Magnesium isotopic analysis of olivine by laser-ablation multi-collector ICP-MS: composition dependent matrix effects and a comparison of the Earth and Moon
M. D.
Norman
ab,
M. T.
McCulloch
a,
H. St. C.
O’Neill
a and
G. M.
Yaxley
a
aResearch School of Earth Sciences, Australian National University, Canberra, ACT 0200, Australia. E-mail: Marc.Norman@anu.edu.au
bLunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058, USA
First published on 18th November 2005
Abstract
Magnesium isotopic compositions of olivine were measured by laser-ablation multi-collector ICP-MS using an ArF 193 nm excimer laser. Analytical precision based on replicate analyses of mantle olivine is ∼0.2 permil (2SD). This is about an order of magnitude better than has been reported by ion microprobe or thermal ionization mass spectrometry, but about a factor of 2–3 less precise than can be obtained by solution aspiration multi-collector ICP-MS with a similar signal intensity. Analysis of synthetic olivines demonstrates a composition-dependent matrix effect in which systematically heavier isotopic compositions are measured in olivines with lower Mg#. The magnitude of this matrix effect varies with laser operating parameters such as spot diameter and repetition rate, and appears to be due to mass-dependent isotope fractionation at the ablation site rather than within the plasma. After correction for matrix effects, the Mg isotopic composition of olivine from the Moon is shown to be identical with that of the Earth’s mantle.
Introduction
Magnesium isotopes potentially offer new insights into a diverse range of geological processes, including the formation of carbonate sediments,1 weathering,2 evaporation and condensation in the solar nebula,3–5 and evidence for extinct radionuclides in the early solar system.6 Previous studies of Mg isotopic compositions have been conducted mainly using ion microprobe secondary ionisation mass spectrometry (SIMS)3,6 and thermal ionization mass spectrometry (TIMS).7 Recently, Mg isotopic compositions have been determined by multi-collector magnetic-sector ICP-MS using both laser ablation and solution aspiration.1,2,4,5 In order to investigate igneous processes on the Earth, Moon, Mars and meteorite parent bodies, and the thermal histories of the terrestrial planets, we have developed methods for measuring magnesium isotopic compositions of the mineral olivine using laser-ablation multi-collector ICP-MS (LA-MC-ICP-MS). This approach provides high spatial resolution, precise isotope ratio measurements, and rapid analysis with a minimum of sample preparation.Olivine is a natural choice for these types of studies as it is one of the most common minerals in rocky bodies and has a restricted range of compositions, being to a good approximation a binary solid solution between Mg2SiO4 and Fe2SiO4. Olivine compositions, expressed as Mg# (=% atomic Mg/(Mg + Fe)), range from ∼70–90 in most mantle-derived rocks. Despite this relatively small range of major element variability, we have discovered what appears to be a composition-dependent matrix effect on the measured Mg isotopic composition of olivine determined with our LA-MC-ICP-MS system. The magnitude of this effect is surprisingly large and could lead to erroneous conclusions if not recognized and properly corrected. Here we describe our methods, and present analytical results that demonstrate a systematic variation of Mg isotopic composition with the Mg# of synthetically prepared olivine. We also present a reconnaissance study of lunar and terrestrial olivines, using the synthetic olivines to apply a correction for composition-dependent matrix effects on the Mg isotopic compositions.
Methods and instrumentation
Separated olivine crystals were mounted in epoxy and polished to expose grain interiors. Major element compositions of the grains were determined by electron microprobe prior to isotopic analysis. Ablation was conducted under a helium atmosphere in a custom-built, small volume sample cell similar to that described by Eggins et al.,8 using a Compex UV pulsed 193 nm ArF excimer laser. The laser was operated at 80 mJ per pulse, with a repetition rate of 5 Hz and 47–62 micron spot diameters. The sample gas flow was mixed with Ar downstream of the ablation chamber and passed through a mixing cell prior to introduction into the ICP.Magnesium isotopic compositions were measured using a Finnigan MAT Neptune MC-ICP-MS operated in medium resolution mode. Data were collected on three Faraday cups using 1011 Ω resistors. Each analysis consisted of 20 cycles of data collected with an integration time of 4.2 s per cycle; the total analysis time was ∼80 s. 26Mg was measured at an off-center, low-mass position (25.972) to avoid potential interference from 12C14N+. An on-peak baseline was measured on the carrier gas without ablation and subtracted from each analysis using the Neptune software. Gas background intensities were typically 2–3 mV on mass 24, and 0.3–0.4 mV on masses 25 and 26. As we were investigating mass-dependent isotopic variations, no corrections for mass bias were applied to the data. Isotopic compositions are reported relative to analyses of natural mantle olivine from San Carlos, New Mexico, measured with the unknowns.
In addition, solutions containing variable mixtures of Mg and Fe were analyzed using a self-aspirating, 100 μl min−1 concentric glass nebulizer and double-pass spray chamber. These solutions all contained 1 ppm of Mg, and 0.26–2.2 ppm of Fe, representing a similar range of Mg# as for the synthetic olivines (Mg# = 50–100). The primary reason for analysing these solutions was to test whether the variation in Mg isotopic composition measured by laser ablation could be related to space-charge effects in the ion beam downstream of the plasma. Similar operating conditions were used for both the laser ablation and solution aspiration analyses, with the carrier gas flow rate adjusted to optimize sensitivity.
Preparation of synthetic olivine
Following preliminary analyses of natural olivines in which the measured 25Mg/24Mg and 26Mg/24Mg ratios appeared to vary systematically with Mg#, we decided to investigate possible matrix effects related to bulk composition using experimentally synthesized olivines with a range of compositions. Olivines with Mg# = 50–90 were synthesised from the oxides MgO, Fe2O3 and “silicic acid” (SiO2·xH2O). Reagents were dried before weighing by heating in platinum crucibles to 1000 °C for at least 24 h. Stoichiometric proportions of these oxides were weighed and thoroughly mixed in an agate mortar under acetone, and pressed into pellets using a tungsten carbide die. These pellets were suspended individually in a vertical muffle furnace equipped for gas mixing, and sintered at 1400 °C for about 24 h under a flowing stream of CO and CO2 in the ratio 1∶1, producing an oxygen fugacity of −8.6 bar. The pellets were then cooled under the same gas mix to 800 °C before quenching to room temperature by lowering them into the cool zone at the bottom of the furnace. In order to achieve a fully dense product, aliquots of each pellet were lightly ground under acetone, loaded into an iron capsule and run for 1 h at 1500 °C (Mg# = 70, 80, 90) or 1400 °C (Mg# 50, 60) and 1.0 GPa in a conventional 15.875 mm diameter piston-cylinder solid-media apparatus, using a NaCl–pyrex cell with a graphite heater.Results
Synthetic olivines and solutions
LA-MC-ICP-MS analysis of the synthetic olivines produced signal intensities of ∼1 to 4 V for 24Mg, with mean intensities strongly correlated with the Mg# of the olivine (Fig. 1). Measured 25Mg/24Mg and 26Mg/24Mg isotopic compositions varied systematically to heavier values with decreasing Mg# of the olivines (Fig. 1). Ablation using smaller laser spot diameters produced more extreme variations in measured Mg isotopic compositions of the synthetic olivines (Fig. 2), as did increasing the repetition rate and the power density of the laser (not shown). Measured 25Mg/24Mg and 26Mg/24Mg ratios of these olivines fall along a 2∶1 trend (Fig. 3), consistent with a mass-dependent process being responsible for the isotope fractionation. Signal intensities for the 1 ppm Mg solutions were ∼0.45 V on 24Mg. In contrast to the synthetic olivines, the solutions showed no systematic variation of Mg isotopic composition over a similar range of Mg# (Fig. 4). These data point toward a laser-induced mechanism as the primary cause for the Mg isotope fractionation observed in the olivines, as discussed below. | ||
Fig. 1
25Mg/24Mg and 26Mg/24Mg isotopic compositions measured by laser ablation MC-ICP-MS on synthetic olivines with Mg# = 50–90, as a function of signal intensity of 24Mg (volts). Data were obtained with a laser spot diameter of 47 µm. Isotope ratios are reported as ε-unit (parts in 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000) deviations from a San Carlos (SC) reference olivine run with the unknowns. 10 ε-units = 1 permil. Error bars are 1 standard error (1 SE) of the measurement. 000) deviations from a San Carlos (SC) reference olivine run with the unknowns. 10 ε-units = 1 permil. Error bars are 1 standard error (1 SE) of the measurement. | ||
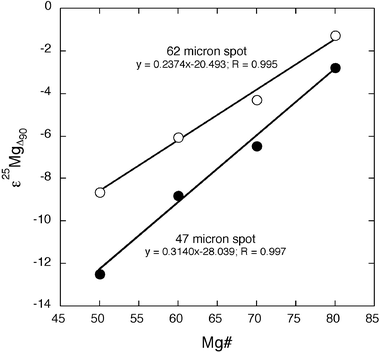 | ||
| Fig. 2 The variation of Mg isotopic composition versus. Mg# of synthetic olivines measured using two laser spot diameters (47 and 62 µm). ε25MgΔ90 is the difference between the 25Mg/24Mg ratio measured in the Mg# = 50–80 experiments and that measured in the Mg# = 90 experiment (see Fig. 1). Larger laser spot diameters produced less extreme variations in measured Mg isotopic compositions. | ||
 | ||
| Fig. 3 Measured 25Mg/24Mg and 26Mg/24Mg ratios of the synthetic olivines fall along a 2∶1 trend, shown by the diagonal line, consistent with a mass-dependent process responsible for the isotope fractionation produced during a laser ablation MC-ICP-MS analysis. The data are reported relative to bracketing analyses of the San Carlos (SC) reference olivine to correct for instrument drift. Error bars are 1 SE of the measurement. | ||
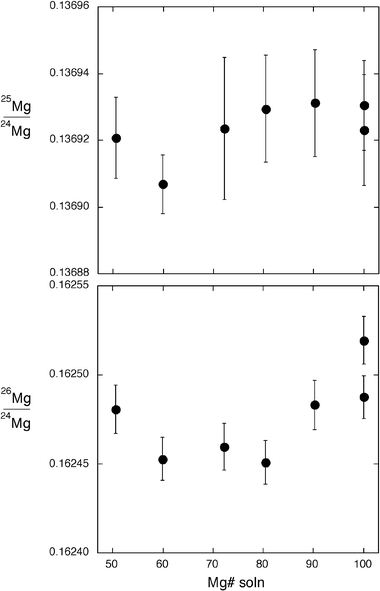 | ||
| Fig. 4 Mg isotopic compositions of solutions with a range of Mg# similar to the synthetic olivines. In contrast to laser ablation analysis of the olivines, solution aspiration showed no variation of measured Mg isotopic composition over a similar range of Mg#. This precludes a space-charge effect in the ion beam as the primary cause of Mg isotope variation in the laser ablation analysis of the olivines. Error bars are 1 SE of the measurement. The 2SD for these 7 solution analyses is ±0.14 permil amu−1. | ||
Natural olivines
The external precision of the laser ablation MC-ICP-MS data was established by analyzing olivines from a peridotite mantle xenolith from Mt. Shadwell, Victoria,9 relative to the San Carlos reference olivine. The 2SD for 10 replicate analyses of the Mt. Shadwell olivines was ±0.2 permil amu−1 for 25Mg/24Mg and 26Mg/24Mg (Fig. 5). This is about an order of magnitude better precision than was reported previously for Mg isotope measurements by ion microprobe6 and thermal ionization mass spectrometry,7 but about a factor of 2–3 worse than has been obtained by solution aspiration MC-ICP-MS for similar signal intensities.1,2,5 As the Mg# of the Mt. Shadwell olivines are identical to the San Carlos reference olivine (Mg# = 90–91), no corrections for matrix effects were applied to these data. | ||
| Fig. 5 Replicate analyses of olivines from Mt. Shadwell peridotite sample MS95-5 relative to the San Carlos reference olivine demonstrate a 2σ standard deviation (2SD, n = 10) precision of ±2 ε-units (0.2 permil) per amu. No corrections for matrix effects were applied to these data. The measured Mg isotopic composition of the Mt. Shadwell olivine is identical to that of the San Carlos reference within analytical uncertainty. Error bars are 1 SE of the measurement. | ||
In addition, Mg isotopic compositions of olivines from four Apollo 12 lunar mare basalts (12![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 009, 12
009, 12![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 035, 12
035, 12![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 040, 12
040, 12![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 075) were measured by LA-MC-ICP-MS to compare the composition of the Earth and Moon. The lunar olivines had Mg# = 48–75,10 significantly lower than the San Carlos reference olivine. In contrast to the relatively restricted range of measured Mg isotopic composition obtained for the San Carlos olivine, the lunar olivines produced a broad range of measured Mg isotopic compositions, with apparently heavier Mg isotopic compositions in grains with lower Mg# as measured by the mean 24Mg signal intensity (Fig. 6). This follows the trend observed in the synthetic olivines and likely reflects a composition-dependent matrix effect rather than real isotopic variability, as discussed in the following section.
075) were measured by LA-MC-ICP-MS to compare the composition of the Earth and Moon. The lunar olivines had Mg# = 48–75,10 significantly lower than the San Carlos reference olivine. In contrast to the relatively restricted range of measured Mg isotopic composition obtained for the San Carlos olivine, the lunar olivines produced a broad range of measured Mg isotopic compositions, with apparently heavier Mg isotopic compositions in grains with lower Mg# as measured by the mean 24Mg signal intensity (Fig. 6). This follows the trend observed in the synthetic olivines and likely reflects a composition-dependent matrix effect rather than real isotopic variability, as discussed in the following section.
 | ||
| Fig. 6 (a) Measured 25Mg/24Mg for San Carlos and lunar olivines relative to their Mg content as indicated by mean signal intensity (volts) of 24Mg. The trend toward heavier isotopic compositions with decreasing Mg content in the lunar olivines reflects a composition-dependent matrix effect rather than real isotopic variability in these samples. (b) Mg isotopic compositions of the lunar olivines corrected to an equivalent value at Mg# = 90 using the relationships given in Fig. 2 and reported relative to the San Carlos reference olivine to correct for instrument drift. Corrected Mg isotopic compositions of the lunar olivines show no residual variation with Mg# and are indistinguishable from the San Carlos reference olivine. The mean corrected Mg isotopic compositions of the lunar olivines are 25MgSC = −0.94 ± 0.98, and 26MgSC = −1.00 ± 0.92. Error bars are 1 SE of the measurement. | ||
Discussion
Composition dependent matrix effect
The systematic variation of measured Mg isotopic composition with Mg# observed in the synthetic olivines (Fig. 1) cannot reflect real differences in Mg isotopic compositions of these olivines, as all experiments were prepared from a common set of starting materials. It appears to reflect a matrix effect in which the measured Mg isotopic composition depends on the Mg# of the olivine. The 2∶1 relationship between 25Mg/24Mg and 26Mg/24Mg (Fig. 3) indicates a mass-dependent process, and shows that the correlation between isotopic composition and signal intensity (which is directly related to Mg abundance in the olivine) is unlikely to be an artifact introduced by the on-peak baseline correction11 or unrecognized interferences.A specific mechanism responsible for the matrix effect has not yet been identified. Hirata et al.12 found that apparent trends to heavier Cu and Fe isotopic compositions during the laser ablation MC-ICP-MS analysis of metals can be instrumental artifacts caused by slow amplifier response to a rapidly changing signal. This mechanism does not explain the composition-dependent matrix effect described here as all of our olivines showed similar relative changes in signal intensity during a laser ablation analysis regardless of their composition. The well-defined mass-dependent correlation of measured 25Mg/24Mg and 26Mg/24Mg ratios (Fig. 3) and the similar analytical precision inferred for both 25Mg/24Mg and 26Mg/24Mg ratios (Fig. 5) further discount slow amplifier response as the primary cause of the matrix effect.
Jackson and Günther13 concluded that laser ablation of native Cu can produce isotopic fractionation both at the ablation site and by incomplete vaporisation and ionisation of particles within the plasma. Incomplete vaporization of large particles within the plasma has also been linked to inter-element fractionation and matrix effects reported for laser ablation ICP-MS trace element analysis of silicate and lithium borate glasses.14,15 By analogy, the matrix effect on Mg isotopic composition that we observed might be related to systematic variations of particle size and isotopic composition with Mg# of the olivine, and incomplete vaporization of these particles in the plasma.
The fact that larger spot diameters reduce the magnitude of the matrix effect (Fig. 2) points to a laser-induced mechanism at the sample site as the primary mechanism responsible for the Mg isotopic fractionation reported here. As illustrated in Fig. 2, a ∼30% increase in the spot diameter (from 47 to 62 µm) results in a ∼30% decrease in the relative magnitude of the Mg isotopic fractionation. This is supported by the observation that changing other analytical conditions to minimize the aspect ratio of the laser pit, such as reducing the laser power density and repetition rate, also minimises the matrix effect. Examination of the time-resolved isotopic variations during laser ablation analysis of synthetic olivines reveals systematic trends toward heavier Mg isotopic compositions with time in samples with a lower Mg# (Fig. 7). This further implicates down-hole isotopic fractionation during the laser ablation process as the primary mechanism responsible for the matrix effect, with Mg isotopic fractionation possibly related to processes such as condensation and remobilization of ablated material on the walls of the ablation pit. Similar effects have been inferred as the cause of time-dependent fractionation of trace elements during laser ablation ICP-MS analyses of silicate glasses and minerals.8,16
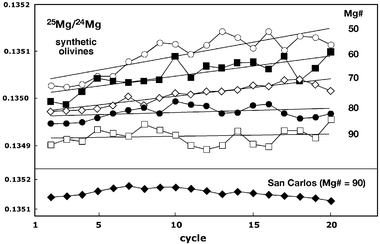 | ||
| Fig. 7 Time-resolved variation of measured 25Mg/24Mg in the synthetic and San Carlos olivines during laser ablation analysis. Isotope ratios measured in the synthetic olivines show systematic trends to progressively heavier isotopic compositions with decreasing Mg# of the olivine. Laser-induced isotopic fractionation during the analysis appears to explain the observed composition-dependent matrix effect inferred from Fig. 1. The trends for the synthetic olivines represent averages of the isotope ratios measured in each cycle for 3 different analyses of each Mg# experiment. The San Carlos trend reflects the average of 27 analyses run with the synthetic olivines. | ||
Mg isotopic composition of lunar basalts
Mg isotopic compositions of olivines from the lunar mare basalts show a systematic trend to heavier Mg isotopic compositions in grains with lower Mg# (Fig. 6). We interpret this as a composition-dependent matrix effect similar to that seen in the synthetic olivines, rather than evidence for a heavier Mg isotopic composition of the Moon compared with the Earth. In order to make a direct comparison with the San Carlos reference olivine, a correction was applied to the lunar data to normalize the Mg compositions to equivalent values at Mg# = 90 using the relationships shown in Fig. 2. After this correction, the lunar olivines were found to have Mg isotopic compositions identical within uncertainty to the San Carlos terrestrial reference olivine (Fig. 6). Based on this preliminary study, we find no measurable difference in the Mg isotopic composition of the Earth and Moon.Conclusions
Magnesium isotopic compositions of olivine were measured by laser-ablation multi-collector ICP-MS with a precision of ∼0.2 permil (2 ε-units), or about an order of magnitude better than results typically obtained by ion microprobe and thermal ionization mass spectrometry. A laser-induced matrix effect was found in which systematically heavier Mg isotopic compositions were measured in olivines with lower Mg abundances. Fortunately, this effect is a regular function of the Mg# of the olivine, which allows corrections to be made. The exact cause of this matrix effect requires additional investigation, but it appears to be related to isotopic fractionation generated at the sample site during the laser ablation analysis. After correction for matrix effects, the Mg isotopic compositions of olivine from the Moon appear to be identical to the Earth’s mantle. This new constraint will need to be considered in models for the formation of the Earth and Moon.Acknowledgements
We thank Les Kinsley for assistance with the isotopic analyses, Steve Eggins for discussions of amplifier response, and Dean Scott and Bill Hibberson for their help in preparation of the synthetic olivines. Two anonymous journal reviews are appreciated.References
- A. Galy, M. Bar-Matthews, L. Halicz and R. K. O’Nions, Earth Planet. Sci. Lett., 2002, 201, 105–115 CrossRef CAS.
- S. de Villiers, J. A. D. Dickson and R. M. Ellam, Chem. Geol., 2005, 216, 133–142 CrossRef CAS.
- F. M. Richter, A. M. Davis, D. S. Ebel and A. Hashimoto, Geochim. Cosmochim. Acta, 2002, 66, 521–540 CrossRef CAS.
- E. D. Young, R. D. Ash, A. Galy and N. Belshaw, Geochim. Cosmochim. Acta, 2002, 66, 683–698 CrossRef CAS.
- E. D. Young and A. Galy, Rev. Mineral. Geochem., 2004, 55, 197–230 Search PubMed.
- S. S. Russell, A. M. Davis, G. J. MacPhearson, Y. Guan and G. R. Huss, Meteor. Planet. Sci., 2000, 35, 1051–1066 Search PubMed.
- T. M. Esat and S. R. Taylor, Int. Geol. Rev., 1999, 41, 31–46 Search PubMed.
- S. M. Eggins, L. P. J. Kinsley and J. M. G. Shelley, Appl. Surf. Sci., 1998, 127–129, 278–286 CrossRef CAS.
- M. D. Norman, Contrib. Mineral. Petrol., 1998, 130, 240–255 CrossRef CAS.
- D. J. Bombardieri, M. D. Norman, V. S. Kamenetsky and L. Danyushevsky, Meteor. Planet. Sci., 2005 Search PubMed , in the press.
- F. Albarede and B. L. Beard, Rev. Mineral. Geochem., 2004, 55, 113–152 Search PubMed.
- T. Hirata, Y. Hayano and T. Ohno, J. Anal. At. Spectrom., 2003, 18, 1283–1288 RSC.
- S. E. Jackson and D. Günther, J. Anal. At. Spectrom., 2003, 18, 205–212 RSC.
- H.-R. Kuhn and D. Günther, J. Anal. At. Spectrom., 2004, 19, 1158–1164 RSC.
- Z. Yu, M. Norman and P. Robinson, Geostand. Newsl., 2003, 27, 67–89 CrossRef CAS.
- J. Kosler, M. Wiedenbeck, R. Wirth, J. Hovorka, P. Sylvester and J. Mikova, J. Anal. At. Spectrom., 2005, 20, 402–409 RSC.
| This journal is © The Royal Society of Chemistry 2006 |
