Electronically interacting single wall carbon nanotube–porphyrin nanohybrids†
G. M. Aminur
Rahman
a,
Dirk M.
Guldi
*a,
Stéphane
Campidelli
b and
Maurizio
Prato
*b
aInstitute for Physical Chemistry, Friedrich-Alexander-Universität Erlangen-Nürnberg, 91058, Erlangen, Germany. and Radiation Laboratory, University of Notre Dame, Notre Dame, IN 46556, USA. E-mail: guldi@chemie.uni-erlangen.de; Fax: +49-(0)9131/85 28307; Tel: +49-(0)9131/85 27341
bDipartimento di Scienze Farmaceutiche, Università di Trieste, Piazzale Europa 1, 34127, Trieste, Italy. E-mail: prato@units.it; Fax: +39-040 52572; Tel: +39-040 558 7883
First published on 25th November 2005
Abstract
Electronically interacting single wall carbon nanotube–porphyrin nanohybrids were confirmed by microscopic and spectroscopic means to exhibit charge transfer features.
More than a decade after their first discovery1 carbon nanotubes (CNT), and especially single wall carbon nanotubes (SWNT), have emerged as one of the most widely studied nanomaterials. These studies demonstrate the extraordinary potential of CNT for applications in the areas of composites, electronics, optoelectronics, and sensors.2,3 However, to prepare functional SWNT-based nanocomposites, it is fundamental to disentangle the tube ropes. The chemical modification of CNT surfaces has attracted growing interest as a powerful means to disperse and debundle SWNT.4 Methodologies to disperse SWNT are typically divided into two different categories: i) synthesis of covalently linked nanoconjugates, and ii) assembly of non-covalently linked nanohybrids. In the context of chemical modifications, particularly important is the development of novel building blocks for photovoltaic nanodevices that are based, for example, on the decoration of CNT with chromophores.5,6 Porphyrins are functional dyes with unique chromophoric, physical, chemical, and biological features that have been shown to strongly interact with the π-electronic surface of graphite.7 CNT contain, analogous to graphite, highly delocalized π-electrons. Thus, the surface of CNT is easily functionalized through π–π interactions with compounds that possess π-electron rich structures. Importantly, this approach does not disrupt the intrinsic electronic structure of CNT and, subsequently, gives rise to structurally intact—but functional—CNT. First reports demonstrate, in fact, the effective non-covalent functionalization of SWNT with metalloporphyrins and free base porphyrins. This was accomplished in either organic solvents or aqueous media.8
In this communication we explore the immobilization of porphyrins onto the SWNT surface towards novel electron donor–acceptor nanohybrid structures. In contrast to our recent work, which focused on the utilization of relatively redox-inactive pyrenes, we will use tetraphenylporphyrins, that is, the free base (H2P) and the corresponding zinc complex (ZnP), see Fig. 1.
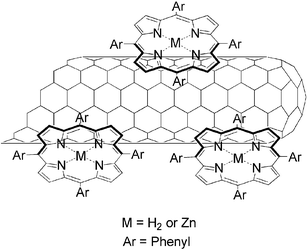 | ||
| Fig. 1 Representation of SWNT/porphyrin nanohybrids. | ||
To suspend SWNT in DMF solutions of either ZnP or H2P the following steps were undertaken. A mixture of 1 mg of purified HIPCO (www.cnanotech.com) SWNT and 2.0 mg of porphyrins (i.e., ZnP or H2P) was vigorously stirred overnight in 10 ml of DMF, followed by strong sonication (i.e., 2 h at 20 °C) and strong centrifugation (i.e., 60 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). In the final step, after removing excess ZnP or H2P, the collected solid was again suspended in DMF by mild sonication (i.e., 5 min at 20 °C). Solubilities of SWNT/ZnP and SWNT/H2P were estimated as 0.1 mg ml−1 and 0.08 mg ml−1, respectively. At the conclusion of our work-up procedure SWNT/ZnP and SWNT/H2P suspensions were generated that are stable for weeks.
000 rpm). In the final step, after removing excess ZnP or H2P, the collected solid was again suspended in DMF by mild sonication (i.e., 5 min at 20 °C). Solubilities of SWNT/ZnP and SWNT/H2P were estimated as 0.1 mg ml−1 and 0.08 mg ml−1, respectively. At the conclusion of our work-up procedure SWNT/ZnP and SWNT/H2P suspensions were generated that are stable for weeks.
In general, suspensions of both porphyrins with SWNT are inherently black colored. In Fig. 2 and ESI Fig. S1,† the porphyrin absorption features are compared in the pristine solutions and in the corresponding SWNT suspensions. Characteristic for ZnP or H2P in these suspensions are the strong Soret-band transitions in the 400–450 nm region accompanied by the weaker Q-band absorptions in the 500–650 nm region. SWNT show the characteristic van Hove singularities in the Vis-NIR part of the spectrum. In the Vis region they are present up to around 450 nm, where the Soret bands of either ZnP or H2P start to dominate the spectrum, while in the NIR region they extend all the way through 1100 nm. These findings are, at first glance, in reasonable agreement with DMF solutions of the individual components, namely, ZnP, H2P, and SWNT. Still, a closer inspection of the absorption spectra reveals some important differences. In particular, broadening of the Soret band and an apparent red-shift are identified. This is especially pronounced for SWNT/H2P. Though the changes are very small, they are outside of the instrumental resolution. Both features (i.e., broadening and red-shift) reflect notable electronic communication between the two π-systems of SWNT and H2P. In comparison, weaker interactions, observed for SWNT/ZnP, are attributed to an appreciable interference between the zinc metal and the SWNT surface. In line with these observations is the fact that SWNT/H2P suspensions are seemingly more stable than SWNT/ZnP.
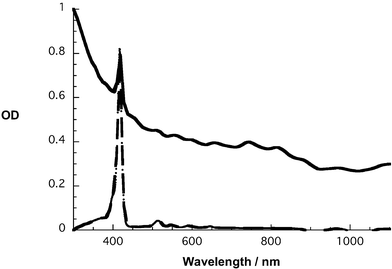 | ||
| Fig. 2 Absorption spectra of H2P (dashed line) and SWNT/H2P (solid line) in DMF. | ||
With the help of conventional transmission electron microscopy the presence of SWNT was further corroborated in SWNT/H2P as well as SWNT/ZnP. A representative image is shown in Fig. 3, which reveals high aspect ratio objects that appear all throughout the scanned area. The mean length of these objects is typically on the order of tens of micrometers, which resembles observations made earlier with SWNT samples of the same origin. The diameters, on the other hand, are found in a wide range starting from a few nanometers and reaching tens of nanometers.
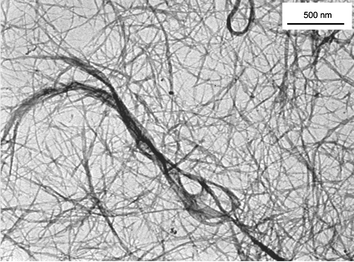 | ||
| Fig. 3 TEM image of SWNT/H2P nanohybrids from a DMF suspension. | ||
Complementary AFM investigations (see Fig. 4) allowed a more detailed analysis of the SWNT/H2P or SWNT/ZnP hybrids. The samples have been prepared by spin coating on silicon wafers from a DMF solution. The two samples are constituted mainly by small bundles of nanotubes (1 to 3 μm length and diameters 10 to 30 nm) and it is also possible to see some individual tubes with diameters as small as 1.5 ± 0.2 nm. Although the two samples are quite similar, the nanotubes in SWNT/ZnP seem to be more aggregated than SWNT/H2P, which suggests again that zinc located in the center of the porphyrin decreases the interactions between the nanotube and the porphyrin π systems. In other words, ZnP is not able to fully exfoliate and/or stabilize individual SWNT in DMF solutions.
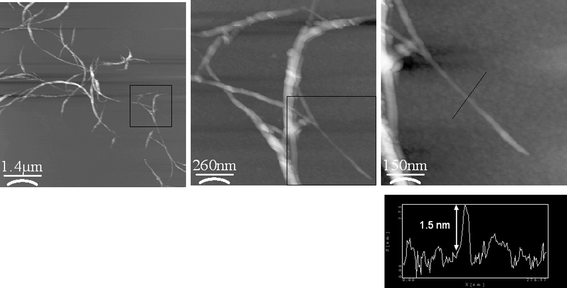 | ||
| Fig. 4 AFM images of SWNT/H2P nanohybrid on silicon wafer. The images (from left to right) show the small bundles of nanotubes which are ended by individual tubes. | ||
In the excited state, mutual electronic interactions between ZnP or H2P and SWNT are deduced from fluorescence spectroscopy, where SWNT/ZnP or SWNT/H2P suspensions were compared with DMF solutions of just ZnP (Φ = 0.04) or H2P (Φ = 0.1) that have matching absorption at the excitation wavelength (i.e., maximum of Soret band absorption). Fig. 5 and ESI Fig. S2† show the typical and widely known fluorescence patterns of ZnP and H2P with maxima at 605/660 nm and 650/715 nm, respectively. They also corroborate that the porphyrin fluorescence is appreciably reduced in both SWNT cases (i.e., SWNT/ZnP and SWNT/H2P). In fact, quenching factors of around 2.6 (SWNT/ZnP) and 5.9 (SWNT/H2P) were deduced. However, a much stronger fluorescence quenching should be expected, since the suspensions contain sizeable amounts of free porphyrins, which are not immobilized onto the SWNT surface, despite the very careful work-up procedure.‡
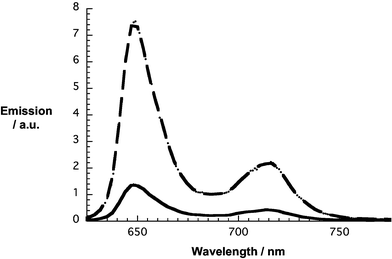 | ||
| Fig. 5 Room temperature fluorescence spectra of H2P (dashed line) and SWNT/H2P (solid line) in DMF with matching absorption at the 418 nm excitation wavelength. | ||
Time-resolved fluorescence spectroscopy further supports the notion that sizeable interactions dominate the excited state behavior of SWNT/ZnP or SWNT/H2P. The fluorescence decays of ZnP (i.e., recorded at 610 nm) and H2P (i.e., recorded at 650 nm) are best represented by mono-exponential fits, from which lifetimes of 2.6 and 12.8 ns, respectively, were derived. Similarly, when fitting the fluorescence decay profiles for SWNT/ZnP or SWNT/H2P with mono-exponential functions, acceptable fits (i.e., χ2 values of around 1 or less) were established that basically yield the same lifetimes, despite the steady-state quenching. This leads us to conclude that the time scales of SWNT/ZnP and SWNT/H2P interactions are outside our instrumental time resolution of approximately 100 ps.
In the final set of experiments, we have focused our work on transient absorption measurements, following the photoexcitation of ZnP or H2P at 387 (150 femtoseconds) or 532 nm (5 nanoseconds) in the absence and presence of SWNT. This was meant to provide insight into the excited state interactions between SWNT and porphyrins, which were not resolved in the aforementioned fluorescence experiments. For both porphyrins—in the absence of any SWNT—singlet–singlet (∼1 ps) and long-lived triplet–triplet features (up to 400 µs) were recorded that include transient bleaching of the main transitions, namely, Soret- and Q-bands. An illustration of the H2P triplet–triplet features is depicted in ESI Fig. S3.† The most important triplet attributes, besides the ground state related bleaching, are maxima in the NIR, which for ZnP are found at 840 nm, while for H2P they are located at 780 nm.
When photoexciting SWNT/H2P at 387 nm the internal conversion from the second singlet excited to the first singlet excited state—similar to just H2P—is seen within the first few picoseconds. While in the absence of SWNT the singlet–singlet transitions of H2P are stable on the 1.6 ns time scale of our experiments, it decays almost instantaneously when SWNT are present. As Fig. 6 points out, the singlet lifetime is reduced to 12.5 ps—compare this value with the H2P singlet lifetime of 11.5 ns. The spectral characteristic of the new species is a broadly absorbing transient (vide infra).
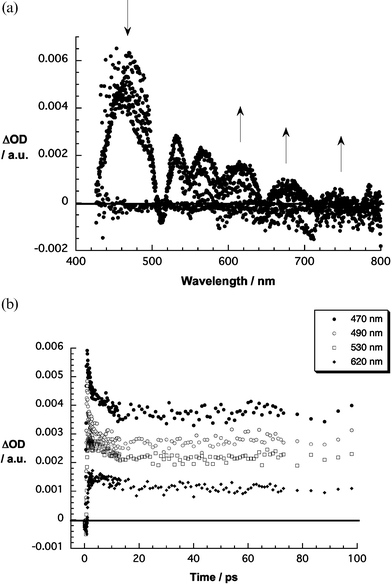 | ||
| Fig. 6 Upper part: differential absorption spectra (visible) obtained upon femtosecond flash photolysis (387 nm) of SWNT/H2P with several time delays between 0 and 50 ps at room temperature. Lower part: time–absorption profiles of the spectra shown above at 470, 490, 530, and 620 nm. | ||
In line with the femtosecond data, SWNT/ZnP or SWNT/H2P suspensions, when photoexcited at 532 nm on the nanosecond scale, also gave rise to characteristic differential absorption changes—compare Fig. 7 with ESI Fig. S3.† Obviously, the triplet signature is mostly absent. Moreover, the transients, which appear much broader in the presence of SWNT, bear some resemblance to those seen for the one-electron oxidized form of either ZnP or H2P. However, a valuable kinetic analysis of the decay dynamics is prevented by the superimposing features of the residual triplet excited state. Important is also the extremely long-lived nature of the porphyrin triplet excited states (i.e., ZnP = 44 µs; H2P = 380 µs). Purging oxygen helped to accelerate the triplet decay, due to nearly diffusion controlled formation of singlet oxygen, and a kinetic analysis provided, at least, for SWNT/H2P a lifetime of around 110 ns.§
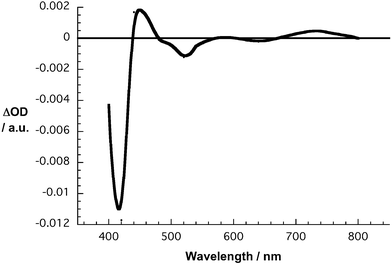 | ||
| Fig. 7 Differential absorption spectrum (near-infrared) obtained upon nanosecond flash photolysis (532 nm) of SWNT/H2P in nitrogen saturated DMF with a 50 ns time delay at room temperature. | ||
In summary, H2P and ZnP were probed to interact directly with the SWNT surface. Hereby, suspendable and stable SWNT/H2P and SWNT/ZnP nanohybrids are realized. A series of microscopic and spectroscopic investigations reveal mutually interacting π-systems, that is, SWNT and H2P or ZnP. Consequences of such electronic interactions are charge transfer features that are even persistent on the time scale of hundreds of nanoseconds.
Acknowledgements
This work was carried out with partial support from the EU (RTN networks “WONDERFULL” and “CASSIUSCLAYS”), MIUR (PRIN 2004, prot. 2004035502), SFB 583, Fonds der Chemischen Industrie, and the Office of Basic Energy Sciences of the U.S. Department of Energy (NDRL 4652).Notes and references
- S. Ijima and T. Ichihashi, Nature, 1993, 363, 603 CrossRef CAS.
- (a) M. Ouyang, J.-L. Huang and C. M. Lieber, Acc. Chem. Res., 2002, 35, 1018 CrossRef CAS; (b) H. Dai, Acc. Chem. Res., 2002, 35, 1035 CrossRef CAS; (c) P. Avouries, Acc. Chem. Res., 2002, 35, 1026 CrossRef CAS; (d) J. J. Davis, K. S. Coleman, B. R. Azamian, C. B. Bagshaw and M. L. H. Green, Chem. Eur. J., 2003, 9, 3732 CrossRef CAS; (e) Y. Lin, S. Taylor, H. Li, K. A. S. Fernando, L. Qu, W. Wang, L. Gu, B. Zhou and Y.-P. Sun, J. Mater. Chem., 2004, 14, 527 RSC.
- (a) P. Calvert, Nature, 1999, 399, 210 CrossRef CAS; (b) R. Dagani, Chem. Eng. News, 1999, 77, 25; (c) R. Andrews, D. Jacques, A. M. Rao, T. Rantel, F. Derbyshire, Y. Chen, J. Chen and R. C. Haddon, Appl. Phys. Lett., 1999, 75, 1329 CrossRef CAS; (d) P. M. Ajayyan, L. S. Schandler, C. Giannaris and A. Rubio, Adv. Mater., 2000, 12, 750 CrossRef CAS.
- (a) A. Hirsch, Angew. Chem., Int. Ed., 2002, 41, 1853 CrossRef CAS; (b) Y.-P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096 CrossRef CAS; (c) D. Tasis, N. Tagmatarchis, V. Georgakilas and M. Prato, Chem. Eur. J., 2003, 9, 4000 CrossRef CAS; (d) N. Tagmatarchis and M. Prato, J. Mater. Chem., 2004, 14, 437 RSC; (e) D. M. Guldi, G. M. A. Rahman, F. Zerbetto and M. Prato, Acc. Chem. Res., 2005, 38, 871 CrossRef CAS; (f) C. A. Dyke and J. M. Tour, Chem. Eur. J., 2004, 10, 812 CrossRef CAS.
- (a) H. Li, R. B. Martin, B. A. Harruff, R. A. Carino, L. F. Allard and Y.-P. Sun, Adv. Mater., 2004, 16, 896 CrossRef CAS; (b) D. Baskaran, J. W. Ways, X. P. Zhang and M. S. Bratcher, J. Am. Chem. Soc., 2005, 127, 6916 CrossRef CAS.
- (a) A. Star, J. F. Stoddart, D. W. Steuerman, M. R. Diehl, A. Boukai, E. W. Wang, X. Yang, S.-W. Chung, H. Choi and J. R. Heath, Angew. Chem., Int. Ed., 2001, 40, 1721 CrossRef CAS; (b) D. W. Steuerman, A. Star, R. Narizzano, H. Choi, R. S. Ries, C. Nicolini, J. F. Stoddart and J. R. Heath, J. Phys. Chem. B, 2002, 106, 3124 CrossRef CAS; (c) A. Star, Y. Liu, K. Grant, L. Ridvan, J. F. Stoddart, D. W. Steuerman, M. R. Diehl, A. Boukai and J. R. Heath, Macromolecules, 2003, 36, 553 CrossRef CAS; (d) L. W. Qu, R. B. Martin, W. J. Huang, K. F. Fu, D. Zweifel, Y. Lin, Y.-P. Sun, C. E. Bunker, B. A. Harruff, J. R. Gord and L. F. Allard, J. Chem. Phys., 2002, 117, 8089 CrossRef CAS; (e) R. B. Martin, L. W. Qu, Y. Lin, B. A. Harruff, C. E. Bunker, J. R. Gord, L. F. Allard and Y.-P. Sun, J. Phys. Chem. B, 2004, 108, 11447 CrossRef CAS.
- (a) N. J. Tao, Phys. Rev. Lett., 1996, 76, 4066 CrossRef CAS; (b) T. Sagara, M. Fukuda and N. Nakashima, J. Phys. Chem. B, 1998, 102, 521 CrossRef CAS.
- (a) H. Murakami, T. Nomura and N. Nakashima, Chem. Phys. Lett., 2003, 378, 481 CrossRef CAS; (b) J. Chen and C. P. Collier, J. Phys. Chem. B, 2005, 109, 7605 CrossRef CAS; (c) H. Li, B. Zhou, Y. Lin, L. Gu, W. Wang, K. A. S. Fernando, S. Kumar, L. F. Allard and Y.-P. Sun, J. Am. Chem. Soc., 2004, 126, 1014 CrossRef CAS; (d) T. Hasobe, S. Fukuzumi and P. V. Kamat, J. Am. Chem. Soc., 2005, 127, 11884 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: absorption and fluorescence spectra. See DOI: 10.1039/b515394h |
| ‡ An additional, second centrifugation/suspension cycle leads to a ZnP fluorescence quenching factor of 7.2 for SWNT/ZnP. |
| § For SWNT/ZnP a meaningful analysis was impossible. |
| This journal is © The Royal Society of Chemistry 2006 |
