Microfluidic diffusive filter for apheresis (leukapheresis)
Palaniappan
Sethu
,
Aaron
Sin
and
Mehmet
Toner
Surgical Services and Center for Engineering in Medicine, Massachusetts General Hospital, Harvard Medical School and Shriners Hospital for Children, MA 02114, Boston, USA. E-mail: spalania@sbi.org; asin@partners.org; mtoner@sbi.org; Fax: (617) 724 2999; Tel: (617) 726 6465
First published on 11th November 2005
Abstract
Apheresis is a procedure used to fractionate whole blood into its individual components. Following fractionation, the desired component is isolated and the remaining blood in many cases is returned to the donor. Leukapheresis is one type of apheresis where leukocytes (white blood cells) are selectively removed. This procedure is commonly used for blood transfusions to remove donor leukocytes from being transferred to the recipient. Apheresis also has several therapeutic applications. In this manuscript we discuss the design, fabrication and testing of a continuous flow diffusive filter, fabricated using simple soft lithographic techniques for depletion of leukocytes. This device employs micro sieves that exploit the size and shape difference between the different cell types to obtain depletion of leukocytes from whole blood. Currently, conventional apheresis methods like centrifugation or fiber mesh filtration are commonly used. A theoretical model was developed to determine the optimal shape of the diffuser to ensure that the volumetric flow through individual sieve elements is equal. This device was designed to serve as a passive device that does not require any external manipulation. Results show that for the given device design, isolation of ∼50% of the inlet erythrocytes (red blood cells), along with depletion of >97% of the inlet leukocytes is possible at a flow rate of 5 µl min−1. Simple modifications to the geometry and dimensions of the sieves can be made to obtain isolation of plasma.
1.0 Introduction
Blood is a complex living tissue that circulates through the heart, arteries, veins, and capillaries carrying nourishment, electrolytes, hormones, vitamins, heat and oxygen to the body's tissues. Blood is also involved in mediation of the immune functions of the body. Whole blood contains erythrocytes, leukocytes, and thrombocytes (platelets) all suspended in plasma. Each component is responsible for different functions; erythrocytes are responsible for oxygen transport, leukocytes perform the immune functions, platelets are responsible for clotting and plasma is the medium which transports various cells and molecules throughout the body.Apheresis is a general term describing removal of blood from a subject; a portion of the blood is fractionated and retained while the rest is returned to the donor. Leukapheresis (or leukodepletion) is a procedure where the leukocytes are isolated or fractionated. Other types of apheresis include plasmapheresis which is a procedure where plasma is separated and manipulated in a variety of ways and peripheral stem cell isolation, a procedure in which stem cells are isolated and retained.
Leukapheresis1,2 is typically performed to decrease a very high white blood cell count in individuals with leukaemia or to remove white blood cells for transfusion. Studies have repeatedly shown that leukocyte contamination during blood transfusion may result in adverse effects3 like febrile transfusion reactions, graft versus host disease, transmission of infectious agents like viruses (cytomegalovirus (CMV), herpes virus, T-cell leukaemia/lymphoma virus), bacteria, toxoplasma gondii, prions, platelet refractoriness and transfusion related immunomodulation. Therefore, the current trend is to ensure that blood used for transfusion is leukodepleted.
Cells in whole blood are predominantly erythrocytes that account for ∼99% of the total cells in blood. Leukocytes make up less than 1% of blood and have three major subpopulations: lymphocytes, monocytes and granulocytes. By taking advantage of the difference in physical properties (Table 1) between the different cell types, selective depletion of leukocytes from whole blood can be accomplished. Erythrocytes are smaller, biconcave in shape and more flexible than the leukocytes. Therefore, the most commonly used technique is filtration using a polymer or wire mesh filter with pore sizes in the order of 3–4 µm. The differences in mass densities of blood cells can also be exploited to achieve separation and depletion of lymphocytes and monocytes from whole blood using methods like differential centrifugation.
| Cell type | Concentration/cells ml−1 | Diameter/µm | Surface area/µm2 | Volume/µm3 | Mass density/g cm−3 |
|---|---|---|---|---|---|
| Erythrocytes (red blood cells) | 4.2 − 5.4 × 109 | 6–9 | 120–163 | 80–100 | 1.089–1100 |
| Leukocytes (white blood cells) | 0.4 − 1.1 × 107 | 6–10 | 300–625 | 160–450 | 1.055–1.085 |
| Neutrophils | 2 − 6 × 106 | 8–8.6 | 422–511 | 268–333 | 1.075–1.085 |
| Eosinophils | 0.4 − 4.8 × 105 | 8–9 | 422–560 | 268–382 | 1.075–1.085 |
| Basophils | 0 − 1.1 × 105 | 7.7–8.5 | 391–500 | 239–321 | 1.075–1.085 |
| Lymphocytes | 1 − 4.8 × 106 | 6.8–7.3 | 300–372 | 161–207 | 1.055–1.070 |
| Monocytes | 1 − 8 × 105 | 9–9.5 | 534–624 | 382–449 | 1.055–1.070 |
| Thrombocytes (platelets) | 2.1 − 5 × 108 | 2–4 | 16–35 | 5–10 | 1.04–1.06 |
There have been several attempts to create microfluidic devices for cell sorting applications. Including applications where the focus is on sorting blood cells. Attempts have been made by different groups using techniques like filtration,4,5 obstacles,6 electrophoresis,7 dielectrophoresis,8 immunomagnetic separation,9 margination,10 laminar flow and surface acoustic waves.11 These techniques are either focussed on low throughput processing for isolation of small number of cells or are not as efficient as bulk techniques that use filters or centrifugation (both continuous centrifugation and non-continuous) where ∼99% of the leukocyte population is removed.
We report the fabrication of a diffusive filter for size based depletion of leukocytes from whole blood (leukapheresis). This device exploits the size and shape difference between different cells to obtain the depletion of leukocytes. The sieves were designed to allow the passage of the biconcave erythrocytes, while providing a barrier to the larger spherical leukocytes. To prevent clogging and facilitate continuous separation, the sieves were arranged on the sides of the channels, connecting the channel to a diffuser. In order to ensure equal volumetric flow through every filter element of the sieve, the shape of the diffuser was modified resulting in a flared geometry. The device operates in the continuous flow mode where blood can be continuously fractionated. The leukocyte depleted blood is isolated and the remaining blood can be returned to the source or donor. This device is passive and does not require any external manipulation. Simple modifications to the sieve size and geometry can be made to isolate plasma.
2.0 Materials and methods
2.1 Device fabrication
The device was fabricated using soft lithographic techniques. A silicon wafer was first treated with oxygen plasma (March Instruments, Concord, MA) and then spin coated with SU-8 a negative photo resist (SU-8 50, MicroChem, Newton, MA). Standard two layer photolithography using a transparency mask (CAD ART Inc., Poway, CA) was drawn using AutoCAD layout software (Autodesk, Inc., San Rafael, CA) and used to create negative replicas of the designed channel structures. Then a silicone elastomer, poly dimethyl siloxane (PDMS, (Dow Corning, Midland, MI)) mixed 10 ∶ 1 with a cross linking agent was poured on the silicon wafer and cured at 60 °C for 12 h in a petri dish. The cured elastomer with the replicated channels was released and access holes to the channels were punched using a blunt gauge 22 syringe needle. The PDMS piece with the channels was then bonded irreversibly to a glass slide after treatment with oxygen plasma. Access tubing (Tygon, Small parts Inc. Miami Lakes, Fl) with a slightly larger diameter than the holes was press fitted into the punched holes.2.2 Blood samples and reagents
Approval for the collection of blood from healthy volunteers was obtained from the institutional review board of Massachusetts General Hospital. Blood was drawn by venipuncture and collected in a 2 ml green BD vaccutainer (Becton Dickinson, Franklin Lakes, NJ) with freeze dried heparin anticoagulant The blood was immediately stored at 4 °C until the experiments were performed.2.3 Modeling and simulation
Computational fluid dynamics simulations were performed using CFDRC finite volume software (CFDRC Corporation, Huntsville, AL). To construct the three-dimensional model, we started with a regular rectangular grid for the bottom cross-section, then extruded in the z direction to form a three-dimensional structure with >5000 hexahedral elements per sieve channel section. Navier–Stokes simulations were then carried out using the algebraic multigrid (AMG) solver to at least 4 orders of magnitude residual reduction, within ∼1000 iterations. The assumptions include no-slip boundary conditions and the fluid was considered to be a newtonian fluid (water). Blood though is best approximated to a non-newtonian fluid. This is due to the fact that the apparent viscosity of blood changes in capillaries and venules where cells have to deform in order to pass through. The overall dimensions of the device are sufficiently large for the newtonian assumption to be reasonable. Also, at low flow rates the pressure is not sufficient to force cells to deform and pass through the filter elements of the sieves and only cells that are small enough pass through. At larger flow rates however, the assumption may not be valid since the pressure is sufficient enough to force larger cells through the sieves resulting in the fluid behaving as a non-newtonian fluid. The simulations were run for half of the device geometry with symmetrical boundary conditions at the top wall of the main channel (mid-point of actual device).2.4 Experimental setup and analysis
The device was mounted onto the stage of a microscope (Nikon Eclipse TE 2000-U) with a CCD camera and a video monitoring system (Panasonic PV-VS4821, Japan). Whole blood was introduced into the device using a 1 ml BD syringe, controlled by a syringe pump (Pico pump, Harvard Apparatus, Holliston, MA). The samples were collected at the outlets. Outlets 1 and 3 contain the leukocyte depleted fractions and outlet 2 contains the leukocyte rich fraction. Cell counts were performed using a standard coulter counter (Beckman Coulter, Franklin Lakes, NJ). Leukocyte counts were obtained after lysis of erythrocytes with Zap-o-globin™ (Beckman Coulter, Franklin Lakes, NJ).Whole blood was delivered into the inlet of the device using a syringe pump. Blood was introduced at flow rates between 5–12 µl min−1. The blood contains different cells suspended in plasma which enter the main channel and encounter the 2.5 µm × 40 µm sieves that line the walls of the main channel.
The experiments were performed with a sample size of N = 5 and all the data is represented as means ±SE.
3.0 Device design
The goal was to accomplish continuous fractionation of erythrocytes from whole blood. In order to achieve this, two primary design objectives had to be accomplished. The first objective was to fabricate a sieve with filter elements designed to allow passage of erythrocytes oriented parallel to the floor of the main channel without any obstruction while providing a barrier to the larger spherical leukocytes. The second objective was to ensure an equal volumetric flow through each and every filter element for optimal device function. The volumetric flow through each element of the sieve depends on pressure gradient (a function of the syringe pump controlled flow rate) and the fluidic resistance (constant for a given geometry). For small flow rates (<5 µl min−1), the size and geometry of erythrocytes allow them to pass through the sieves unobstructed whereas leukocytes are larger and cannot pass through. At higher flow rates the pressure gradient across the filter elements of the sieves becomes large enough to support the passage of smaller leukocytes. In order to obtain efficient operation of the device (isolation of majority of the erythrocytes without leukocyte contamination) all filter elements must be functional while not supporting the transport of leukocytes.A schematic of the designed device is illustrated in Fig. 1. Whole blood enters the device at the inlet and flows through the main channel. The dimensions of the main channel are 2 cm × 200 µm × 50 µm (L × W × H). This channel is connected to a diffuser on both sides through sieves with filter elements as shown in Fig. 1 (insert). The dimensions of each filter element are 40 µm wide × 2.5 µm high (W × H), and each device has a total of 500 filter elements. For filter elements less than 1 µm tall, soft lithographic techniques may be unsuitable. Other materials like silicon or glass may be necessary. The geometry (Fig. 1, insert) of the filter elements accomplishes the design objective that only the biconcave erythrocytes pass through the sieves into the diffuser and the larger spherical leukocytes are contained in the main channel under normal flow conditions. Therefore if a cell encounters a sieve element and cannot pass through it is pushed along the main channel to the outlet due to the flow in the main channel. For this particular device design, at flow rates greater >5 µl min−1, the pressure gradient across the filter elements becomes large enough to deform and force some of the leukocytes through into the diffuser.
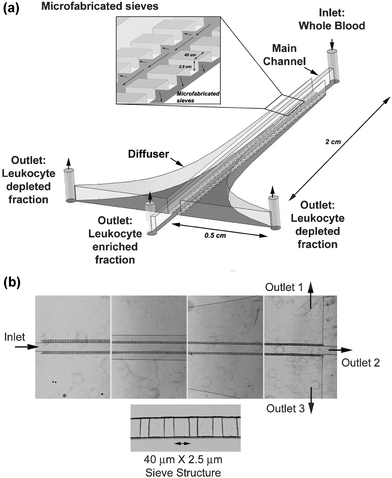 | ||
| Fig. 1 Device design: (a) Schematic of the diffusive filter for size based continuous flow fractionation of erythrocytes from whole blood. Insert shows the 40 µm × 2.5 µm sieve structure and the arrangement connecting the main channel to the diffuser. (b) Phase contrast microscopic images of the top view of the device at different locations with a magnified image of the 2.5 µm × 40 µm sieves. | ||
3.1 Modeling
A microfluidic device can be modeled as a fluidic circuit with discrete elements. The fluid flow in the circuit depends on the pressure difference between the inlet and the outlet and the volumetric flow through each element is a function of both the pressure gradient and the fluidic resistance. The fluidic resistance of a rectangular channel for a fluid with viscosity μ can be determined from its dimensions (length L, width W and height H) (eqn (1)). This equation holds good only if W > H. At locations close to the inlet for the first 20 filter elements of the sieves we had conditions where H > W. For these locations, the values of H and W were interchanged in the formula.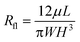 | (1) |
The diffuser and main channel were broken up into a series of rectangular blocks to simplify the theoretical model as shown in Fig. 2. The device can be represented as a series of independent elements arranged in a network as shown in Fig. 3(a). Each element is associated with a fluidic resistance. The volumetric flow through each element depends on the fluidic resistance of that element and the corresponding pressure difference or pressure gradient across that element (ΔP). The network can be further simplified into discrete repetitive modules as shown in Fig. 3(b). Each module represents the portion of the device between filter elements m and m + 1 (where m = 1 to n
− 1, and n is the total number of filter elements). The module consists of the section of the main channel and the diffuser on either side connected by the filter elements m and m + 1. Since the device is axially symmetrical the module can be further reduced to the closed loop shown in Fig. 3(c). In order to ensure constant volumetric flow ‘Y’ through the each of the filter elements, the fluidic resistance has to be controlled by appropriate diffuser design to provide the necessary resistance. If ‘X’ defines the output flow through the outlet of the main channel (outlet 2) then the ratio of flow through a corresponding set of filter elements to output flow through the main channel ratio can be defined by a dimensionless number ‘![[Fraktur R]](https://www.rsc.org/images/entities/char_e18f.gif) ’, where:
’, where:
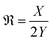 | (2) |
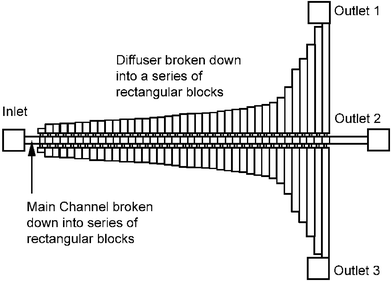 | ||
| Fig. 2 Simplification of device geometry: In order to simplify the design of the device the diffuser was broken down as a series of rectangular blocks such that the fluidic resistance of each block can be easily determined. | ||
 | ||
| Fig. 3 Device modelling: (a) Microfluidic circuit represented as a network of resistors with each resistor representing the resistance to fluid flow. X and nY are outputs at the outlets and X + 2nY is the volume of sample through the inlet. (b) This network can be divided into repetitive modules with each module containing a part of the channel, diffuser and two sets of filter elements. (c) Each module can be further reduced to a closed loop where ΔPc, ΔPd, ΔPm and ΔPm+1 are the pressure gradients across the section of the channel, diffuser and filter elements m and m + 1. | ||
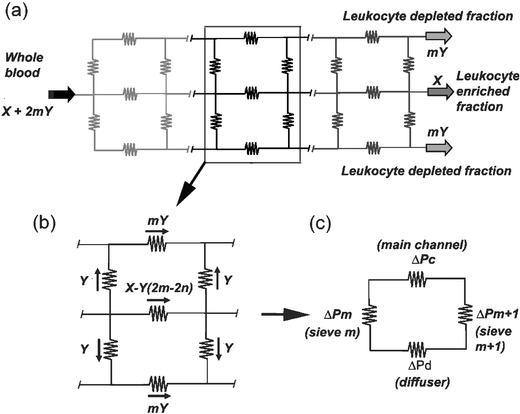
Solving for the condition that the volumetric flow through each and every filter element is a constant ‘Y’, we can then obtain an approximation for the width of the diffuser element wdiffuser for each discrete module using the equation shown below. This breaks down the diffuser into a series of rectangular blocks with increasing widths thereby varying the fluidic resistance appropriately, ensuring uniform volumetric flow through each and every filter element.
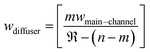 | (3) |
4.0 Simulation and experimental results
In order to verify the developed analytical model, computational fluid dynamics simulations were performed using CFDRC software. The device geometry was generated based on the developed analytical expression to determine the width of the diffuser at different locations. This device was used to run simulations to determine the total flow through each sieve in the device. Finite volume modelling and simulations of a device with 100 sieves was performed and Fig. 4 shows the magnitude of total velocity of fluid flow (U) at a height of 1.25 µm from the bottom of the device, calculated using the formula: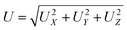 | (4) |
 | ||
| Fig. 4 Simulation results: Finite volume modelling and simulation of the absolute velocity of fluid flow through each sieve element at a height of 1.25 µm from the bottom of the device. The simulation was performed for a device with 100 sieve elements. Normalization of flow velocities to a certain extent of the fluid flow through each sieve element can be achieved using the modified design. | ||
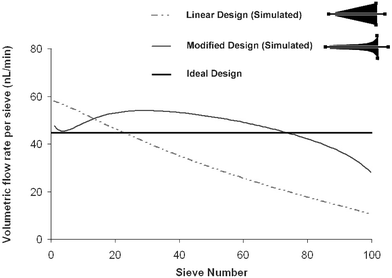 | ||
| Fig. 5 Modified design: Shown are the volumetric flow rates through sieve elements for a device with 100 sieves. The flow through each sieve element depends on the shape of the diffuser. Simulation results show that the using the modified design the volumetric flow rate through individual sieves can be normalized to a certain extent. | ||
Devices were fabricated and tested. From experimental results it can be seen that for smaller flow rates the filter elements allow only the biconcave erythrocytes oriented parallel to the floor of the channel to pass through unobstructed. The leukocytes are spherical and larger than the cross sectional area of the sieves and can only pass through with significant deformation. For flow rates >5 µl min−1 the pressure gradient across the sieves becomes large enough to deform and force some leukocytes to pass through the sieves.
In the experiments performed, 0.5 ml of whole blood was introduced into the device at different flow rates and after transit through the device the samples were collected at the 3 outlets. Sample from outlets 1 and 3 contain the fraction of sample that passed through the sieve structure into the diffuser whereas the sample from outlet 2 contains the fraction of sample that transited through the main channel alone. The sample from outlets 1 and 3 were combined and compared to the sample from outlet 2. The experiments were performed at sample inlet flow rates of 5, 7 and 12 µl min−1. The samples were collected and cell counts were performed on each sample. Fig. 6 shows the erythrocyte and leukocyte counts obtained for the sample collected from each of the outlets for the three different flow rates. The percentages were obtained by comparing the cell counts at the outlet to the original sample. The device and tubing itself account for a very small source of cell loss (∼1%), but at flow rates of 7 and 12 µl min−1 it can be seen from Fig. 6 that there is loss of cells, possibly due to lysis caused by the high shear experienced by the cells in the filter elements of the sieves.
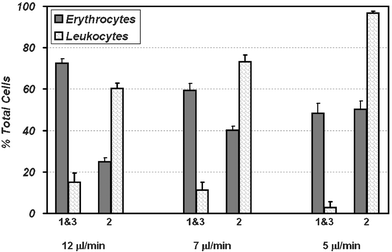 | ||
| Fig. 6 Erythrocyte fractionation results: Erythrocyte fractionation obtained at operating flow rates of 12, 7 and 5 µl min−1. Optimal fractionation occurs at 5 µl min−1; outlets 1 and 3 contain ∼50% of the total erythrocytes while containing only <3% of the total leukocytes. At higher flow rates the pressure difference across the sieves improves the erythrocyte yield through outlets 1 and 3. This happens at the cost of increased leukocyte contamination. | ||
5.0 Discussion
Apheresis in general refers to techniques used to fractionate blood into its individual components. The two commonly performed apheresis procedures involve isolation of plasma or plasmapheresis and the depletion of leukocytes or leukapheresis. In most apheresis procedures the required component is isolated and the remaining components are returned to the donor. In order for seamless fractionation the devices or techniques used for apheresis must operate in the continuous flow mode. The two techniques that are widely used today include filtration and centrifugation. Filtration does not work in the continuous flow mode whereas; centrifugation subjects leukocytes to mechanical stress. In this paper we have described the design, fabrication and testing of an inexpensive, continuous flow fractionation device for leukapheresis. By exploiting the geometrical differences between erythrocytes and leukocytes we obtained size based fractionation for leukapheresis. Leukocyte depletion prior to blood transfusion can be beneficial. The presence of donor leukocytes in blood used for transfusion can result in several adverse effects including febrile transfusion reactions, graft versus host disease, transmission of infectious agents like viruses (cytomegalovirus (CMV), herpes virus, T-cell leukaemia/lymphoma virus), bacteria, toxoplasma gondii and prions, platelet refractoriness and transfusion related immunomodulation. This device with the integration of a simple on-chip pump (like the polymeric pump described by Unger et al.12) has the capability to be directly interfaced with the donor through venous catheterization to provide continuous fractionation. The leukocyte depleted blood is collected through outlets 1 and 3 and the leukocyte enriched fraction can be continuously returned to the donor.The fluid flow through the device is controlled using a constant flow rate syringe pump. The pump delivers blood into the inlet of the device and travels through the device into the main channel and through the sieves on either side into the diffuser. The volume of blood flow through the main channel and through the sieves depends on the fluidic resistance and the pressure difference. The pressure difference between the main channel and the diffuser drives fluid through the sieves into the diffuser. The fluidic resistance of the sieve-diffuser combination determines the ratio of the volume of blood obtained at the different outlets. In order to ensure that the volumetric flow through individual filter elements is uniform the diffuser was designed with a flared geometry to create a uniform volumetric flow through each filter element. This model works well for fluids. However, for fluids with particles/cells in suspension the operating conditions have to be sufficiently controlled to ensure proper operation.
The device takes advantage of the geometrical differences between erythrocytes and leukocytes to obtain fractionation. The operating flow rates must be optimized such that the pressure gradient across the filter elements does not support the passage of leukocytes into the diffuser. Erythrocytes that are oriented parallel to the floor of the channel can pass through the filter elements without any obstruction. Leukocytes on the other hand can only pass through the filter elements by deforming and squeezing through the filter elements. At flow rates <5 µl/min the pressure gradients across the sieves is not sufficient to support forced transport of leukocytes across the filter elements. However, at higher flow rates the pressure gradient necessary for forced leukocyte transport is available. Further, in order to ensure that the volumetric flow through all of the filter elements was equal an analytical model was developed. An expression to determine the width and hence the optimal shape of the diffuser was determined to provide the necessary fluidic resistance for the given pressure difference to ensure uniform volumetric flow through the sieves. Further, computational fluid dynamics simulations were performed on CFDRC finite volume software. Navier–Stokes simulations were then carried out using the algebraic multigrid (AMG) solver to at least 4 orders of magnitude residual reduction, within ∼1000 iterations. The results show that optimization to a certain extent can be accomplished using this mathematical model. This compares favorably with a linear diffuser (the flared geometry replaced with a linear geometry).
The expression to determine the width of the diffuser ‘wdiffuser’ depends on: the total number of filter elements ‘n’, the width of the channel ‘wchannel’ and the flow ratio ‘![[Fraktur R]](https://www.rsc.org/images/entities/char_e18f.gif) ’. ‘
’. ‘![[Fraktur R]](https://www.rsc.org/images/entities/char_e18f.gif) ’ is the ratio of flow through the main channel to the flow through a single set of filter elements. This ratio can be fixed to obtain the desired device performance. This way we can control the percentage of fluid obtained through the main channel and percentage of fluid obtained through the outlets of the diffusers. Since our end goal is to filter out all erythrocytes we set this ratio low (as in our case 1∶4). The observed value of ‘
’ is the ratio of flow through the main channel to the flow through a single set of filter elements. This ratio can be fixed to obtain the desired device performance. This way we can control the percentage of fluid obtained through the main channel and percentage of fluid obtained through the outlets of the diffusers. Since our end goal is to filter out all erythrocytes we set this ratio low (as in our case 1∶4). The observed value of ‘![[Fraktur R]](https://www.rsc.org/images/entities/char_e18f.gif) ’ is higher than the set value due to the nature of the blood. The flow through the filter elements ‘Y’ becomes smaller due to obstruction or temporary clogging of the sieves due cells at the interface of the main channel and the filter elements (erythrocytes that are improperly oriented and larger leukocytes). The high concentration of cells in blood ensures that erythrocytes are in most cases not oriented parallel to the floor of the channel. The cells near the walls of the main channel are drawn to the sieves due to the pressure gradient that exists across the filter elements. Erythrocytes, if oriented properly travel through the sieves without any delay. Whereas, erythrocytes not oriented properly pause and reorient themselves in order to travel through the sieve. Both leukocyte and erythrocyte interaction with the filter elements strongly depends on the pressure gradient across the filter elements, which is a function of the operating flow rate. At low flow rates, the interaction of leukocytes with the filter elements is weak and the flow through the main channel is sufficient to break this interaction and move them along the main channel. At faster flow rates leukocytes interact strongly with the filter elements and can be forced through into the diffuser and even erythrocytes not oriented properly are forced through the filter elements.
’ is higher than the set value due to the nature of the blood. The flow through the filter elements ‘Y’ becomes smaller due to obstruction or temporary clogging of the sieves due cells at the interface of the main channel and the filter elements (erythrocytes that are improperly oriented and larger leukocytes). The high concentration of cells in blood ensures that erythrocytes are in most cases not oriented parallel to the floor of the channel. The cells near the walls of the main channel are drawn to the sieves due to the pressure gradient that exists across the filter elements. Erythrocytes, if oriented properly travel through the sieves without any delay. Whereas, erythrocytes not oriented properly pause and reorient themselves in order to travel through the sieve. Both leukocyte and erythrocyte interaction with the filter elements strongly depends on the pressure gradient across the filter elements, which is a function of the operating flow rate. At low flow rates, the interaction of leukocytes with the filter elements is weak and the flow through the main channel is sufficient to break this interaction and move them along the main channel. At faster flow rates leukocytes interact strongly with the filter elements and can be forced through into the diffuser and even erythrocytes not oriented properly are forced through the filter elements.
Experiments to determine the fractionation efficiency of the device were performed. Leukocyte depleted blood according to the World Apheresis Association (WAA) is defined as a sample of blood containing <1 × 104 leukocytes ml−1 which corresponds to ∼99% depletion of leukocytes. The experiments were performed at three different flow rates and the recovery of erythrocytes and leukocytes through each of the outlets was documented. As can be seen from the results we can achieve close to 99% depletion of leukocytes and isolation of ∼50% of the total erythrocytes at a flow rate of 5 µl min−1 where our device compares well with commercially available devices (∼99% depletion) for leukocyte depletion used for blood transfusion. Higher flow rates result in isolation of a larger percentage of erythrocytes at the cost of greater leukocyte contamination of the sample.
By reducing the height of the filter elements from 2.5 µm to <0.5 µm this device can be used for fractionation of plasma or plasmapheresis. The use of elastomeric materials might not be suitable for filter elements with sub-micron heights necessary for plasmapheresis. Rigid materials like silicon, glass or polymers with high glass transition temperatures might be necessary. Plasmapheresis applications include plasma exchange (PE), extracorporeal immunoadsorption using columns and low density lipoprotein (LDL) apheresis. Plasmapheresis for therapeutic applications or TPE is commonly performed in patients with autoimmune diseases. TPE can be used to treat both acute and chronic conditions of autoimmune diseases to provide temporary relief by depletion of certain immune factors and complexes.
6.0 Summary
We have developed a microfluidic device for continuous fractionation of blood into its components. This device has the capability to interface directly with the donor and provide continuous fractionation while returning the remaining components into circulation. The device was designed to perform leukapheresis for blood transfusion. We developed an analytical model to optimize the device function and performed computational fluid dynamic simulations to verify the model. Results show that a leukocyte depleted blood with quality comparable to commercially available devices or techniques can be achieved with this device. Simple modifications can be made to the sieve dimensions to allow fractionation of plasma for plasmapheresis.Acknowledgements
The authors would like to acknowledge and thank Daniel Irimia and Kevin R. King of the Center for Engineering in Medicine, and Dr Bryan Hurley, Michael Pazos and Dr Beth McCormick from the Pediatric Mucosal Immunology Laboratory for helpful discussions and providing us with blood samples. We would also like to thank Octavio Hurtado for invaluable help and support with microfabrication and for the excellent facilities. A.S. thanks the Croucher Foundation postdoctoral fellowship for support.References
- J. M. Garcia Gala, P. Rodriguez Vicente, S. Gonzalez Muniz, M. Moran Alcala and J. M. Del Blanco Rodriguez, Utility of red blood cell apheresis in autologous blood donation., Transfus. Sci., 2000, 23, 69–73 CrossRef.
- M. Masse, Universal leukoreduction of cellular and plasma components: process control and performance of the leukoreduction process, Transfus. Clin. Biol., 2001, 8, 297–302 CrossRef CAS.
- R. W. Chu, Leukocytes in blood transfusion: adverse effects and their prevention, Hong Kong Med. J., 1999, 5, 280–284 Search PubMed.
- M. Caggana, J. M. Conroy and K. A. Pass, Rapid efficient method for multiplex amplification from filter paper, Hum. Mutat., 1998, 11, 404–409 CrossRef CAS.
- K. Lindmark and K. G. Engstrom, Use of polyester leukocyte elimination filters in blood filterability research, Biorheology, 1998, 35, 131–140 CrossRef CAS.
- R. H. Carlson, J. P. Brody, S. Chan, C. Gabel, J. Winkleman and R. H. Austin, Self-sorting of white blood cells in a lattice, Phys. Rev. Lett., 1997, 79, 2149–2152 CrossRef CAS.
- Y. Huang, K. L. Ewalt, M. Tirado, R. Haigis, A. Forster, D. Ackley, M. J. Heller, J. P. O'Connell and M. Krihak, Electric manipulation of bioparticles and macromolecules on microfabricated electrodes, Anal. Chem., 2001, 73, 1549–1559 CrossRef CAS.
- J. Yang, Y. Huang, X. B. Wang, F. F. Becker and P. R. Gascoyne, Cell separation on microfabricated electrodes using dielectrophoretic/gravitational field-flow fractionation, Anal. Chem., 1999, 71, 911–918 CrossRef CAS.
- V. I. Furdui and D. J. Harrison, Immunomagnetic T cell capture from blood for PCR analysis using microfluidic systems, Lab Chip, 2004, 4, 614–618 RSC.
- S. S. Shevkoplyas, T. Yoshida, L. L. Munn and M. W. Bitensky, Biomimetic autoseparation of leukocytes from whole blood in a microfluidic device, Anal. Chem., 2005, 77, 933–937 CrossRef CAS.
- F. Petersson, A. Nilsson, C. Holm, H. Jönsson and T. Laurell, Separation of lipids from blood utilizing ultrasonic standing waves in microfluidic channels, Analyst, 2004, 129, 938–943 RSC.
- M. A. Unger, H.-P. Chou, T. Thorsen, A. Scherer and S. R. Quake, Monolithic microfabricated valves and pumps by multilayer soft lithography, Science, 2000, 288, 113–116 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
