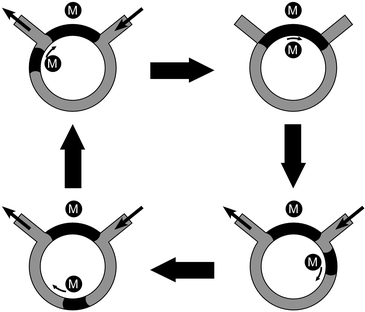 | ||
| Fig. 5 The principle of a circular ferrofluid pump, redrawn with permission from ref. 59. Two magnets (M) were employed to manipulate ferrofluid plugs in a circular microchannel. | ||
Thermal and magnetic fields were combined for a magneto-caloric pump.64 Materials lose their magnetic properties when heated to their Curie temperature (TC). Conventional ferrofluids have a TC so high that any liquid would boil before demagnetisation occurred. Here, ferrofluids containing Mn and Zn were investigated with a TC below 80 °C. A uniform magnetic field and a temperature gradient were applied over a plug of such a ferrofluid. As the ferrofluid reached its TC, the attraction towards the magnetic field was lost and the hot fluid was displaced by cooler fluid. With the resulting pressure gradient, a flow of 2 µm s−1 was achieved.
Other pumps
Fluid movement in a microchannel can also be induced by rotating small bar magnets in a reservoir adjacent to the channel. A micro stirrer bar integrated into a polymer (parylene) microchannel was rotated using a conventional benchtop stirrer plate.17 The 400 µm long and 15 µm thick permalloy bar was positioned in the centre of a 420 µm diameter and 25 µm depth microchamber adjacent to a microchannel of 200 µm width. Flow velocities of up to 200 µm s−1 were achieved. However, a back pressure as small as 10 Pa was enough to reverse the flow. With the rather complex fabrication of the integrated stirrer bar, the usefulness of such a pump in its present form is questionable.A biomimetic pump based on vortices was reported by Atencia and Beebe.65 In the same way as animals utilise vortices to swim or fly with a minimum amount of energy, local vortices can be employed to propel fluid through shallow fluidic networks. A magnetic bar integrated into a microfluidic structure was restricted by posts or channel walls, so that the bar oscillated when a rotating magnetic field was applied. The oscillation induced local vortices which created fluid flow of between 3 to 600 µL min−1 inside the channel.
Valves and switches
Valves based on ferrofluid plugs have already been mentioned with ferrofluid-based pumps. In Hatch et al.’s design,59 a plug held in a fixed position with an external magnet blocked fluid flow and forced liquid to take an alternative route (Fig. 5). Hartshorne et al. used ferrofluid valves for water as well as air.61 With a 280 mT magnet the valve could operate against a pressure of 0.5 bar, with a 580 mT magnet operation was possible up to 1.2 bar.Magnetically modified PDMS can also be used for valves. Mixing iron powder with the PDMS precursor solution results in a flexible magnetic material. A PDMS microchannel network was fabricated with 5 mm long sections of magnetic PDMS66 and attached to a circuit board containing electromagnets. When a magnet was switched on, the elastic magnetic PDMS was deformed and blocked the channel. With 87 mT flux from the magnet, the valve could resist water at a pressure of 0.15 bar. A cycle time of 1 s was reported.
Magnetohydrodynamic flow can act as a switch to guide flow along a channel network. MHD pumps were integrated into the two branches of a Y-shaped channel network.67 Flow could be switched between the branches by activating one MHD pump and setting the other to counter-acting pressure. No physical barriers or moving parts were required. However, the pump could not withstand large back pressures and careful adjustment of voltages and currents were necessary. Bau et al. also controlled flow through a microfluidic network using MHD pumps.68 Individual pairs of gold electrodes were located on opposing channel walls of each of the branches and a uniform magnetic field was applied over the entire network with a Nd magnet (400 mT). By addressing the appropriate electrodes, a plug of fluorescent dye was shown to flow along the desired branches of the fluidic network. Flow velocities of up to cm s−1 could be attained.
Mixing
Mixing with magnetic stirrers
A very simple and effective combination of macroscopic stirrer bars and microfluidic mixing was demonstrated by placing two 5 mm long stirrer bars into the inlet reservoirs at opposing sides of a microchamber.10 The bars were rotated with a conventional stirrer plate which initiated flow through the chamber. The reaction efficiency of a DNA array could be increased 5-fold.Stirrer bars could also be integrated directly into a channel. Magnetic permalloy rotors were fabricated on a glass substrate and enclosed by a PDMS channel network.11 Rotation was initiated with a conventional stirrer plate at 1.5 mT in the rotor plane. When a 400 µm wide rotor was used in a 750 µm wide channel, little mixing was achieved at the edges, where the rotor did not reach. After some design improvements,17 a permalloy bar of 400 µm width could be fitted into a microchamber of 420 µm diameter (Fig. 6). With this design, continuous flow mixing of two dyes was feasible over the entire channel width.
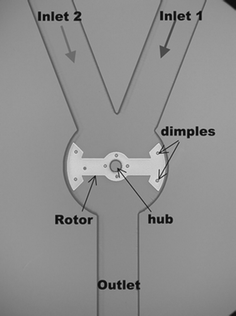 | ||
| Fig. 6 Magnetic mixing with a permalloy rotor (400 µm length) controlled with a conventional benchtop stirrer plate.17 | ||
Using a less elaborate fabrication regime, a steel blade (3 mm long, 800 µm wide) was positioned at the junction of a Y-shaped fluid network and rotated by a benchtop stirrer plate at 2200 rpm.37 Two dyes were pumped through the device and mixed at velocities between 1 and 240 µm s−1. The authors identify applications for mixing in channels where diffusion based mixing alone is too slow.
Small magnetic nickel bars of 40 µm length and 1 µm diameter controlled by three external electromagnets were also suggested for mixing in microchannels.69 The mixing efficiency of these bars has not yet been tested.
Mixing with magnetic particles
An easier way to integrate a miniaturised stirrer bar into a microchannel is to assemble a chain of magnetic micro-particles.70 To increase the stability of the chain, the particles were bonded together with linker molecules. The channel network contained three inlet channels merging into one outlet channel. A rotating magnetic field (60 to 600 rpm, 1.5 mT) was generated by two pairs of electromagnets that were supplied with sinusoidal ac currents. A dye and two buffer streams all containing particle chains were merged and mixed quickly upon rotation of the chains. Also, an acid and a base were mixed in the presence of a fluorescent pH indicator. Flow rates were between 0.3 and 1.4 mm s−1.In another approach, two principles of efficient mixing, i.e. fast magnetic rotation and utilising an array of rotors, were combined. A PDMS device was fabricated with an array magnetic flux concentrators at the bottom of a mixing chamber.71 These concentrators were 25 to 100 µm diameter sections of magnetically doped PDMS integrated into regular PDMS. A suspension of iron filings (5 µm) was filled into the chamber. When a permanent magnet (50 mT) was rotated below the device, the magnetic field was concentrated to the regions with the magnetic PDMS. The iron filings aggregated above the concentrators into needle-like structures and rotated around their bases. The mixing efficiency of these rotating spikes was tested by pumping dye solutions through the chamber. Best results were achieved in a pear-shaped chamber with semicircular wall indentations.
Magnetic particles moving at random were also suggested for mixing of two fluid streams.13,72 A plug of magnetic particles (1 µm) was formed in a microchannel by means of an electromagnet. The magnet poles were tapered to increase the force on the particles (see Fig. 2(b), eqn (1)). The particles however did not form a static plug. Instead they were moved over short distances due to the ever changing magnetic field which was modified by an arbitrary wave form generator. A mixing efficiency of 95% was reported for a fluorescent dye and water, measured at a point 400 µm downstream of the particle plug with flow rates of 5 mm s−1.
Chaotic mixing was reported in a serpentine microchannel with copper wire electromagnets, embedded transverse to the direction of flow.73 The magnetic field generated from these wires was used to attract nearby magnetic particles. Time varying magnetic fields in combination with the serpentine geometry lead to efficient mixing.
Magnetohydrodynamic (MHD) mixing
Magnetohydrodynamic flow was described above as a pumping method. The same principle can also be utilised for mixing fluids by integrating several MHD pumps into a microfluidic device and by utilising time varying flow patterns in order to achieve mixing. For example, a microchamber with several electrodes along the walls and additional electrodes at the bottom was subjected to uniform magnetic field from a large Nd-magnet.68,74 Circulatory fluid motion was induced in the chamber by applying potential differences between the electrodes. Time and space variations in the electric field led to chaotic motion and thus mixing, as visualised with a dye. In another example, the parabolic flow profile of MHD-flow was exploited for mixing in circular channels.75,76 Two solutions were injected into the channel and MHD flow was initiated. The parabolic flow profile led to a large increase in interfacial surface area and thus to mixing. For example, fluorescein and 1 M KCl solution were mixed within 30 s at flow rates of 8 mm s−1.Trapping and transporting
Transport with electromagnets
Magnetic particles can be transported by pulling them from one field maximum to the next. In an early example, a plug of 2.8 µm particles was moved along a capillary by consecutive switching of several external tapered electromagnets.15 The same principle was used on a smaller scale to transport particles along meandering gold wires (Fig. 7(a)).28 Here, local field maxima were obtained by combining the field from the wires with a uniform background field. A plug of 4.5 µm particles was trapped from the suspension within the 200 µm × 200 µm area of a meander and moved along by switching between the wires. On a larger scale, transport of magnetic beads (1 µm) over a distance of 40 mm was reported using overlapping electromagnets, each 3.5 mm in diameter.20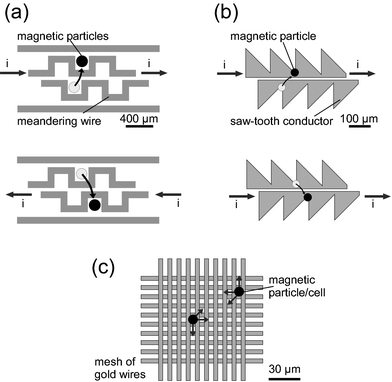 | ||
| Fig. 7 Magnetic particles can be transported with time-varying electromagnetic fields. This can be achieved (a) along a track of meandering gold wires,28 (b) along a saw tooth gold wire18 and (c) along a gold wire mesh.29 All figures were redrawn with permission. | ||
Very refined magnetic field maxima were obtained with saw-tooth shaped current carriers fabricated from gold (Fig. 7(b)).18 A single bead (2 µm) was trapped at the location of highest field gradient, i.e. at the edge of a ratchet element. By alternating the current between the wires at 0.1 Hz, the bead was pulled from one ratchet element to the next at 20 µm s−1.
Loops and meshes of gold wires were effective for trapping of magnetic particles,21 magnetically labelled yeast cells77 and magnetotactic bacteria.29 A loop of 120 µm diameter could trap magnetic particles (1–2 µm) from a drop of suspension.21 A smaller ring trap of 5 µm diameter, at the bottom of a PDMS microchannel was employed to capture magnetotactic bacteria.29 Meshes of 7 × 7 and 10 × 10 straight gold wires (Fig. 7(c)), each individually addressable, generated localised maxima of up to 100 mT.29 Cooling was necessary to prevent boiling. Magnetic particles as well as magnetically labelled yeast cells were trapped in these localised field maxima, i.e. with a precision of less than 10 µm. Particles could be transported in all directions across the mesh. Two groups of particles could be merged into a single group. Similarly, a group of cells was separated into two groups. Even single cell manipulation was feasible. Magnetotactic bacteria were trapped, lysed and the magnetic nanocrystals were retained.
Magnetic tweezers have been investigated for trapping of particles in microfluidic channels. Exact positioning was reported of magnetic particles (2.8 µm) in a capillary with a tapered electromagnet that was mounted onto a mechanical positioning stage.78 Magnetic nickel bars (250 nm wide and 9 µm long) were also suggested as a clamping system.14
Manipulation of diamagnetic objects
As mentioned earlier (eqn (1)), diamagnetic objects can be trapped in magnetic field minima and the trapping force can be increased by using a paramagnetic buffer. Polymer particles in a paramagnetic 0.6 M MnCl2 solution and human blood cells in a 0.1 M Mn2+ solution were trapped inside a capillary.79 A magnetic field minimum over the channel was realised by using two tapered NdFeB magnets with their north poles facing each other. Blood cells could be trapped from flows travelling up to 30 µm s−1. Trapping and manipulation of cells suspended in a paramagnetic buffer with 40 mM Gd3+ was also demonstrated.7 Again, two magnets were arranged with their north poles facing each other and tapered pole shapes were used to localise the field minimum. Polystyrene particles and a variety of cells were trapped from drops of suspension. The smallest item to be trapped was 2.5 µm in diameter. Transport over 50 µm was possible by moving the magnets and shifting the field minimum.Diamagnetic objects could also be levitated and transported along a 10 mm long channel.52 Droplets (30 µm) of glycerine/water in air were levitated and transported with 300 nm accuracy. To achieve levitation, two NdFeB magnets (500 mT) were arranged with their north poles facing each other such that a magnetic field minimum was present in the centre of the gap. The droplets overcame gravity and were levitated into this minimum. At the bottom of the gap, 25 µm wide electrodes were positioned in order to generate localised field minima. Sequential switching of these electromagnets enabled transport of the levitated droplets. Even rotation and merging of two droplets was possible. Other objects were levitated including microparticles, nanotube powders and blood cells. The trapping of diamagnetic objects has been proposed as an alternative to optical tweezers.7,52 This technique has a number of advantages: the capturing volume is large, objects larger than 10 µm can be trapped, trapping of a wider range of materials is feasible and heat problems due to high optical flux density do not occur.
Sorting and separation
High gradient magnetic separations are commonly performed in tubes or capillaries for separation of magnetic particles or cells.49,80,81 Field-flow fractionation (FFF)82,83 and split-flow thin (SPLITT) fractionation84,85 are continuous flow particle separation methods in which several forces, such as gravity, thermal gradients, electric or magnetic fields are combined.On the microfluidic scale, H-shaped channel networks can be used for magnetic separation. In one example (Fig. 8(a)), electromagnets A and B, 220 mT each, were positioned at each end of the connecting channel.86 Magnetic particles (2.8 µm) were pumped through one of the parallel channels with the magnet adjacent to this channel, i.e. magnet A, switched on. However, when magnet A was switched off and the magnet adjacent to the opposite parallel channel, i.e. magnet B, was switched on, particles at the channel junction were dragged through the connecting channel into the neighbouring channel. About 30 particles were isolated into the opposite parallel stream during each cycle. Another H-shaped device with two inlet channels merging into a wider channel and splitting into two outlet channels was also suggested for the separation of magnetic particles87 (Fig. 8(b)). A sample stream with particles could be introduced into one of the inlets, buffer solution through the other inlet. Without magnetic forces, the two streams left unmixed through the corresponding outlet channels. Application of a magnetic field gradient over the middle channel would drag magnetic particles out of their original stream into the buffer stream. The device has not yet been quantitatively evaluated.
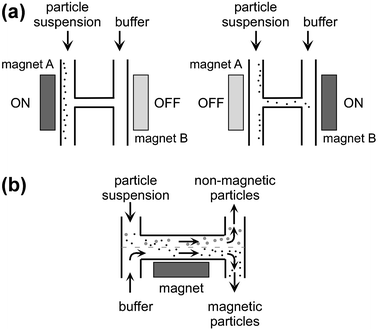 | ||
| Fig. 8 Principle of H-shaped separators: (a) for particle isolation into separate streams86 and (b) for continuous flow separation.87 Redrawn with permission. | ||
A continuous flow method capable of separating magnetic from non-magnetic particles as well as separating different magnetic particles from each other was termed on-chip free-flow magnetophoresis.88 Laminar flow was generated over a flat separation chamber by a number of inlet and outlet channels (Fig. 9(a)). Perpendicular to the direction of flow, a magnetic field was applied by an assembly of NdFeB magnets, resulting in a field gradient over the separation chamber. Non-magnetic particles left the chamber opposite the sample inlet. Magnetic particles however were dragged into the magnetic field and left the chamber via one of the other outlet channels (Fig. 9(b)). This deflection depended on the particle's magnetic susceptibility and the particle size (eqn (1)). The complete separation of 2.8 µm and 4.5 µm magnetic particles was demonstrated.89 Biological cells such as tumour cells and macrophages, internally labelled with magnetic nanoparticles, were separated according to their magnetic loading.90 In another example, ferromagnetic strips at the bottom a separation chamber assisted the separation of magnetic cells in continuous flow.16 The 10 µm wide nickel strips ran at an angle of 11° to the fluid flow. The flux lines from an external magnet (100 mT) were concentrated by the nickel strips, leading to local field gradients. Magnetically labelled leucocytes were attracted to the strips and found to flow along the strips rather than to follow the direction of fluid flow. Similar to free-flow magnetophoresis,88 flow rates had to be <1 mm s−1 in order for the magnetic force to be sufficient to induce deflection.
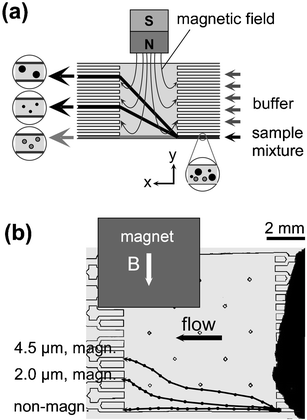 | ||
| Fig. 9 (a) The principle of free-flow magnetophoresis. (b) Separation of different types of magnetic particles from each other as well as from non-magnetic particles88 (redrawn with permission). | ||
Zabow et al.91 used magnetic forces in combination with capillary forces and surface tension to focus magnetic particles into the centre of a microchannel. An aqueous suspension of magnetic particles and air were pumped through a PDMS channel with a hydrophilic bottom and a hydrophobic top. The aqueous phase at the bottom bulged upwards. With a magnetic field applied at the top, the particles were pulled upwards to the air/water interface and subsequently they were focussed at the point of highest magnetic force along the interface, i.e. at the centre of the bulge.
Magnetic particles as solid supports for bioassays
Magnetic microparticles coated with biomolecules (Fig. 4) are often used as solid supports for biochemical reactions. Particle plugs can be formed in microchannels, featuring high surface to volume ratios and low diffusion distances for the reagents to the particle surface.4 Magnetic particles can be trapped without physical barriers by simply stopping them in flow with an external magnet. They can be released on demand by removing the magnetic field (Fig. 10).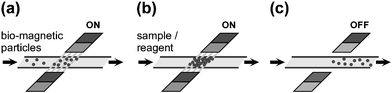 | ||
| Fig. 10 (a) Loading of magnetic particles into a microchannel. (b) Flushing with sample and reagents for bioanalysis. (c) Release of particles for downstream applications or disposal. | ||
Plug formation in microchannels
Choi, Ahn and co-workers reported several microfluidic devices with integrated electromagnets for trapping magnetic particles in flow. They fabricated 3D electromagnets with poles adjacent to a microchannel31 and planar magnets with serpentine27 and spiral shapes.26 Depending on the magnet design, currents of 500 mA31 to 30 mA27 were required to capture magnetic particles (1 µm) and hold them at the magnet poles. Particles could be retained at flow rates <1 mm s−1. A change in inductance was observed, when the particles were immobilised on the magnet, which could be exploited for particle quantification. Smistrup et al. also fabricated spiral electromagnets beneath a microchannel (Fig. 3)30 and applied 360 mA to the coil. Magnetic particles (1 µm) were captured in the region of strongest magnetic field on the magnet surface at a flow rate of 74 µm s−1. The capture efficiency was simulated to be about 90% but has not yet been verified experimentally.Nickel posts integrated at the bottom of a channel were also demonstrated for the capture of magnetic particles in flow.36 PDMS was bonded to a wafer which was patterned with nickel posts of 7 µm in height and 15 µm in diameter. Using an external NdFeB magnet (500 mT), the nickel posts were magnetised. With the strong field gradients over the posts, 4.5 µm diameter magnetic particles flowing through the channel could be captured, even though the posts were much smaller than the channel height. At a flow rate of 4.4 mm s−1 a capture efficiency of 95% was reported. Magnetic particles were also isolated from a mixture containing non-magnetic particles.
Immunoassays
Commercial capillary electrophoresis instrumentation in combination with external magnets was utilised for assays on magnetic particles.92 Dynabeads (2.8 µm) were coated with antibodies and stopped in flow with a cobalt magnet. Several enzymatic assays and antibody isolation were performed on the 2–3 mm long plug. Hayes et al. conducted immunoassays inside capillaries and glass chips.93 Magnetic particles coated with an appropriate antibody were trapped with a NdFeB magnet (240 mT). Antigen containing sample was slowly flushed through the channel, at about 1 mm s−1, to allow for antibody–antigen binding. The fast reaction times (few min) and the small reaction volume (µL) enabling high sensitivity detection were highlighted. Assays for fluorescein isothiocyanate (FITC), parathyroid hormone (PTH) and interleukin-5 (IL-5) were also demonstrated.Choi, Ahn and co-workers demonstrated an enzyme immunoassay94 by immobilising particles with a spiral electromagnet26 and detecting the assay reaction product with an integrated electrochemical sensor positioned at the top of the channel above the magnet. The sandwich assay was based on 2.8 µm Dynabeads coated with an antibody. These were trapped, reacted with antigen solution and subsequently with a secondary antibody, which was labelled with an enzyme. The authors emphasise the fast reaction time of 20 min for the entire assay procedure and low sample volumes of a few µL.
DNA and RNA hybridisation
Fast DNA hybridisation was reported on plugs of magnetic particles within a microfluidic device.95 Magnetic particles (2.8 µm) were coated with target DNA, pumped through a microfluidic network with eight parallel channels and trapped with external permanent magnets (600 mT) to form a plug of less than 1 mm in length. Complementary probe DNA was then pumped through the channel network for reaction with the particle surface. Captured probe DNA could also be released from the plug by heating to 87 °C. Thus, the beads could be re-used up to 12 times.mRNA was isolated from total RNA with capture efficiencies of 50% using a plug of magnetic particles in a microfluidic channel.96 Magnetic particles (2.8 µm) coated with oligo-dT were immobilised at a flow rate of 4.8 mm s−1. Specific capturing of RNA from Dengue fever virus in a polymer microchip was also reported.97 Magnetic particles (2.8 µm) were coated with an appropriate DNA probe and immobilised using a small permanent magnet (250 mT) positioned near the microchannel. The flow rate was 3 mm s−1. The authors stress the high sensitivity of their sensor in comparison to conventional sensors for the same pathogen.
Tryptic digestion
Tryptic digestion of proteins was demonstrated on-chip with a plug of magnetic particles that were coated with trypsin.98 A protein solution was passed through the 6 mm long particle plug at 3.5 µm s−1. Digestion of glycoproteins was possible within 15 min, in a very small reaction volume.Cells
Magnetically labelled cells have been investigated only recently. Jurkat cells were isolated from blood with anti-CD3 coated magnetic particles (1–2 µm diameter).99 Blood and particles were mixed off-chip and then trapped in the microchannel at flow rates between 0.14 and 1.4 mm s−1. The capture rate was about 50% from a 2 µL sample. In another device, low abundance T-cells were isolated from whole blood.9 A small NdFeB magnet was employed to capture anti-CD3 coated Dynabeads. Subsequently, whole blood was pumped through the particle bed at 190 µm s−1. Best capture rates were achieved in a device with 8 parallel channels, but efficiencies did not exceed 37%. Optimisation was a trade-off between a sufficiently dense particle bed and the high shear stresses that would ultimately push the magnetic particles out of the trap. A more complex microdevice for cell capture on chip and PCR combined with DNA detection was employed for E. coli analysis.100 The bacteria cells contained in a blood sample were attached to 2.8 µm Dynabeads during a 20 min incubation step. Subsequently the mixture was pumped into the microdevice and the magnetic E. coli cells were captured and isolated from the blood sample with an external magnet. This was followed by on-chip PCR and DNA detection.Self-assembly and patterning
The term self-assembly has often been used to describe the formation of particle plugs or chains in microchannels. Dynamic self-assembly has been used to describe the behaviour of magnetic particles in time-varying or rotating magnetic fields. Here, the emphasis is on the study of basic phenomena of magnetic particle assembly and on controlling the assembly into specific features and patterns.Studies on micron-scale magnetic behaviour
Microfluidic devices offer the possibility to study fundamental aspects of magnetism that are impossible to access in the bulk. Researchers have calculated and verified experimentally the formation of chains or columns of magnetic particles within microfluidic chambers and how these columns interact with each other to form characteristic patterns.101,102 Physical laws were found to explain the spacing of particle columns in droplets of ferrofluids.103The behaviour of magnetic particle columns was examined under the influence of electroosmotic and pressure driven flows.104 In a uniform magnetic field (500 mT) the particles formed columns in the direction of the field lines. Upon application of a pressure driven flow of 20 µm s−1, the particle columns were pushed through the channel, but no deformation was observed as might have been expected from the parabolic flow profile. Aggregation of magnetic particles in the presence of pressure driven flow was also studied.105 A suspension of particles (0.83 µm) was introduced into a microchannel and a uniform magnetic field (17 mT or 40 mT) was applied parallel to the flow direction with SmCo magnets. The particles did not form any aggregates, because there was no field gradient and thus no force on the particles (see eqn (1)). When flow was applied with rates between 21 and 333 µm s−1, the particles started to move past each other in close proximity and formed particle chains along the field lines of the applied magnetic field, before they were dragged away by the fluid. The authors investigated this behaviour theoretically and experimentally.
Magnetic microparticles can be forced to assemble into pyramids106 and small rings107 (Fig. 11). In a homogeneous magnetic field particles generally form chains. Self-assembly into pyramids is favourable, if the particles forming the base of the pyramid are fixed, for example at the liquid/air interface of a droplet. The authors verified this theory with a particle suspension (2.8 µm) and a magnetic field of about 1 mT, which is less than the magnetic saturation of the beads. In another example, a domain wall of a magnetic film, i.e. a bismuth line in a ferrite film was used for patterning. Particles were attracted towards the domain wall. At flux densities between 0.5 mT and 1.25 mT, pyramids of 3, 6, 10, 15 or even 21 particles formed, the domain wall being at the base of the pyramid. At fields larger than 1.25 mT, colloidal chains were formed. The concept of domain walls was investigated further by arranging these domain walls into stripes and mazes.108 Magnetic particles accumulated at the domain boundaries and were observed to self-assemble into patterns depending on the complexity of the domain pattern. Patterns changed when the applied magnetic field strength was changed. Rings of magnetic particles were obtained from an emulsion of decane droplets (10 to 100 µm diameter) in water.107
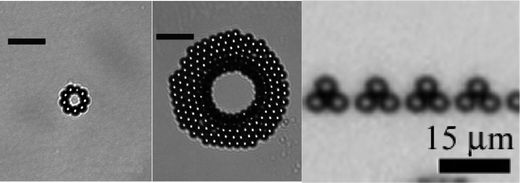 | ||
| Fig. 11 Magnetic microparticles (2.8 µm Dynabeds) assembled into rings107 and pyramids106 (with permission of the ACS). | ||
DNA separation on self-assembled magnet columns
Magnetic particle columns have been used as a sieving matrix for DNA separation as an alternative to gels or tediously fabricated nanocolumns. Viovy's group investigated this approach109,110 by assembling magnetic particles (0.5 or 1 µm) into columns inside a microchannel using a homogeneous magnetic field (10 mT). A fairly regular inter-column distance was observed which could also be fine tuned by changing the particle concentration or the magnetic flux density. A mixture of DNA molecules was pumped through the channel by electroosmotic flow and the DNA molecules became entangled with the columns. The longer the DNA molecule, the longer it was retarded by the column, thus DNA strands of different lengths could be separated from each other. This was demonstrated with the rapid separation of λ-phage DNA, 2 λ-DNA and bacteriophage T4 DNA.110 The particle bed could be flushed out of the microchannel easily and a new plug of particles could be introduced and assembled.Patterning of surfaces
A modulated magnetic field (see Fig. 2(c)) was employed to pattern diamagnetic particles and cells onto a surface.5,6 The modulated field was obtained by a stack of alternating iron and aluminium sheets, each layer 300 µm thick, which was placed into a homogeneous magnetic field of 1 T. Due to the different magnetic susceptibilities, the field lines were denser in the iron layers. Hence, the flux density was lower above the iron, and higher above the aluminium layers. When a suspension containing diamagnetic polymer particles was placed above this modulator, the particles accumulated above the iron layers, in the form of lines with 300 µm width.6 The process took about 15 min. When manganese chloride was added to the buffer, the magnetic susceptibility of the buffer was increased and hence the force on the polystyrene particles was increased (see eqn (1)). The particles were then observed to move almost immediately to the area above the iron layers. The same principle was also applied to biological cells.5Detection
In most of the examples above, magnetic particles were observed using optical microscopy. In some cases, bioassays were carried out using fluorescence signals. However, the magnetic properties of the particles could also be used for detection. The technical challenge is to develop detectors that are sensitive enough to count a single magnetic particle, ideally in continuous flow.Giant magnetoresistive (GMR) sensors
GMR sensors consist of a film made from alternating magnetic and non-magnetic layers. The electrical resistance of this film undergoes a large change as a function of the applied magnetic field strength. When a magnetic particle is in the vicinity of the sensor, the magnetic field on the sensor will be slightly altered and this can be detected.Miniaturised GMR sensors have so far been mostly employed for the detection of DNA hybridisation. Typically, DNA probes have been immobilised on the insulating layer above such a sensor. Then, sample DNA was flushed over the sensors and allowed to hybridise with complementary DNA. The hybridised double strands were then labelled with magnetic particles which could be detected by the GMR sensor. In one example, a flow cell was positioned over an array of 64 GMR sensors, divided into eight groups for the detection of up to eight DNA sequences.44 Dynabeads of 2.8 µm diameter were used for magnetic labelling. The beads could be detected with the GMR sensor and the signal intensity indicated the amount of particles. However, detection was not possible at the single bead level. Sensitivity could be improved by using a thinner insulating layer above the sensor, or by using particles with a greater magnetic susceptibility. In a more recent paper, the detection of as little as ten Dynabeads per sensor was achieved.45 Single bead detection was possible with a highly magnetic uncoated nickel–iron particle (3.3 µm), which unfortunately, was not biocompatible. In another example, an array of 206 sensor elements was fabricated. Each element had a diameter of 70 µm.111 Three types of commercially available magnetic particles with 0.35, 0.86 and 0.90 µm diameters were compared. Detection was feasible with a surface coverage of at least 5%, corresponding to about 2000 magnetic particles.
Pekas et al. demonstrated the use of GMR sensors for magnetic detection in continuous flow within a microfluidic channel.112 In this case, plugs of ferrofluid in a non-magnetic oil were passed through a PDMS channel. Four GMR sensors (20 µm × 4 µm) were located at the bottom of this channel. The ferrofluid plugs were 86 µm long, each containing about 5 × 108 magnetic nanoparticles. 5 plugs could be measured in 50 ms. Measurement of plug velocities and size and counting of the number of plugs were possible.
Spin-valve sensors
Spin-valve sensors also consist of multiple layers of magnetic and non-magnetic metals. Their magnetoresistance (MR) changes depending on the magnetic field. Magnetic particles in the vicinity of the sensor can be directly detected as an electrical signal. Traditionally, such sensors have been used as read-heads of hard-disk drives.The velocity detection of 250 nm paramagnetic particles within microfluidic channels was investigated.113 A PDMS channel was fitted on a substrate with two integrated spin-valve sensors, 1.65 mm apart, each sensor with an area of 2 µm by 6 µm. The particles were pumped through the channel and magnetised in flow by two aluminium wire electromagnets positioned next to the sensors. A field gradient pulled the particles close to the sensor, so that the passing particles could be detected. The velocity was measured from the time it took the particles to pass between two sensors. In this case flow rates between 50 and 300 µm s−1 were used. Detection of single particles was not possible. Instead the authors reported detection of particle plugs that were pumped through the channel alternately with water plugs. DNA hybridisation has also been detected using spin-valve sensors.114 Probe DNA was immobilised on the sensor surface and the target DNA was labelled with magnetic beads (250 nm). Aluminium wire electromagnets were again used to attract the magnetic particles into close proximity to the sensor and to allow for the hybridisation reaction to take place. After washing away of any unbound molecules, the remaining magnetic-DNA molecules could be detected by the sensor. About 50 to 100 magnetic particles were sufficient for detection. These reactions were performed in 10 to 20 µL droplets.
Superconducting quantum interference devices (SQUID)
SQUID microscopes can scan surfaces and provide magnetic mapping with very high sensitivity (nT) at modest spatial resolution (10 µm). Instrumental demands include magnetically shielded rooms and cooling for the superconductors to work. Katsura et al. worked on applications that are close to microfluidics.115 An 8 mm long plug of ferrofluid (11 nm diameter particles) was pumped through a plastic tube at flow rates of 0.3 to 1.1 mm s−1 and this could be detected by the microscope. The minimum number of particles for detection was 108. They also performed a DNA hybridisation assay on a glass slide with magnetic particles used as labels. After washing off any unbound label, they scanned the surface and were able to detect hybridisation.Miniaturised hall sensors
The Hall effect occurs when a current and a magnetic field are applied perpendicular to each other over a conducting material. Perpendicular to both fields, a change in voltage can be detected that is proportional to the magnetic field strength.Microfabrication of Hall sensors is somewhat easier than SQUID or GMR sensors. Hall sensors with a footprint area of several tens to 100 µm2 were fabricated from nickel or perm-alloy connected to aluminium wires. With a micromanipulator, a single 2.8 µm Dynabead was placed on the sensor and could be detected.116 Clusters of nanoparticles (250 nm) and single 2 µm particles could also be detected from droplets.117,118 The authors stressed that Hall sensors should exhibit a lower noise level than GMR or SQUID sensors. They envisage an array of such sensors to be used for mapping, for example in combination with DNA hybridisation arrays.
In conclusion, there have been promising developments in magnetic particle detection in recent years. Single particle detection is now feasible, if a particle is in close vicinity to a sensor. Continuous flow detection was possible for plugs of ferrofluids. However, the counting of single particles in continuous flow has not yet been achieved.
NMR on microchips
NMR spectroscopy is one of the most powerful methods for the structural elucidation of molecules. Microfluidics in combination with NMR would allow for measuring small sample volumes. Kakuta et al. used a microfluidic mixing chip that was directly connected to a microcoil NMR probe in order to study the kinetics of protein conformation changes.119Several groups, however, have attempted to miniaturise the coil for excitation and detection of NMR signals and fabricate it in close proximity to a microfluidic channel.120 A particular challenge is the fact, that the magnetic field must be strong as well as uniform in order get sufficient sensitivity and spectral resolution. This imposes certain requirements for the coil design and also for the flow cell.
Trumbull et al. reported the first example of on-chip NMR.34 They integrated a planar NMR coil onto the back surface of a glass microfluidic chip. The fluidic network contained a reservoir of 1.4 mm diameter and 20 µm depth. A single turn coil copper electromagnet was located below the reservoir and connected to a PCB board for electrical control. The chip was inserted into the bore of a 5.9 T superconducting magnet. The miniaturised integrated coil was used for pulse transmission and for receiving the free induction decay (FID) signals. Proton spectra of a 30 nL sample of water/ethanol were measured with a linewidth of 1.39 Hz. Massin et al. reported NMR spectroscopy with a three-turn copper microcoil integrated into glass microfluidic chips with chambers of 30 nL, 120 nL or 470 nL volume.23,121 The coils had a diameter of 2 mm or less and the distance between the channel and the coil was 65 µm. Again the microchip was connected to a PCB board and the assembly was inserted into a modified conventional NMR machine. Spectra were taken of 160 µg of sucrose in D2O for proof of concept with a line width of much larger than 1 Hz.
Sorli et al. fabricated a copper microcoil of 500 µm × 500 µm at 200 µm distance to a microchannel of a silicon/glass device.22 The device was inserted into a 2 T magnetic field. Spectra of water and ethanol were reported with a linewidth of 12 Hz. Wensink et al. fabricated a coil at 80 µm distance from a microchannel.24 The detection volume above the coil was 56 nL and residence times were between 9 s and 30 min, depending on the applied flow rates. The chip was placed into a superconducting magnetic field of 1.4 T. Two chemicals were introduced into the microfluidic chip (benzaldehyde and aniline) and merged just upstream of the coil section. The formation of the reaction products (imine and water) was studied at different flow rates.
Walton, Goleshevsky and others fabricated a dual coil microfluidic NMR cell.25,120 Three-turn coils with diameters of about 4 mm were located above and below a microchannel. The authors took 13C spectra of labelled methanol with a linewidth of 15 Hz, as well as 31P spectra of phosphoric acid with a linewidth of 12 Hz. Even a 13C COSY pulse sequence of acetic acid was achieved with a sampling time of one hour.
Outlook
Magnetism is now a widely applicable item in the microfluidicist's tool box. Many applications have been investigated, but not all of them are competitive with the more conventionally used methods. For example, some of the magnetic mixers are complex to fabricate in comparison to other microfluidic mixers. Some of the magnetic pumps are too slow or generate poor pressure heads. As always, the question lies in the requirements for a particular application. The unique advantages of magnetic manipulation lie in the possibility of externally controlling matters inside a microchannel. This can be a magnetic rotor for mixing or a single magnetic particle or cell for trapping and transport.Further developments are likely in several areas. One trend within the microfluidics community is the design of integrated devices in which sample pre-treatment, isolation, separation and/or detection are combined. Labelling with magnetic particles for isolation is an elegant option in such devices.100 Another trend is cell analysis on microfluidic platforms. Magnetic forces can be employed to trap and position cells7,9 and more work is likely to emerge in this field. Reactions on plugs of magnetic particles have so far been limited to one plug. In the future, a sample could be flushed through several particle beds with different surface chemistries, in order to test for several analytes simultaneously.122 Several fundamental studies on magnetic particle behaviour have been published. With increasing insight into the fabrication of stronger and/or smaller magnetic particles, more studies are likely to be undertaken, possibly even in nanochannels to study single domains. The effect of magnetic fields on bioreactions123 and cells, as well as on the growth of crystals and films124 is of great interest and could be studied in microfluidic devices. Self-assembly of magnetic objects into complex three-dimensional structures125 or into small machines126 is only starting to be investigated and may soon be transferred to the micro- or nanoscale. New materials, such as magnetically doped PDMS,61 magnetic microgels,127 magnetic wires, magnetic nanotubes are or will soon be finding their way into microfluidic applications.
The potential of magnetic forces for microfluidic applications has been realised and many proof of principle devices have been reported. With advances in this field on so many fronts, more sophisticated devices will emerge and be part of integrated and hopefully widely applicable micro-total analysis systems.
Acknowledgements
The author would like to thank Alexander Iles for comments on the manuscript and the Japanese Ministry for Education, Culture, Sports, Science and Technology (MEXT) for funding.References
- T. Vilkner, D. Janasek and A. Manz, Anal. Chem., 2004, 76, 3373 CrossRef CAS
.
- D. R. Reyes, D. Iossifidis, P. A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623 CrossRef CAS
.
- P. A. Auroux, D. Iossifidis, D. R. Reyes and A. Manz, Anal. Chem., 2002, 74, 2637 CrossRef CAS
.
- E. Verpoorte, Lab Chip, 2003, 3, 60N RSC
.
- T. Kimura, Y. Sato, F. Kimura, M. Iwasaka and S. Ueno, Langmuir, 2005, 21, 830 CrossRef CAS
.
- T. Kimura, M. Yamato and A. Nara, Langmuir, 2004, 20, 572 CrossRef CAS
.
- A. Winkleman, K. L. Gudiksen, D. Ryan, G. M. Whitesides, D. Greenfield and M. Prentiss, Appl. Phys. Lett., 2004, 85, 2411 CrossRef CAS
.
- A. Homsy, S. Koster, J. C. T. Eijkel, A. van den Berg, F. Lucklum, E. Verpoorte and N. F. de Rooij, Lab Chip, 2005, 5, 466 RSC
.
- V. I. Furdui and D. J. Harrison, Lab Chip, 2004, 4, 614 RSC
.
- P. K. Yuen, G. S. Li, Y. J. Bao and U. R. Muller, Lab Chip, 2003, 3, 46 RSC
.
- L. H. Lu, K. S. Ryu and C. Liu, J. Microelectromech. Syst., 2002, 11, 462 CrossRef CAS
.
- B. A. Grzybowski and C. J. Campbell, Chem. Eng. Sci., 2004, 59, 1667 CrossRef CAS
.
- A. Rida and M. A. M. Gijs, Anal. Chem., 2004, 76, 6239 CrossRef CAS
.
- M. Barbic, J. Magn. Magn. Mater., 2002, 249, 357 CrossRef CAS
.
- J. Joung, J. Shen and P. Grodzinski, IEEE Trans. Magn., 2000, 36, 2012 CrossRef
.
- D. W. Inglis, R. Riehn, R. H. Austin and J. C. Sturm, Appl. Phys. Lett., 2004, 85, 5093 CrossRef CAS
.
- K. S. Ryu, K. Shaikh, E. Goluch, Z. F. Fan and C. Liu, Lab Chip, 2004, 4, 608 RSC
.
- R. Wirix-Speetjens and J. de Boeck, IEEE Trans. Magn., 2004, 40, 1944 CrossRef
.
- Q. Ramadan, V. Samper, D. Poenar and C. Yu, J. Magn. Magn. Mater., 2004, 281, 150 CrossRef CAS
.
- A. Rida, V. Fernandez and M. A. M. Gijs, Appl. Phys. Lett., 2003, 83, 2396 CrossRef CAS
.
- C. S. Lee, H. Lee and R. M. Westervelt, Appl. Phys. Lett., 2001, 79, 3308 CrossRef CAS
.
- B. Sorli, J. F. Chateaux, M. Pitaval, H. Chahboune, B. Favre, A. Briguet and P. Morin, Meas. Sci. Technol., 2004, 15, 877 CrossRef CAS
.
- C. Massin, F. Vincent, A. Homsy, K. Ehrmann, G. Boero, P. A. Besse, A. Daridon, E. Verpoorte, N. F. de Rooij and R. S. Popovic, J. Magn. Reson., 2003, 164, 242 CrossRef CAS
.
- H. Wensink, F. Benito-Lopez, D. C. Hermes, W. Verboom, H. Gardeniers, D. N. Reinhoudt and A. van den Berg, Lab Chip, 2005, 5, 280 RSC
.
- J. H. Walton, J. S. de Ropp, M. V. Shutov, A. G. Goloshevsky, M. J. McCarthy, R. L. Smith and S. D. Collins, Anal. Chem., 2003, 75, 5030 CrossRef CAS
.
- J. W. Choi, T. M. Liakopoulos and C. H. Ahn, Biosens. Bioelectron., 2001, 16, 409 CrossRef CAS
.
- J. W. Choi, C. H. Ahn, S. Bhansali and H. T. Henderson, Sens. Actuators, B, 2000, 68, 34 CrossRef
.
- T. Deng, G. M. Whitesides, M. Radhakrishnan, G. Zabow and M. Prentiss, Appl. Phys. Lett., 2001, 78, 1775 CrossRef CAS
.
- H. Lee, A. M. Purdon, V. Chu and R. M. Westervelt, Nano Lett., 2004, 4, 995 CrossRef CAS
.
- K. Smistrup, O. Hansen, H. Bruus and M. F. Hansen, J. Magn. Magn. Mater., 2005, 293, 597 CrossRef CAS
.
- C. H. Ahn, M. G. Allen, W. Trimmer, Y. N. Jun and S. Erramilli, J. Microelectromech. Syst., 1996, 5, 151 CrossRef CAS
.
- J. A. Rogers, R. J. Jackman and G. M. Whitesides, J. Microelectromech. Syst., 1997, 6, 184 CrossRef
.
- D. J. Sadler, T. M. Liakopoulos and C. H. Ahn, J. Microelectromech. Syst., 2000, 9, 460 CrossRef CAS
.
- J. D. Trumbull, I. K. Glasgow, D. J. Beebe and R. L. Magin, IEEE Trans. Biomed. Eng., 2000, 47, 3 CrossRef CAS
.
- E. Mirowski, J. Moreland, A. Zhang, S. E. Russek and M. J. Donahue, Appl. Phys. Lett., 2005, 86 Search PubMed
.
- T. Deng, M. Prentiss and G. M. Whitesides, Appl. Phys. Lett., 2002, 80, 461 CrossRef CAS
.
- G. A. Mensing, T. M. Pearce, M. D. Graham and D. J. Beebe, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 1059 CrossRef
.
- Q. A. Pankhurst, J. Connolly, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2003, 36, R167 CrossRef CAS
.
- M. A. M. Gijs, Microfluidics Nanofluidics, 2004, 1, 22 Search PubMed
.
- X. Q. Liu, Y. P. Guan, H. Z. Liu, Z. Y. Ma, Y. Yang and X. B. Wu, J. Magn. Magn. Mater., 2005, 293, 111 CrossRef CAS
.
- Z. Y. Ma, Y. P. Guan, X. Q. Liu and H. Z. Liu, J. Appl. Polym. Sci., 2005, 96, 2174 CrossRef CAS
.
- X. Q. Liu, Y. P. Guan, Z. Y. Ma and H. Z. Liu, Langmuir, 2004, 20, 10278 CrossRef CAS
.
- X. Q. Liu, Y. P. Guan, Y. Yang, Z. Y. Ma, X. B. Wu and H. Z. Liu, J. Appl. Polym. Sci., 2004, 94, 2205 CrossRef CAS
.
- M. M. Miller, P. E. Sheehan, R. L. Edelstein, C. R. Tamanaha, L. Zhong, S. Bounnak, L. J. Whitman and R. J. Colton, J. Magn. Magn. Mater., 2001, 225, 138 CrossRef CAS
.
- J. C. Rife, M. M. Miller, P. E. Sheehan, C. R. Tamanaha, M. Tondra and L. J. Whitman, Sens. Actuators, B, 2003, 107, 209 CrossRef
.
- D. R. Baselt, G. U. Lee, M. Natesan, S. W. Metzger, P. E. Sheehan and R. J. Colton, Biosens. Bioelectron., 1998, 13, 731 CrossRef CAS
.
- I. Safarik and M. Safarikova, J. Chromatogr., B: Biomed. Appl., 1999, 722, 33 CrossRef CAS
.
- C. Wilhelm, F. Gazeau, J. Roger, J. N. Pons and J. C. Bacri, Langmuir, 2002, 18, 8148 CrossRef CAS
.
- M. Zborowski, P. S. Malchesky, T. F. Jan and G. S. Hall, J. Gen. Microbiol., 1992, 138, 63 CAS
.
- S. Hutzler, D. Weaire, F. Elias and E. Janiaud, Philos. Mag. Lett., 2002, 82, 297 CrossRef CAS
.
- M. D. Simon and A. K. Geim, J. Appl. Phys., 2000, 87, 6200 CrossRef CAS
.
- I. F. Lyuksyutov, D. G. Naugle and K. D. D. Rathnayaka, Appl. Phys. Lett., 2004, 85, 1817 CrossRef CAS
.
- J. Jang and S. S. Lee, Sens. Actuators, A, 2000, 80, 84 CrossRef
.
- J. H. Zhong, M. Q. Yi and H. H. Bau, Sens. Actuators, B, 2002, 96, 59 CrossRef
.
- A. V. Lemoff and A. P. Lee, Sens. Actuators, B, 2000, 63, 178 CrossRef
.
- J. West, B. Karamata, B. Lillis, J. P. Gleeson, J. Alderman, J. K. Collins, W. Lane, A. Mathewson and H. Berney, Lab Chip, 2002, 2, 224 RSC
.
- J. C. T. Eijkel, C. Dalton, C. J. Hayden, J. P. Burt and A. Manz, Sens. Actuators, B, 2003, 92, 215 CrossRef
.
- J. C. T. Eijkel, A. van den Berg and A. Manz, Electrophoresis, 2004, 25, 243 CrossRef CAS
.
- A. Hatch, A. E. Kamholz, G. Holman, P. Yager and K. F. Bohringer, J. Microelectromech. Syst., 2001, 10, 215 CrossRef CAS
.
- J. J. Ahn, J. G. Oh and B. Choi, Microsyst. Technol.—Micro- Nano- Inf. Storage Process. Syst., 2004, 10, 622 Search PubMed
.
- H. Hartshorne, C. J. Backhouse and W. E. Lee, Sens. Actuators, B, 2004, 99, 592 CrossRef
.
- W. He, S. J. Lee, D. C. Jiles, D. H. Schmidt, M. D. Porter and R. Shinar, J. Appl. Phys., 2003, 93, 7459 CrossRef CAS
.
- Y. Melikhov, S. J. Lee, D. C. Jiles, D. H. Schmidt, M. D. Porter and R. Shinar, J. Appl. Phys., 2003, 93, 8438 CrossRef CAS
.
- L. J. Love, J. F. Jansen, T. E. McKnight, Y. Roh and T. J. Phelps, IEEE Trans. Nanobiosci., 2004, 3, 101 Search PubMed
.
- J. Atencia and D. J. Beebe, Lab Chip, 2004, 4, 598 RSC
.
- W. C. Jackson, H. D. Tran, M. J. O'Brien, E. Rabinovich and G. P. Lopez, J. Vac. Sci. Technol., B, 2001, 19, 596 CrossRef CAS
.
- A. V. Lemoff and A. P. Lee, Biomed. Microdev., 2003, 5, 55 Search PubMed
.
- H. H. Bau, J. Z. Zhu, S. Z. Qian and Y. Xiang, Sens. Actuators, B, 2003, 88, 207
.
- M. Barbic, J. J. Mock, A. P. Gray and S. Schultz, Appl. Phys. Lett., 2001, 79, 1399 CrossRef CAS
.
- S. L. Biswal and A. P. Gast, Anal. Chem., 2004, 76, 6448 CrossRef CAS
.
- C. J. Campbell and B. A. Grzybowski, Philos. Trans. R. Soc. London, Ser. A, 2004, 362, 1069 CrossRef
.
- A. Rida and M. A. M. Gijs, Appl. Phys. Lett., 2004, 85, 4986 CrossRef CAS
.
- H. Suzuki, C. M. Ho and N. Kasagi, J. Microelectromech. Syst., 2004, 13, 779 CrossRef
.
- S. Z. Qian, J. Z. Zhu and H. H. Bau, Phys. Fluids, 2002, 14, 3584 CrossRef CAS
.
- J. West, J. P. Gleeson, J. Alderman, J. K. Collins and H. Berney, Sens. Actuators, B, 2003, 96, 190 CrossRef
.
- J. P. Gleeson, O. M. Roche, J. West and A. Gelb, Siam J. Appl. Math., 2004, 64, 1294 Search PubMed
.
- H. Lee, A. M. Purdon and R. M. Westervelt, Appl. Phys. Lett., 2004, 85, 1063 CrossRef CAS
.
- M. Barbic, J. J. Mock, A. P. Gray and S. Schultz, Appl. Phys. Lett., 2001, 79, 1897 CrossRef CAS
.
- H. Watarai and M. Namba, J. Chromatogr. A, 2002, 961, 3 Search PubMed
.
- M. Zborowski, L. R. Moore, P. S. Williams and J. J. Chalmers, Sep. Sci. Technol., 2002, 37, 3611 CAS
.
- I. Safarik and M. Safarikova, J. Chromatogr., B: Biomed. Appl., 1999, 722, 33 CrossRef CAS
.
- A. H. Latham, R. S. Freitas, P. Schiffer and M. E. Williams, Anal. Chem., 2005, 77, 5055 CrossRef CAS
.
- P. C. Gascoyne, C. Das, J. Vykoukal, R. Weinstein, A. Gandini, D. Parks and R. Sawh, Abstr. Pap. – Am. Chem. Soc., 2003, 225, U138
.
- C. B. Fuh and S. Y. Chen, J. Chromatogr., A, 1998, 813, 313 Search PubMed
.
- C. B. Fuh and S. Y. Chen, J. Chromatogr., A, 1999, 857, 193 Search PubMed
.
- S. Ostergaard, G. Blankenstein, H. Dirac and O. Leistiko, J. Magn. Magn. Mater., 1999, 194, 156 CrossRef CAS
.
-
N. Chronis, W. Lam and L. Lee, Micro Total Analysis Systems 2001, ed. J. M. Ramsey and A. van den Berg, Kluwer Academic Publishers, Monterey, USA, 2001, p. 497 Search PubMed
.
- N. Pamme and A. Manz, Anal. Chem., 2004, 76, 7250 CrossRef CAS
.
-
N. Pamme and A. Manz, Micro Total Analysis Systems 2004, ed. T. Laurell et al., Kluwer Academic Publishers, Malmö, Sweden, 2004, p. 49 Search PubMed
.
-
N. Pamme and C. Wilhelm, Micro Total Analysis Systems 2005, ed. K. Jensen, Kluwer Academic Publishers, Boston, USA, 2005, p. 1389 Search PubMed
.
- G. Zabow, F. Assi, R. Jenks and M. Prentiss, Appl. Phys. Lett., 2002, 80, 1483 CrossRef CAS
.
- L. G. Rashkovetsky, Y. V. Lyubarskaya, F. Foret, D. E. Hughes and B. L. Karger, J. Chromatogr., A, 1997, 781, 197 Search PubMed
.
- M. A. Hayes, N. A. Polson, A. N. Phayre and A. A. Garcia, Anal. Chem., 2001, 73, 5896 CrossRef CAS
.
- J. W. Choi, K. W. Oh, J. H. Thomas, W. R. Heineman, H. B. Halsall, J. H. Nevin, A. J. Helmicki, H. T. Henderson and C. H. Ahn, Lab Chip, 2002, 2, 27 RSC
.
- Z. H. Fan, S. Mangru, R. Granzow, P. Heaney, W. Ho, Q. P. Dong and R. Kumar, Anal. Chem., 1999, 71, 4851 CrossRef CAS
.
- G. F. Jiang and D. J. Harrison, Analyst, 2000, 125, 2176 RSC
.
- S. Kwakye and A. Baeumner, Anal. Bioanal. Chem., 2003, 376, 1062 CAS
.
- L. Korecka, Z. Bilkova, M. Holeapek, J. Kralovsky, M. Benes, J. Lenfeld, N. Minc, R. Cecal, J. L. Viovy and M. Przybylski, J. Chromatogr., B, 2004, 808, 15 CrossRef CAS
.
- V. I. Furdui, J. K. Kariuki and D. J. Harrison, J. Micromech. Microeng., 2003, 13, S164 CrossRef
.
- R. H. Liu, J. N. Yang, R. Lenigk, J. Bonanno and P. Grodzinski, Anal. Chem., 2004, 76, 1824 CrossRef CAS
.
- T. Ukai and T. Maekawa, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2004, 69
.
- Y. Nagaoka, H. Morimoto and T. Maekawa, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2005, 71
.
- J. Liu, M. Lawrence, A. Wu, M. L. Ivey, G. A. Flores, K. Javier, J. Bibette and J. Richard, Phys. Rev. Lett., 1995, 74, 2828 CrossRef CAS
.
- M. A. Hayes, N. A. Polson and A. A. Garcia, Langmuir, 2001, 17, 2866 CrossRef CAS
.
- E. Brunet, G. Degre, F. Okkels and P. Tabeling, J. Colloid Interface Sci., 2005, 282, 58 CrossRef CAS
.
- L. E. Helseth, Langmuir, 2005, 21, 7276 CrossRef CAS
.
- L. E. Helseth, R. M. Muruganathan, Y. Zhang and T. M. Fischer, Langmuir, 2005, 21, 7271 CrossRef CAS
.
- L. E. Helseth, T. Backus, T. H. Johansen and T. M. Fischer, Langmuir, 2005, 21, 7518 CrossRef CAS
.
- N. Minc, C. Futterer, K. Dorfman, A. Bancaud, C. Gosse, C. Goubault and J. L. Viovy, Anal. Chem., 2004, 76, 3770 CrossRef CAS
.
- P. S. Doyle, J. Bibette, A. Bancaud and J. L. Viovy, Science, 2002, 295, 2237 CrossRef CAS
.
- J. Schotter, P. B. Kamp, A. Becker, A. Puhler, G. Reiss and H. Bruckl, Biosens. Bioelectron., 2004, 19, 1149 CrossRef CAS
.
- N. Pekas, M. D. Porter, M. Tondra, A. Popple and A. Jander, Appl. Phys. Lett., 2004, 85, 4783 CrossRef CAS
.
- H. A. Ferreira, D. L. Graham, P. Parracho, V. Soares and P. P. Freitas, IEEE Trans. Magn., 2004, 40, 2652 CrossRef CAS
.
- D. L. Graham, H. A. Ferreira, N. Feliciano, P. P. Freitas, L. A. Clarke and M. D. Amaral, Sens. Actuators, B, 2005, 107, 936 CrossRef
.
- S. Katsura, T. Yasuda, K. Hirano, A. Mizuno and S. Tanaka, Supercond. Sci. Technol., 2001, 14, 1131 CrossRef CAS
.
- P. A. Besse, G. Boero, M. Demierre, V. Pott and R. Popovic, Appl. Phys. Lett., 2002, 80, 4199 CrossRef CAS
.
- L. Ejsing, M. F. Hansen, A. K. Menon, H. A. Ferreira, D. L. Graham and P. P. Freitas, Appl. Phys. Lett., 2004, 84, 4729 CrossRef CAS
.
- L. Ejsing, M. F. Hansen, A. K. Menon, H. A. Ferreira, D. L. Graham and P. P. Freitas, J. Magn. Magn. Mater., 2005, 293, 677 CrossRef CAS
.
- M. Kakuta, D. A. Jayawickrama, A. M. Wolters, A. Manz and J. V. Sweedler, Anal. Chem., 2003, 75, 956 CrossRef CAS
.
- A. G. Goloshevsky, J. H. Walton, M. V. Shutov, J. S. de Ropp, S. D. Collins and M. J. McCarthy, Rev. Sci. Instrum., 2005, 76
.
- C. Massin, C. Boero, F. Vincent, J. Abenhaim, P. A. Besse and R. S. Popovic, Sens. Actuators, A, 2002, 97–8, 280 CrossRef
.
-
N. Pamme and Y. Miyahara, Proceedings of the 19th International Symposiuim on Microscale Bioseparations (MSB Kobe), ed. Y. Baba and K. Otsuka, Kobe, 2005, p. 113 Search PubMed
.
- E. Katz, O. Lioubashevski and I. Willner, J. Am. Chem. Soc., 2005, 127, 3979 CrossRef CAS
.
- F. Kimura, T. Kimura, M. Tamura, A. Hirai, M. Ikuno and F. Horii, Langmuir, 2005, 21, 2034 CrossRef CAS
.
- M. Boncheva, S. A. Andreev, L. Mahadevan, A. Winkleman, D. R. Reichman, M. G. Prentiss, S. Whitesides and G. M. Whitesides, Proc. Natl. Acad. Sci. USA, 2005, 102, 3924 CrossRef CAS
.
- J. M. K. Ng, M. J. Fuerstman, B. A. Grzybowski, H. A. Stone and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 7948 CrossRef CAS
.
- A. Pich, S. Bhattacharya, Y. Lu, V. Boyko and H. A. P. Adler, Langmuir, 2004, 20, 10706 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2006 |
