Implementation of radiotelemetry in a lab-in-a-pill format
Erik A.
Johannessen
a,
Lei
Wang
a,
Stuart W. J.
Reid
b,
David R. S.
Cumming
a and
Jon M.
Cooper
*a
aDept. Electronic and Electrical Engineering, University of Glasgow, Rankine Building, 79-85 Oakfield Avenue, Glasgow G12 8LT, UK. E-mail: jmcooper@elec.gla.ac.uk; Fax: + 44 (0)141 330 4907; Tel: +44 (0)141 330 4931
bFaculty of Veterinary Medicine, University of Glasgow, 464 Bearsden Road, Glasgow, G61 1QH, UK. E-mail: S.Reid@vet.gla.ac.uk; Fax: +44 (0) 141 330 5729; Tel: +44 (0)141 330 5741
First published on 21st November 2005
Abstract
A miniaturised lab-in-a-pill device has been produced incorporating a temperature and pH sensor with wireless communication using the 433.92 MHz ISM band. The device has been designed in order to enable real time in situ measurements in the gastrointestinal (GI) tract, and accordingly, issues concerning the resolution and accuracy of the data, and the lifetime of the device have been considered. The sensors, which will measure two key parameters reflecting the physiological environment in the GI (as indicators for disease) were both controlled by an application specific integrated circuit (ASIC). The data were sampled at 10-bit resolution prior to communication off chip as a single interleaved data stream. This incorporated a power saving serial bitstream data compression algorithm that was found to extend the service lifetime of the pill by 70%. An integrated on–off keying (OOK) radio transmitter was used to send the signal to a local receiver (base station), prior to acquisition on a computer. A permanent magnet was also incorporated in the device to enable non-visual tracking of the system. We report on the implementation of this device, together with an initial study sampling from within the porcine GI tract, showing that measurements from the lab-on-a-pill, in situ, was within 90% of literature values.
1. Introduction
Despite the development of a variety of sophisticated methods, including traditional endoscopic techniques,1 radionucleotide tracers,2,3 computed tomography,4 magnetic resonance imaging, MRI,5 ultrasound6 and X-ray imaging,7 the relative inaccessibility of the human gastrointestinal (GI) system still serves to restrict our knowledge of the general physiology and disease conditions of the functional gut. The adaptation of wireless communication systems for body monitoring8 has enabled a new class of radiotelemetric diagnostic pills to be developed,9–15 in order to make routine physiological measurements during normal passage through the GI system. These instruments potentially provide access to the entire length of the gut, and represent an alternative to the traditional diagnostic techniques16 with a proven feasibility.17–20In general, such devices are all “passive” moving with gastrointestinal peristalsis, although some self-propelled mechanisms have been studied .21 In all cases, the information from these devices is relayed to an external receiver by radio frequency transmission, were the data can be stored and analysed as part of a diagnostic procedure.
The position of the lab-in-a-pill relative to the outside of the body can be found using techniques analogous to conventional direction finding.10 However, the entwined nature of the gut makes it impossible to determine the precise whereabouts of the capsule with respect to the GI tract at a given time. In order to better understand this problem, the location of a radio capsule has previously been determined visually by X-ray imaging at predefined intervals,11,22 or has relied upon the use of a thread attached to the capsule to provide information of the length travelled.23
An automated tracking system was proposed over a decade ago, by Jay et al.24 Several methods have since been studied to enable tracking of a remote sensor system without visual aid, based either on the use of external magnetic field generators and an implanted receiver coil,25 or in the opposite sense—an external receiver coil and an implanted transmitter.26 The ability to process the data and store the tracking record is paramount in order to determine the location of the device in the gut.
Our current research has sought to build upon previous9–15,17–20 developments (which have used discrete component sensors for single channel recordings) to produce a class of more sophisticated devices, with added functionality.27–29 The emphasis has been on using aspects of lab-on-a-chip methods, microfabrication and system-on-a-chip technology to enable a microsystem capable of performing simultaneous multi-parameter physiological analysis.
In this paper we present the further development of a lab-on-a-chip device, which exploits sensor integration and onboard data processing through an ASIC to produce a lab-in-a-pill. The device incorporates a microfabricated pH and temperature sensor chip with a custom made ASIC with an implemented power saving feature for extended service lifetime. A permanent magnet has been incorporated in order to track the device in situ. The magnet does not consume any additional power from the lab-in-a-pill, and, by using an external array of magnetoresistive sensors, its field can be used to determine both the location of the device in three dimensions (x, y and z) as well as its orientation.
We have previously shown that both wire and wireless data capture27–29 provides the same experimental measurement, i.e. we are confident that data is neither corrupted nor altered by wireless transmission (data previously published). We now present a series of analytical measurements from within the porcine GI tract, which include both wireless (transmitted) data, wired (direct) communications and control in vitro measurements, made ex situ. Measurements were also performed demonstrating the ability of the magnetoresistive sensors to locate the pill. In all cases, the porcine gastrointestinal tract was used as the model experimental system.
2. Instrumentation
2.1. The implementation of the sensors
The sensors used in this paper were a modified research product, which are described in more detail in a prior publication.27 These comprised a pH ISFET with a microfabricated Ag|AgCl reference electrode and a silicon diode temperature sensor. The sensor chip was 4.75 mm × 5 mm, and was encapsulated by a 50 µm thick layer of polyimide (Durimide 7020, Arch Semiconductor, Belgium). A “window” was opened in the thin polyimide layer enabling microfluidic access to the pH sensor. The dynamic range of the ISFET was tuned from pH 1 to 10, with a temperature range from 10 to 50 °C, offering tolerances well beyond the physiological parameters that need to be measured in the GI tract.2.2. The design of the ASIC
The system control unit (ASIC) was fabricated as a 44 pin 4.5 mm × 4.5 mm silicon die using a 3 V, 2-poly, 3-metal 0.6 µm CMOS process by Austria Microsystems (AMS, Austria), Fig. 1. The three functional blocks (sensor interface, timer and system scheduler) were designed to minimise the overall size and power consumption of the device. The timer implemented a RC relaxation oscillator, which clocked the system at 32 kHz. A finite state machine represented the core of the system scheduler. This implemented a 10 bit analogue to digital converter and related digital logic which performed several tasks such as sensor cycling and sampling.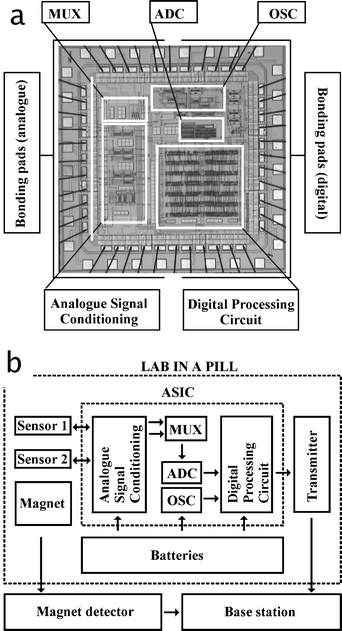 | ||
| Fig. 1 (a) Layout plot of the 4.5 mm × 4.5 mm application specific integrated circuit control chip (ASIC), and the associated flow chart (b) illustrating the integration of the ASIC in the lab-in-a-pill. The acronyms explained are MUX (multiplexer), ADC (analogue to digital converter), and OSC (32 kHz oscillator). The large bonding pads allow repetitive use of wire bonding for test and verification prior to integration in the lab-in-a-pill. | ||
2.3. The power saving algorithm
A sleep mode and a serial bitstream data compression algorithm were developed to enhance the power saving features of the pill. In this context, the rate of movement of the pill through the gut (by peristalsis) and the subsequent change in an analytical signal was considered. For example, the rate of change in the magnitude of an analytical signal can vary greatly. During the pill's transit within the stomach, small intestine or colon, one would not expect large changes in measured values. However during transit between these regions (e.g. between the stomach and small intestine, through a sphincter) or upon encountering a pathophysiological feature (such as an ulcer or an abcess), the change in the measured signal may be much larger. During the collection of a stream of data, the most recent analytical measurements were therefore compared to the previous signals, at a sample interval of 1 s. The algorithm calculated at which point to start transmission by comparing the difference between the most recent and the previous data sets: if this was below a predefined tolerance limit of ±1 least significant bits (LSB), the scheduler did not make a transmission, and the data was stored on a local buffer until the difference between samples was more than the tolerance limit, or when the buffer was full (180 samples, or 3 minutes). Whilst not making a transmission, the system lies at rest (using only 30% of full power). The digitised data was fed directly into a miniaturised AM transmitter module running at the licence free 433.92 MHz ISM band. The data implemented the Manchester code, which translated combined clock and data signals at 4 kb s−1.2.4. Data capture
The sensor data was captured either by a wire connection, or by using an external base station as a receiver (comprising a simplex wireless interface module, a process core module and a data presentation module). All components were battery operated. The data presentation was performed with a portable PC (Toshiba, Japan), which was managed using a DAQ type PCMCIA card (National Instruments, USA) with a wireless interface developed as a miniaturised AM receiver (RF Solutions, UK). The transmitter and the receiver pair were chosen with a fixed reception frequency of 433.92 MHz. The data processing module ran on a Matlab 6 (Mathworks, USA) routine, which was designed to be synchronous with the hand-held stand-alone magnet detector system. This has the potential to be incorporated into a portable base station.302.5. System integration and construction
The constructed lab-on-a-pill was 12 mm × 36 mm in size and weighed 8 g. The backbone of the system was a 0.8 mm thick PCB onto which all of the components were attached. Copper tracking on both sides of the PCB board was used to define the power supply rails, contact points for the sensors and an integrated single loop antenna corresponding to 1/16 λ of the wireless transmission frequency. A schematic of the pill is shown in Fig. 2 (a), illustrating the different components. The sensors and ASIC were connected to one side of the PCB, whilst the 5 mm × 4 mm large permanent magnet, and the transmitter was connected to the opposing side. The 3 V power supply constituted two 75 mA h Ag2O battery cells (SR48), activated by a rear mounted 7 pin 0.8 mm pitch socket (Radio Spares, UK). The three reference voltages of the power supply (positive, virtual ground and negative) were required by the circuitry. Wire bonding connected the sensors and the ASIC, whilst the two batteries and the magnet were attached to slots made in the PCB. The contact points from the batteries were “cold soldered” using conductive glue (Chemtronics, Kennesaw, GA), and the wires running from the batteries and the transmitter were tin soldered onto the PCB.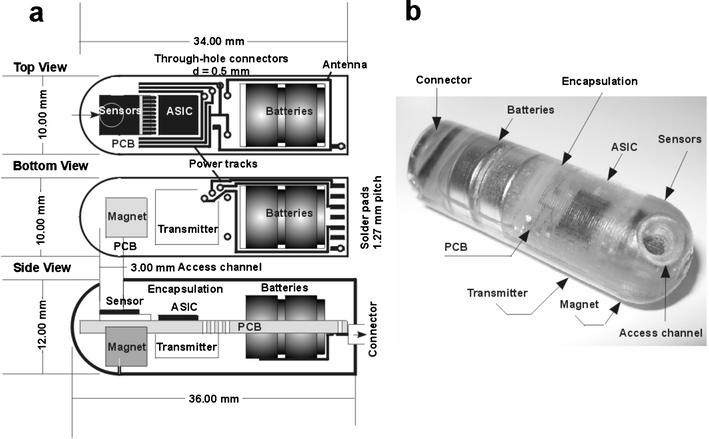 | ||
| Fig. 2 (a) CAD schematic illustrating the architecture of the lab-in-a-pill. The sensor chip, incorporating a dual temperature and pH sensor, is located at the front, followed by the ASIC and batteries. The magnet and the transmitter are assembled on the reverse side. (b) The whole unit is encapsulated in epoxy resin (Araldite 2020), with the external connector enabling powering both from the batteries and external sources. | ||
2.6. Packaging
The tracks and wires were cast in high strength epoxy (Radio Spares, UK) for protection against the encapsulation material. The sensors were protected by a custom made 2 mm high and 3 mm wide polypropylene “cup” attached by 3140 silicone rubber (Dow corning, US). The pre-assembled device was placed in a mould of PTFE, for encapsulation by a low viscosity epoxy resin, Araldite 2020, (Vantigo AG, Switzerland), which cured to a hard chemically resistant transparent polymer. The sensors were accessed by machining a 3 mm diameter aperture into the pre-mounted plastic “cup”. This was then removed to reveal a channel running through the epoxy to the microsensor array, Fig. 2(b).2.7. Tracking
As stated, tracking the device required a permanent magnet to be incorporated inside the lab-in-a-pill device with an array of magneto-resistive sensors located outside the body to detect the emitted magnetic field,34Fig. 3. The DC magnetic field generated by a permanent magnet having a magnetic dipole moment m, located at a position r, is given by:32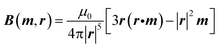 | (1a) |
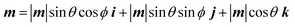 | (1b) |
| r = xi + yj + zk | (1c) |
 | ||
| Fig. 3 Tracking studies. Illustrating the relationship between dipole moment (m) and the detection of the related magnetic field (B) from the permanent magnet located at a distance (r) from the detector. The magnet was moved relative to the stationary detector in order to estimate the range over which the magnet could be tracked, taking into account the background field (BE) concomitant with the Earth's magnetic field. | ||
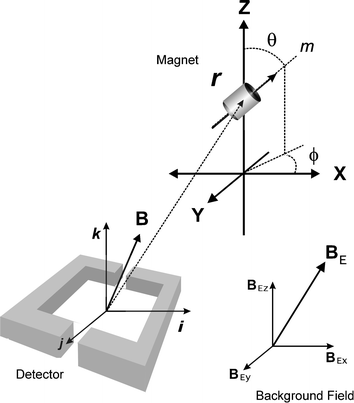
The magnitude of the magnetic moment is fixed for any particular magnet (neglecting thermal effects) and is given by:
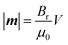 | (2) |
It can therefore be seen that the magnetic field generated by the magnet (eqn (1)) is determined by five variables, which are the five degrees of freedom of the magnet: x, y, z, ϕ (orientation of r from the x-axis in the xy-plane) and θ (orientation of r from the z-axis). In addition, the background (Earth's) magnetic field , BE, has to be considered, since the ∼50 μT flux density33 may be larger than the equivalent field from the magnet used for tracking. In principle this background field, consisting of three components, BEx, BEy and BEz, in addition to the five directly associated with the magnet, can be determined using just three external 3-axis magnetic field sensors, whose positions relative to one another are fixed. These sensors were used to make nine independent measurements enabling all eight unknowns (x, y, z, ϕ, θ, BEx, BEy, BEz) to be calculated (in point of fact, only 8 are required in theory but redundancy helps provide iterative solutions).
The permanent magnet (Magnequench, USA) used to enable tracking of the device was made of sintered Nd12Fe4B. A cylindrical volume of this material of length 4 mm and 5 mm in diameter yielded a magnetic moment of |m| = 0.087 A m2. The magnetic field, which could be measured at a distance of 15 cm (nominal distance from the pill and the body surface is 5.15 µT in the direction of m).
A Zortran® prototype detection system developed by Lucent Medical Systems, US,34 for tracking catheters and feeding tubes, was used to enable a proof-of-principle of this tracking method. The detector comprised an array of five 3-axis magnetic field sensors, signal conditioning electronics and proprietary embedded software which enable eqn (1) to be calculated so that the location and orientation for the indwelling magnet is found.
Two important parts to this device were an estimation processor and a neural network, which enabled the real-time solution to a large set of simultaneous equations. The estimation processor made use of the known sensor positions and an estimate of the magnet position (x, y, z, ϕ and θ) to predict values of B(r,m) and make iterative comparisons with the measured values.
A neural network was used to provide the initial estimate of the magnet position, obtained during a calibration step. The more accurate this estimate, the more reliable and efficient the algorithm was. A field strength limit of 0.5 µT was expected for the algorithm to function correctly, with fields > 2 µT giving the best position accuracy (<±1 cm). The hardware interface for the magnet detector system was through a RS232 serial port, where the positional data obtained for the initial performance evaluation was gathered using a PC with HyperTerminal software. The data was updated at a rate of 10–15 times per second.
3. Experimental
The sensors, radio transmission and the tracking protocols were optimised independently of each other prior to integration. When wireless data was being collected, the transmission distance was kept within 1 m from the receiving antenna and the signals from the sensors were recorded by the base-station and with the data presented in real time.The efficiency of the power saving features was assessed under different measurement conditions representing analytical solutions (pH buffers), artificial gut solutions (gastric and intestinal juice, intestinal chyme), and temperature. The artificial gut solutions have been described in our previous work.31 The measurement periods over which the efficiency of the power saving features were assessed ranged from 3 to 15 hours. All characterisation experiments were performed at room temperature (21 °C).
The lab-in-a-pill was tested within fresh animal carcasses of large white male porcine (25 kg), aged 6 months. A midline incision was made along the abdomen starting at the xiphoid process of the sternum and ending at the proximal inguinal teat allowing access to all parts of the digestive tract.
Incisions were made in the GI tract and lab-in-a-pill measurements were made by the positioning and subsequent manual relocation of the device. Subsequently, digestive juices were collected from the stomach, the duodenum (approximately 5 centimetres below the pyloric sphincter), the jejunum, ileum, caecum, and the proximal and distal colon. These samples were aliquoted into beakers and used for making analytical control measurements using a standard lab pH meter, (Consort n.v., Belgium). Results were presented as mean values from triplicate readings.
4. Results
The power consumption of the lab-in-a-pill was 4.75 mW in the standby mode (of which 3.9 mW was attributed to the ASIC), whereas the power increased to 15.5 mW with the transmitter powered up for signal transmission. The compression ratio of the power saving features averaged 3.4, which represented a power reduction of 70% compared to the transmitter running in continuous operation. The data acquired with the pill under different in vitro conditions is presented in Table 1, showing the different compression ratios for different measurements. The compression ratio increases in solutions in which the device reaches equilibrium fastest, such as low viscous analytical solutions (pH buffers), and slowest in high viscosity analytes (chyme). Long-term measurement protocols took into account temperature changes overnight, in which room temperature changes increased the transmission rate, and thereby reduced the overall compression ratio.In the bench-top proof-of-principle tests, the magnet was moved away from the center of the detector and the distance, z, was measured with the magnet oriented as shown in Fig. 3, with x = 0. The position calculated by the detector was compared with the measured value, Fig. 4(a). A benchmark of an error of less than ±1 cm was used (this is the error for locating specially designed, magnet-tipped Peripherally Inserted Central Catheters or Central Venous Catheters). It can be seen that, within the range 1 cm ≤ z ≥ 18 cm, the z position can be determined with better than ±1 cm accuracy. Good repeatability of the results can be seen from data points obtained as the magnet was returned along the same path back up to the detector (there is negligible hysteresis). The increase in error at a large distance is consistent with the field falling below the expected 2 µT detection level.
![Difference between detector reading (a) and manual reading of magnet position from centre of the detector along the z-axis [z(detector) −
z(ruler)]. Difference between detector reading (b) and manual reading of magnet position from the centre of the detector along the x-axis [x(detector) −
x(ruler)] at a distance of z = 3 cm.](/image/article/2006/LC/b507312j/b507312j-f4.gif) | ||
| Fig. 4 Difference between detector reading (a) and manual reading of magnet position from centre of the detector along the z-axis [z(detector) − z(ruler)]. Difference between detector reading (b) and manual reading of magnet position from the centre of the detector along the x-axis [x(detector) − x(ruler)] at a distance of z = 3 cm. | ||
The magnet was also moved across the width of the detector, and the distance, x, measured with the results shown in Fig. 4(b). For x ≤ 10 cm from the centre line of the detector, the magnet position could be determined with better than ±0.5 cm accuracy. It should be noted that these measurements were taken with the magnet at a depth of only 3 cm (z = 3 cm), the range over which the position can be tracked within ±1 cm will clearly decrease with increasing depth.
In order to assess the effect of rate of magnet movement on the tracking capability of the detector, the magnet was moved through a path in the x–y plane at a distance z of 14 cm below the detector at a speed of 100 cm min−1 and 10 cm min−1 (Fig. 5). It should be noted that a freely moving lab-in-a-pill, traveling under peristalsis, will move fastest in the esophagus (∼150 cm min−1) and slowest in the small intestine (∼2 cm min−1).35 Within these boundary conditions, it can be seen that the response time of the tracking sensor array does facilitate accurate tracking of the magnet in the small intestine, whilst the fast loop also shows that position data could be obtained for a lab-in-a-pill moving freely in the esophagus, although with a larger tolerance. Importantly, no points lie outside the possible path, demonstrating the ability of the detectors algorithm to either generate valid solutions even when the magnet has moved several centimeters since the last update (previous algorithm solution) or to reject solutions, which generate a large error function.
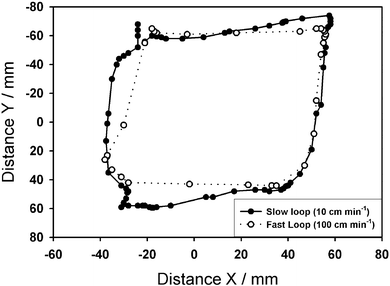 | ||
| Fig. 5 Qualitative comparison of the detector tracking capability with a magnet moved at two different rates of 10 cm s−1 (solid) and 100 cm s−1 (dotted) in approximate rectangular paths (x–y plane) at a distance of z = 14 cm. | ||
Results collected ex situ showed that an apparent shift in the magnet's location of between 1–2 cm could also be observed with the magnet fixed at a distance of 9 cm relative to the detector while moving the whole arrangement in both horizontal and vertical loops of 1 m diameter. This laboratory based measurement showed that local variations (i.e. on length scales less than the distance between field sensors in the detector) in the magnitude of the background field resulted in a measurement error (since they cannot be distinguished from changes in the field due to the magnet itself). The variation in average background field components, BEx, BEy, BEz, were determined to be ±5 µT during the course of these detector movements, thus confirming that significant variation in background field was present in the test environment.
The recordings from samples from the porcine GI tract are presented in Fig. 6. The three sets of sensors registered an average pH of 4.69 ± 0.45 in the stomach, 5.4 ± 0.7 in the duodenum, 6.74 ± 0.55 in the jejunum, 7.32 ± 0.80 in the ileum, 7.03 ± 0.35 in the caecum, 6.95 ± 0.30 in the proximal colon and 6.85 ± 0.31 in the distal colon. In comparison, the readings from the lab pH meter were 4.67 ± 0.42, 6.02 ± 0.71, 7.01 ± 0.8, 6.63 ± 0.74, 6.65 ± 0.27, 6.76 ± 0.20 and 6.95 ± 0.28 respectively. The data lay within 90% of literature values, with the exemption of the stomach (see discussion). Temperature measurements were constant (21 °C) throughout the course of the experiment.
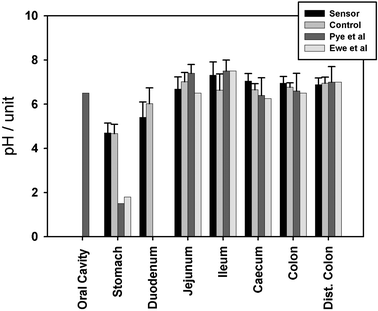 | ||
| Fig. 6 Gut measurements from the porcine gastrointestinal tract concomitant with control and literature values. See text for details. | ||
5. Discussion
Tracking the lab-in-a-pill with a stationary detector demonstrated a detection range for the magnet, to a depth of 18 cm from the centreline directly below the detector, and which could extend to a radius of 10 cm from this centre line (measured with z = 3 cm). The apparent increase in error when the magnet was closest to the detector (Fig. 4a) was probably a result of saturation of one or more of the magnetic field sensors (>200 µT). This proximity-effect would be avoided in the real application, where the magnet (lab-in-a-pill) will be located many centimeters away from the detectors within the GI tract.The system also demonstrated an ability to track the device at the expected passage speeds of the gut. The results indicate a constraint to the range of abdominal region within which the lab-in-a-pill could be detected with the system used in these studies. However, it is emphasised that the experiments represent only a first step towards what can be achieved with present state of the art using, a passive magnet as the source object consuming zero power from the device.
The lab-in-a-pill would ideally be used by a patient carrying out their usual daily activities, such as moving from room to room or even traveling to and from a place of work. This prototype detector was developed for use with a stationary patient. When there are dynamic changes in background field due to ferri/ferromagnetic objects, some additional consideration needs to be taken.
Alternative tracking systems, such as the use of external AC magnetic field generators and an implanted receiver coil25 has the disadvantage that the field sensor is located inside the body. Subsequently, all the electronics required for power, signal conditioning and analogue to digital conversion would need to be incorporated into the lab-in-a-pill. The AC signal is also susceptible for attenuation by different amounts, depending on the depth and type of body tissue/bone through which the signal must pass—thus limiting the position sensing accuracy. In contrast, the use of external receiver coils and an implanted transmitter26 has the additional limitation, that power must be provided to the RF transmitter. However, the transmitter used to convey the pH and temperature data from the lab-in-a-pill might also be used to track the device. The variable signal attenuation with this method may limit the accuracy with how the capsule can be detected.
The GI tract measurements were conducted from fresh porcine digestive tracts, to minimise the effect from post mortem changes, in which the gastrointestinal juices from the different regions of the gut will intermix as the sphincters dilate and the secreting organs supplying the gut with juices stops functioning. Post-mortem, there will be a net influx of water due to the higher osmotic pressure of the gut contents, and thereby affecting the pH values. Comparing the GI tract measurements with literature from earlier radiotelemetric devices36,37 one can clearly see a comparable trend (Fig. 6).
The porcine model is considered to be close to the human due to comparable size and physiology (non-ruminant) of the GI system. Measurements starting at the oral cavity of human subjects36 shows the pH close to neutral (pH 6.5) due to action of the saliva. This decreases in the stomach of fasting individuals to 1.5 and 1.8 due to the secretion of stomach acid.36,37 In contrast, our measurements (pH 4.7) were performed on an animal that had not been subject to fasting, and thus represented a realistic value from the dilution of stomach acid after ingestion of food (and any further dilution, post-mortem). The increase in pH to 5.4 in the duodenal environment, will be a result of pancreatic secretions rich in enzymes and bicarbonate. The trend is manifested further in the upper part of the small intestine (jejunum), as the bicarbonate accumulates with values measured to pH 6.7 with literature values ranging from pH 6.5 to pH 7.4.36,37
The most alkaline part of the GI tract (pH 7.3) was measured in the lower part of the small intestine (ileum) which compares favourably with the literature value of pH 7.5.36 The noticeable reduction in pH of the large intestine is due to the activity of the intestinal microflora. Likewise, in the caecum, the transverse colon and the distant colon our measured values compare well with the literature.36,37 The data from the lab-in-a-pill should be considered to have a smaller error, given the nature of the ISFET sensor, when compared to the large glass pH membrane used either ex situ or in the literature.
6. Conclusion
In this work, a miniaturised lab-in-a-pill for wireless communication within the 433.92 MHz ISM band, has been designed. The built in power saving features extended the service lifetime by 70%. Our preliminary studies with a tracking system demonstrated that a permanent (passive) magnet incorporated into the lab-in-a-pill could be detected with an accuracy of ±1 cm up to a depth of 18 cm. However the limited radial detection range and errors introduced for ambulatory measurements will present challenges for this type of detection system. Options for improvement would entail: a larger magnet volume; an extended detector calibration range; optimisation of magnetoresistive sensors; and improvement in the algorithm specification.Acknowledgements
The authors acknowledge technical staff at the University of Glasgow for their assistance. The authors further acknowledge Ms Amy Koterbay, Mr David Ellson, Mr Richard M. Irvine and Mr Michael McGuigan from the Veterinary School at the University of Glasgow for experimental support. The authors also thank Dr Robert Golden of Lucent Medical Systems for loan of the prototype magnet tracking system, and Honeywell, UK, for the funding of aspects of this work.References
- T. N. Moyana and J. Xiang, Ann. Clin. Lab. Sci., 1999, 29, 200 Search PubMed.
- A. C. Perkins and M. Frier, Curr. Pharm., 2004, 10, 2907 Search PubMed.
- S. Halligan and B. Saunders, Best Pract. Res. Clin. Gastroenterol., 2002, 16, 595 Search PubMed.
- L. Ruess, A. A. Frazier and C. J. Sivit, Radiographics, 1995, 15, 89 Search PubMed.
- D. J. Lomas, Eur. Radiol., 2003, 13, 1058 CAS.
- I. Waxman and C. E. Dye, Cancer J., 2002, 8, S113 Search PubMed (suppl).
- Z. Lefkovitz, M. S. Cappell, M. Kaplan, H. Mitty and P. Gerhard, Gastroenterol. Clin. North Am., 2000, 29, 489 Search PubMed.
- T. E. Budinger, Ann. Rev. Biomed. Eng., 2003, 5, 383 Search PubMed.
- R. S. Mackay, in Biomedical Telemetry, IEEE Press, Piscataway, N.J., 2nd edn, 1970, p. 540 Search PubMed.
- S. Mackay, Science, 1961, 134, 1196 CrossRef.
- H. S. Wolff, New Scientist, 1961, 12, 419.
- M. R. Andres and J. R. Bingham, C. M. A. J., 1970, 102, 1087 Search PubMed.
- R. H. Colson, B. W. Watson, P. D. Fairlclough, J. A. Walker-Smith, C. A. Campell, D. Bellamy and S. M. Hinsull, Biotelem. Patient Monit., 1981, 8, 213 Search PubMed.
- G. Iddan, G. Meron, A. Glukhovsky and P. Swain, Nature, 2000, 405, 417 CrossRef CAS.
- S. A. Barrie, in A Textbook of Natural Medicine, Murray & Barrie, Pizzorno, 1992, pp. 5–13 Search PubMed.
- R. Eliakim, World J. Gastroenterol., 2004, 10, 1238 Search PubMed.
- G. Qvigstad, O. Flottum and H. L. Waldum, Tidskrift for den Norske Laegeforening, 2005, 125, 163 Search PubMed.
- R. Eliakim, Dig. Liver Dis., 2004, 36, 519 Search PubMed.
- W. A. Voderholzer, M. Ortner, P. Rogalla, J. Beinholzl and H. Lochs, Endoscopy, 2003, 35, 1009 Search PubMed.
- J. Soares, Endoscopy, 2004, 36, 1060 Search PubMed.
- D. Chi and G. Yan, J. Med. Eng. Technol., 2003, 27, 71 CrossRef.
- S. Mackay and B. Jacobson, Nature, 1957, 179, 1239.
- W. C. Watson and E. Paton, Gut, 1965, 6, 606 CrossRef CAS.
- J. Jay, R. Fouquet and R. Rougny, Med. Biol. Eng. Comput., 1993, 31, 201 CAS.
- Navion, Catheter depth, position and orientation location system, in http://www.navionbiomedical.com, 1997, USA.
- Micronix, Medical instrument location means, in http://www.micronix.com.au, 1992, USA.
- E. A. Johannessen, L. Wang, L. Cui, T. B. Tang, M. Ahmadian, A. Astaras, S. W. J. Reid, P. S. Yam, A. F. Murray, B. W. Flynn, S. P. Beaumont, D. R. S. Cumming and J. M. Cooper, IEEE Trans. Biomed. Eng., 2004, 51, 525 CrossRef.
- L. Wang, E. Johannessen, P. Hammond, L. Cui, J. M. Cooper, S. W. J. Reid and D. R. S. Cumming, IEEE Trans. Biomed. Eng., 2005, Search PubMed in press.
- T. B. Tang, E. A. Johannessen, L. Wang, A. Astaras, M. Ahmadian, A. F. Murray, J. M. Cooper, S. P. Beaumont, B. W. Flynn and D. R. S. Cumming, IEEE Sens. J., 2002, 2, 628 Search PubMed.
- G. Coldani, G. Dandolfi, P. Ghidetti, F. Leporati and R. Lombardi, in Proceedings on Euromicro Symposium on Digital Systems Design, 2001 Search PubMed.
- E. A. Johannessen, L. Wang, P. Yam, S. W. J. Reid, D. R. S. Cumming and J. M. Cooper, IEEE Trans. Biomed. Eng., Search PubMed submitted.
- V. Schlageter, Sens. Actuators, A, 2001, 2951, 1.
- D. M. Brownstone, in The World Book Encyclopedia, World Book International, London, 1995, vol. 13 Search PubMed.
- Lucent-Medical-Systems, in www.lucentmedical.com. 2001, USA.
- L. Sherwood, in Human physiology from cells to systems, West Publishing Company, Minneapolis, 2nd edn, 1993, pp. 745 Search PubMed.
- G. Pye, Gut, 1990, 31, 1355 CrossRef CAS.
- K. Ewe, S. Schwartz, S. Petersen and A. Press, Dig. Dis. Sci., 1999, 44, 1434 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
